Sympathetic nervous system and chronic bladder pain: a new tune for an old song
Introduction
Chronic bladder pain (CBP) is a condition characterized by supra-pubic pain or discomfort in the pelvic area related to bladder filling, of at least 6 months of duration (1). It is often accompanied by wide range of lower urinary tract symptoms, such as frequency, nocturia, and urgency. Patients may refer pain or pressure in the pelvic area and in other areas of the body, including the back and the neck (2). Some patients present typical cystoscopic findings including bladder glomerulations during hydrodistention and ulcerative areas of the bladder mucosa, known as Hunner’s lesion (1). Bladder biopsy may confirm the diagnosis and exclude confusable diseases (1). Typical pathological findings include bladder inflammation, mast cell accumulation in the bladder wall and urothelial thinning or disruption (1).
The aetiology of CBP is unknown. Taking in consideration the morphological changes in the urothelium, such as urothelial thinning, decreased urothelial cell cohesion and loss of the protective glycosaminoglycan layer led to the common belief that CBP is linked to an increase permeability of the urothelium. As a consequence, the diffusion of urine constituents into the bladder wall may induce an intense inflammatory infiltrate, mast cells accumulation and activation of nociceptive fibres (3). However the causes impairing the ability of the urothelium to maintain a barrier and undergo repair following injury are not clear. The release of an anti-proliferative compound slowing urothelial growth, autoimmune disorders, chronic infection by unknown bacteria, bladder ischemia, changes in nitric oxide metabolism or unknown toxin agents, all have been forward as possible mechanism to the urothelial changes (3). The leaky urothelium, whatever the cause, it is expected to self-perpetuate bladder inflammation and nociceptive fibres activation (3).
CBP is frequently associated with other painful syndromes, such as irritable bowel syndrome (IBS), chronic fatigue and fibromyalgia (4). The reason for such association is unclear, although it might suggest a common systemic background. Here, we will review the evidence supporting the involvement of sympathetic in the etiology of CBP.
Evidence of the sympathetic nervous system dysfunction in patients with CBP
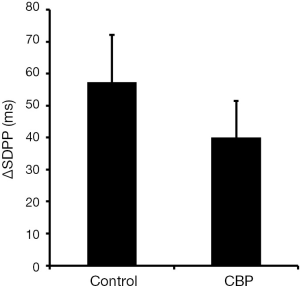
In a recent work, Charrua and co-workers have analysed the activity of sympathetic nervous system by performing the TILT test in ten female patients with CBP (5). Ten aged match healthy woman were used as controls. The TILT test analysis the increase in sympathetic nervous system activity generated by the change in body position, measuring the variation of the standard deviation of the P wave interval (∆SDPP, a parameter that inversely correlates with sympathetic activity), the root-mean-square difference among successive normal R-R intervals in heart period series (rMSSD, a parameter that measures the parasympathetic activity) and the average of all baroreflex sequences registered as the test progresses (BRS, a parameter that measures the parasympathetic activity) (5). Patients with CBP presented a ∆SDPP value of 24.2±18 ms, which was lower than the 57.2±23.0 ms presented by controls, showing an overactivity of sympathetic nervous system (Figure 1) (5). The values of rMSSD, 5.6±8.4 ms, and of BRS, 7.7±8.2 ms/mmHg, presented by CBP patients did not differ from values presented by controls (6.3±2.8 ms and 7.1±3.8 ms/mmHg, respectively) (5).
Similarly, Lutgendorf and co-workers examined 14 patients with CBP and 14 healthy individuals and observed that the former had higher resting heart rate and elevated resting diastolic blood pressure, suggesting that these patients harbour an autonomic dysregulation (6). Also, Williams and co-workers analysed changes in heart rate variability in 26 subjects with CBP and 32 healthy subjects and found that CBP patients had lower vagal activity and a shift toward sympathetic nervous system dominance (7).
The levels of noradrenaline were analysed by high-performance liquid chromatography (HPLC), in the blood and urine 24 h collected from 18 patients with CBP and ten aged matched controls (5). Also, plasmatic noradrenaline levels were also analysed after performing the TILT test in CBP patients. It was observed that in supine position, the levels of plasma noradrenaline were higher in CBP patients than healthy subjects (5). However, no changes in plasma noradrenaline were observed between patients with CBP and healthy subjects in the upright position (5). Concerning the 24 h urine, CBP patients presented higher levels of noradrenaline than healthy subjects (5). Altogether, these findings corroborate the overactivity of sympathetic nervous system of CBP patients.
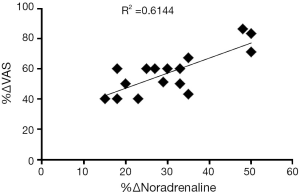
Pinto and co-workers studied if there was a correlation between CBP and the observed increase in noradrenaline levels (8). For that, they assessed visual analogue scale for pain and analysed urinary noradrenaline levels before and 1 month after intra-trigonal injection of 100 U onabotulinum type A in 16 patients with CBP (8). These authors have observed a positive correlation between the improvement of visual analogue scale for pain and the decrease in the 24 h urinary norepinephrine excretion, upon onabotulinum type A treatment (Figure 2) (8).
The above mentioned data is in agreement with other studies that have suggested a relation between CBP and the sympathetic nervous system. Stein and co-workers quantified by ELISA the levels of noradrenaline in the urine of 111 CBP patients and of 92 healthy volunteers (9). CBP patients had a higher urinary noradrenaline level than healthy volunteers. Urinary noradrenaline levels were investigated in patients presenting the two classical phenotypes of bladder pain syndrome/interstitial cystitis: those with Hunner’s lesion and those without lesions (9). Noradrenaline levels were similar between CBP patients that present and do not present ulcers (9).
There are also several lines of evidence showing changes in sympathetic innervation in the bladder of CBP patients. Using immunohistochemical stains for the nonselective neuronal marker, protein gene product (PGP) 9.5, Christmas and co-workers observed that the sub-urothelium and the muscular layer of CBP patients presented more nerve fibres than the bladder of healthy subjects (10). Later on, Hohenfellner and co-workers using bladder tissue from ten patients with CBP, performed immunohistochemical staining against vasoactive intestinal polypeptide (a marker of vasodilator cholinergic nerves) and neuropeptide Y (a marker of vasoconstrictor adrenergic neurons), and verified that CBP patients presented an increase in the number of nerves positive for these targets, showing the existence of sympathetic sprouting (11). Peeker and co-workers have also observed an increase in the density and number of nerve fibers immunoreactive for tyrosine hydroxylase (an enzyme essential for neuronal catecholamine synthesis and therefore, a marker of adrenergic activity) in the bladders of CBP patients (12). Hence, CBP has been associated with increased urinary bladder sympathetic innervation.
In normal human bladder, noradrenaline-containing autonomic nerve fibers are found near smooth muscle cells of the vesico-urethra junction (13,14). It is not known if, during CBP, the sprouting of sympathetic fibres carries them to other bladder regions. In fact, in an animal model of CBP, Charrua and co-workers have observed the sprouting of sympathetic fibres to other bladder regions were sympathetic fibres were scarcely found (5).
Lundeberg and co-workers have observed more nerve fibres within the sub-urothelium and detrusor muscle in CBP patients with ulcers than in CBP patients who did not present these bladder changes (15). However, Peeker and co-workers did not observe any differences in the expression of sympathetic fibres among these two populations of CBP patients (12).
These data suggest that there is an increase in sympathetic nerve fibres expression where activity in the urinary bladder of patients with CBP. However, these studies have not examined whether this sympathetic overactivity triggers or exacerbates CBP.
The sympathetic nervous system and mast cell activation
CBP is often accompanied by bladder inflammation. Different authors have evaluated the migration and activation of mast cell in the bladder wall of patients with CBP and observed an increase in these cells number and activity (16-24). When activated, mast cells release molecules that activate/sensitize bladder primary afferents in its close vicinity, promoting the release of neuropeptides that will have a paracrine action further stimulating the mast cells, an event thought to aggravate pain in CBP patients (15,25-33). Contrary to the observed role of mast cell—sensory fibres cross-talk in CBP, the cross-talk between mast cells and sympathetic nerve fibres is poorly studied. Hohenfellner and co-workers verified a sprouting of sympathetic fibres in the urinary bladder of patients with CBP (11). Keith and co-workers showed that the sprouted sympathetic fibres were located near serotonin-immunoreactive mast cells (33). Also, using an animal model of CBP, Charrua and co-workers observed that chronic adrenergic stimulation lead to an increase in the number of mast cells in the bladder mucosa (5). Altogether, these data suggest that sympathetic nervous system overactivity promotes mastocytosis, and consequently, augments and likely sustains pain.
Animal models of CBP: sympathetic nervous system implications
Most recently, Charrua and co-workers have developed a model of chronic adrenergic stimulation that mimic most of the signs and symptoms observed in patients with CBP (5). In this model, female rats subcutaneously received 2.5 mg phenylephrine (PHE)/kg, for 14 days (5). Visceral pain behaviour, changes in bladder motility and morphology were analysed in rats receiving chronic adrenergic stimulation and in saline treated controls (5). It was observed that rats receiving chronic adrenergic stimulation had higher pain scores than controls (5). At day 14, treated animals responded to 9±2 g filaments and non-treated animals responded to 54±13 g, showing a decrease in mechanical pain threshold (5). Curiously, chronic adrenergic stimulation-induced pain was blocked by depleting nociceptive fibres with a systemic capsaicin (CAP) pre-treatment (5).
BPS patients tend to empty their bladder at lower volumes than healthy individuals, due to pain associated with bladder filling. Using chronic adrenergic stimulation, Charrua and co-workers also observed that treated animals had increase bladder frequency (8.2±3.6 spots/hour and reflex activity (1.47±0.24 contractions/minute) than control (2.8±1.1 spots/hour and 0.43±0.11 contractions/minute, respectively) (5). As observed in patients with CBP, animals submitted to chronic adrenergic stimulation presented patchy impaired urothelium, with reduced thickness due to loss of umbrella cells, as observed by lack of cytokeratin 20 staining, and the presence of the pro-apoptotic markers caspase and bax (5). Also, the urothelial barrier function from animals submitted to chronic adrenergic stimulation is also compromised has it stained for trypan blue (5). These observations were absent in control animals (5). Therefore, these data indicates a possible adrenergic contribution for the development of CBP and associated symptoms.
Nevertheless, there are other animal models to study CBP, although all of them presenting important limitation (34). The most widely used is the intravesical application chemical irritants, such as cyclophosphamide-induced cystitis (35), lipopolysaccharide-induced cystitis (36), bladder irritation by intravesical instillation of acetone (37), turpentine (38) or acetic acid (39), or feline interstitial cystitis (FIC) model. Recently, animal models of stress, such as water avoidance stress model, have also been used to study CBP (5,40,41).
Taking in consideration that during joint inflammation, there is an increase in the density of sympathetic nerve fibres (42), Charrua and co-workers have investigated whether the same was observed in the rat inflamed bladder (5). Hence, using a rat model of cystitis induced by intravesical instillation of lipopolysaccharide, these authors studied the vesicle monoamine transporters two expression, a membrane protein that transports monoamines, in the bladder wall and measured urinary noradrenaline (5). An increase in sympathetic nerve fibres density in the body and dome of muscular and suburothelial layers of inflamed bladders compared to controls was observed (5). Also, these authors found a great increase in urinary levels of noradrenaline in animals with cystitis, compared to the control (5), suggesting a possible paracrine effect of noradrenaline in nearby cellular population.
Cats have been shown to exhibit CBP syndrome known as FIC (43). FIC has similarities with human CBP syndrome, such as pain, decrease in urinary glycosaminoglycan and urothelium structure and function impairment (43-47). Reche Júnior and Buffington analysed tyrosine hydroxylase expression in locus coeruleus from six FIC cats, in the quiescent period, and six healthy cats (48). These authors observed that FIC cats expressed more tyrosine hydroxylase than healthy cats, which suggests an alteration in the sympathetic outflow (48). Later on, Buffington and Pacak analysed plasma norepinephrine levels using blood samples from eight cats with FIC and eight healthy cats, and concluded that this catecholamine levels were higher in the former group (49).
The role of stress in CBP is becoming more evident. Lee and co-workers have demonstrated that rats submitted to chronic water avoidance stress resulted in a bladder hyperalgesia (41). After 10 days of exposing rats in a pedestal placed in the centre of a container filled with water, the animals had higher frequency of responses to lower strength filaments than sham animals (41), they have showed that these animals presented referred bladder hyperalgesia. Furthermore, these authors also demonstrated that only chronic stress, but not acute stress, could induce such change in pain behaviour (41), suggesting that the bladder nociceptive pathways activated by chronic stress stimulus differ from acute stress stimulus. Also, these authors have observed changes in colon activity after chronic stress stimuli, showing a possible cross-talk between visceral organs due to their cross-innervation (41). In fact, previous works have shown the existence of a colon-bladder cross-talk due to activation of colon nociceptive fibres (50-55). These data are in accordance with the observation that stress worsens both bladder and gastrointestinal symptoms, such as chronic pain felt by CBP patients (6,56-59). The mechanisms that lead to colon-bladder cross-talk during chronic stress are mediated by an increase in plasma corticosterone levels (60). Curiously, the increase of corticosterone levels early in life leads to a chronic alteration of the sympathetic nervous system that result in increased levels of plasmatic noradrenaline (61), indicating a possible role of sympathetic overactivity in stress-induced CBP.
Spanos and co-workers have observed that chronic stress promotes the activation of bladder mast cells (62). The activation is induced by CAP sensitive fibres that sprout in the bladder wall near mast cells (62). Interestingly, Cikler and co-workers have observed that the increase in mast cells activation in the skin of rats subjected to the water avoidance stress model was prevented by the pre-treatment with melatonin (63), a molecule known to have a reverse effect in plasmatic noradrenaline levels. These results seem to indicate that there is a relation between CBP, mast cells activation, nociceptive fibres activation and sympathetic activity. Further corroborating this hypothesis, in another study using an animal model of chronic adrenergic stimulation, it was observed that PHE induced an increase in the number of mast cells present in the bladder mucosa, accompanied with CBP (5). The pre-treatment with CAP reversed the PHE induced CBP (5) and mast cell infiltration (Figure 3).
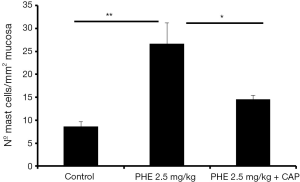
In some cases, stress may lead to urothelial degeneration (detachment and loss of urothelial cells, focal decrease in the urothelium cell layer and GAG layer irregularity), promoting dilated tight junctions (64,65). Another study showed that this effect was reversed both by melatonin and CAP pre-treatment, indicating that they are downstream events to sympathetic and nociceptive fibre activation (64,65). The chronic stress-induced mast cell activation and urothelial disruption can also be reverted by taurine (66). Taurine is known to suppress sympathetic nervous system activity (67), which further suggests the involvement of sympathetic nervous system in CBP induced by stress.
Sympathetic nervous system and other chronic pain disorders
CBP is often associated with other painful conditions such as IBS and fibromyalgia (4), which seems to be indicative of a common systemic etiology. There is ample support for an involvement of the sympathetic nervous system in triggering and maintaining of pain associated with these pathologies.
Heitkemper and co-workers interviewed and followed 24 female IBS patients and 25 healthy female aiming to describe and compare their levels of urinary catecholamines (68). Similar to what was observed to CBP patients, IBS patients also presented higher urine noradrenaline levels than controls (68). Burr and co-workers compared plasma catecholamine levels during sleep of 30 female patients with IBS with 31 healthy controls, and also observed that patients with IBS presented higher plasmatic noradrenaline levels than controls (69). Winston and co-workers have shown that noradrenaline induces visceral hypersensitivity upon colorectal distension induced by stress, through a nerve growth factor dependent mechanism (70). The increase in nerve growth factor levels may sensitizes primary afferents in the absence of an inflammatory response (70). In addition, Mazur and co-workers performed resting and functional autonomic nervous system tests and percutaneous electrogastrography in 30 patients with IBS and 30 healthy volunteers and observed that these patients presented increased sympathetic activation (71). These authors also collected blood samples to analyse plasmatic noradrenalin and also observed that IBS patients had substantially higher plasma catecholamine concentration, which further support a sympathetic nervous system dysfunction (71).
Fibromyalgia symptoms also suggest an impairment of sympathetic nervous system associated with chronic pain. In a study involving fibromyalgia patients and healthy individuals, Anderberg and co-workers analysed the levels of the sympathetic neurotransmitter neuropeptide Y in the plasma of those patients (72). These authors observed an increase in plasmatic neuropeptide Y levels compared to healthy subjects (72). Furthermore, in order to study a possible correlation between plasmatic neuropeptide Y levels changes and pain, the patients were followed for 28 days and 15 different symptoms were daily registered (72). Although there was a correlation between physical symptoms and plasmatic neuropeptide Y levels, the change in neuropeptide levels did not correlate with pain (72). Curiously, Yunus and collaborators have analysed plasmatic and urinary catecholamines in 30 patients with primary fibromyalgia and 30 healthy controls, without significant pain, and observed that there was no differences in between catecholamines levels in these groups (73). These results suggest that noradrenaline release by sympathetic fibres in fibromyalgia is secondary to fibromyalgia symptoms, without excluding its associated with pain arousal (73). Using 20 patients with fibromyalgia and 20 healthy controls, Martinez-Lavin and co-workers have injected noradrenaline in a forearm and saline solution in the opposite forearm of the studied subjects and registered maximum local pain elicited using a visual analogue scale (74). The norepinephrine-evoked pain was more frequent and intense in fibromyalgia patients than in healthy subjects (74). This observation further indicates that pain associated with fibromyalgia is driven by sympathetic nervous system (74). Recently, Zamunér and co-workers assessed whether there is a relationship between sympathetic activity and pain intensity. For that, cardiac activity and post-ganglionic sympathetic discharge activity were analysed in 25 patients with primary fibromyalgia (75). These authors observed a positive correlation between the sympathetic drive and the magnitude of chronic pain (75), further confirming the role of sympathetic overactivity in chronic pain arousal and maintenance.
The CBP-independent chronic regional pain syndrome also seems to present a correlation between chronic pain and a sympathetic dysfunction. This association is based on the observed vasomotor instability and a positive response to sympathetic blockade (76-80). Also, it has been described that intradermal injection of PHE or noradrenaline in complex regional pain syndrome patients induced abnormal pain response and mechano-allodynia around the injection site, contrary to the brief stinging pain observed in healthy individuals (81).
Conclusions
Abnormalities in autonomic (sympathetic) activity have been demonstrated in CBP and this has been described both at a clinical and at an experimental level. Although several studies suggest a link between sympathetic overactivity and CBP, additional research is necessary to understand the underlying mechanisms. The confirmation of such hypothesis may help our understanding of how abnormalities in autonomic function may contribute to the pathophysiology of a number of chronic pelvic pain disorders.
Acknowledgements
Funding: A Charrua is supported by Fundação para a Ciência e Tecnologia (FCT) fellowship SFRH/BPD/68716/2010. A Charrua research is supported by the PFD Research Foundation and ICA IC/PBS Research Grant. LA Birder is consultant/investigator for Astellas and Menarini, and research is supported by National Institutes of Health (NIH) R01 DK57284 and R37 DK54824. F Cruz is consultant/investigator/speaker for Astellas, Ipsen, Recordati and Allergan.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Fall M, Baranowski AP, Elneil S, et al. EAU guidelines on chronic pelvic pain. Eur Urol 2010;57:35-48. [PubMed]
- Tripp DA, Nickel JC, Wong J, et al. Mapping of pain phenotypes in female patients with bladder pain syndrome/interstitial cystitis and controls. Eur Urol 2012;62:1188-94. [PubMed]
- Hanno P, Lin A, Nordling J, et al. Bladder Pain Syndrome Committee of the International Consultation on Incontinence. Neurourol Urodyn 2010;29:191-8. [PubMed]
- Bullones Rodríguez MÁ, Afari N, Buchwald DS, et al. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol 2013;189:S66-74. [PubMed]
- Charrua A, Pinto R, Taylor A, et al. Can the adrenergic system be implicated in the pathophysiology of bladder pain syndrome/interstitial cystitis? A clinical and experimental study. Neurourol Urodyn 2015;34:489-96. [PubMed]
- Lutgendorf SK, Latini JM, Rothrock N, et al. Autonomic response to stress in interstitial cystitis. J Urol 2004;172:227-31. [PubMed]
- Williams DP, Chelimsky G, McCabe NP, et al. Effects of Chronic Pelvic Pain on Heart Rate Variability in Women. J Urol 2015;194:1289-94. [PubMed]
- Pinto RM, Lopes TA, Silva JF, et al. 997 Urinary levels of noradrenaline are increased in patients with bladder pain syndrome/interstitial cystitis and are decreased by intratrigonal onabotulinum toxin type A injection. Eur Urol Suppl 2012;11:e997-e997a.
- Stein PC, Torri A, Parsons CL. Elevated urinary norepinephrine in interstitial cystitis. Urology 1999;53:1140-3. [PubMed]
- Christmas TJ, Rode J, Chapple CR, et al. Nerve fibre proliferation in interstitial cystitis. Virchows Arch A Pathol Anat Histopathol 1990;416:447-51. [PubMed]
- Hohenfellner M, Nunes L, Schmidt RA, et al. Interstitial cystitis: increased sympathetic innervation and related neuropeptide synthesis. J Urol 1992;147:587-91. [PubMed]
- Peeker R, Aldenborg F, Dahlström A, et al. Increased tyrosine hydroxylase immunoreactivity in bladder tissue from patients with classic and nonulcer interstitial cystitis. J Urol 2000;163:1112-5. [PubMed]
- Klück P. The autonomic innervation of the human urinary bladder, bladder neck and urethra: a histochemical study. Anat Rec 1980;198:439-47. [PubMed]
- Gosling JA, Dixon JS, Lendon RG. The autonomic innervation of the human male and female bladder neck and proximal urethra. J Urol 1977;118:302-5. [PubMed]
- Lundeberg T, Liedberg H, Nordling L, et al. Interstitial cystitis: correlation with nerve fibres, mast cells and histamine. Br J Urol 1993;71:427-9. [PubMed]
- Larsen S, Thompson SA, Hald T, et al. Mast cells in interstitial cystitis. Br J Urol 1982;54:283-6. [PubMed]
- Kastrup J, Hald T, Larsen S, et al. Histamine content and mast cell count of detrusor muscle in patients with interstitial cystitis and other types of chronic cystitis. Br J Urol 1983;55:495-500. [PubMed]
- Feltis JT, Perez-Marrero R, Emerson LE. Increased mast cells of the bladder in suspected cases of interstitial cystitis: a possible disease marker. J Urol 1987;138:42-3. [PubMed]
- Moore KH, Nickson P, Richmond DH, et al. Detrusor mast cells in refractory idiopathic instability. Br J Urol 1992;70:17-21. [PubMed]
- Letourneau R, Sant GR, el-Mansoury M, et al. Activation of bladder mast cells in interstitial cystitis. Int J Tissue React 1992;14:307-12. [PubMed]
- Christmas TJ, Rode J. Characteristics of mast cells in normal bladder, bacterial cystitis and interstitial cystitis. Br J Urol 1991;68:473-8. [PubMed]
- Liu HT, Shie JH, Chen SH, et al. Differences in mast cell infiltration, E-cadherin, and zonula occludens-1 expression between patients with overactive bladder and interstitial cystitis/bladder pain syndrome. Urology 2012;80:225.e13-8.
- Enerbäck L, Fall M, Aldenborg F. Histamine and mucosal mast cells in interstitial cystitis. Agents Actions 1989;27:113-6. [PubMed]
- Fall M, Johansson SL, Aldenborg F. Chronic interstitial cystitis: a heterogeneous syndrome. J Urol 1987;137:35-8. [PubMed]
- Theoharides TC, Kempuraj D, Sant GR. Mast cell involvement in interstitial cystitis: a review of human and experimental evidence. Urology 2001;57:47-55. [PubMed]
- Park CS, Bochner BS. Potential targeting of siglecs, mast cell inhibitory receptors, in interstitial cystitis. Int Neurourol J 2011;15:61-3. [PubMed]
- Elbadawi A. Interstitial cystitis: a critique of current concepts with a new proposal for pathologic diagnosis and pathogenesis. Urology 1997;49:14-40. [PubMed]
- Frenz AM, Christmas TJ, Pearce FL. Does the mast cell have an intrinsic role in the pathogenesis of interstitial cystitis? Agents Actions 1994;41:C14-5. [PubMed]
- Rudick CN, Bryce PJ, Guichelaar LA, et al. Mast cell-derived histamine mediates cystitis pain. PLoS One 2008;3:e2096. [PubMed]
- Pang X, Boucher W, Triadafilopoulos G, et al. Mast cell and substance P-positive nerve involvement in a patient with both irritable bowel syndrome and interstitial cystitis. Urology 1996;47:436-8. [PubMed]
- Theoharides TC, Pang X, Letourneau R, et al. Interstitial cystitis: a neuroimmunoendocrine disorder. Ann N Y Acad Sci 1998;840:619-34. [PubMed]
- Pang X, Marchand J, Sant GR, et al. Increased number of substance P positive nerve fibres in interstitial cystitis. Br J Urol 1995;75:744-50. [PubMed]
- Keith IM, Jin J, Saban R. Nerve-mast cell interaction in normal guinea pig urinary bladder. J Comp Neurol 1995;363:28-36. [PubMed]
- Izgi K, Daneshgari F. Animal Models for Interstitial Cystitis/Painful Bladder Syndrome (IC/PBS). Erciyes Med J 2013;35:103-7.
- Boucher M, Meen M, Codron JP, et al. Cyclophosphamide-induced cystitis in freely-moving conscious rats: behavioral approach to a new model of visceral pain. J Urol 2000;164:203-8. [PubMed]
- Bjorling DE, Jacobsen HE, Blum JR, et al. Intravesical Escherichia coli lipopolysaccharide stimulates an increase in bladder nerve growth factor. BJU Int 2001;87:697-702. [PubMed]
- Ghoniem GM, Shaaban AM, Clarke MR. Irritable bladder syndrome in an animal model: a continuous monitoring study. Neurourol Urodyn 1995;14:657-65. [PubMed]
- Farquhar-Smith WP, Rice AS. Administration of endocannabinoids prevents a referred hyperalgesia associated with inflammation of the urinary bladder. Anesthesiology 2001;94:507-13; discussion 6A.
- Yoshimura N, Seki S, Novakovic SD, et al. The involvement of the tetrodotoxin-resistant sodium channel Na(v)1.8 (PN3/SNS) in a rat model of visceral pain. J Neurosci 2001;21:8690-6. [PubMed]
- Robbins M, DeBerry J, Ness T. Chronic psychological stress enhances nociceptive processing in the urinary bladder in high-anxiety rats. Physiol Behav 2007;91:544-50. [PubMed]
- Lee UJ, Ackerman AL, Wu A, et al. Chronic psychological stress in high-anxiety rats induces sustained bladder hyperalgesia. Physiol Behav 2015;139:541-8. [PubMed]
- Longo G, Osikowicz M, Ribeiro-da-Silva A. Sympathetic fiber sprouting in inflamed joints and adjacent skin contributes to pain-related behavior in arthritis. J Neurosci 2013;33:10066-74. [PubMed]
- Buffington CA, Chew DJ, DiBartola SP. Interstitial cystitis in cats. Vet Clin North Am Small Anim Pract 1996;26:317-26. [PubMed]
- Buffington CA, Blaisdell JL, Binns SP Jr, et al. Decreased urine glycosaminoglycan excretion in cats with interstitial cystitis. J Urol 1996;155:1801-4. [PubMed]
- Lavelle JP, Meyers SA, Ruiz WG, et al. Urothelial pathophysiological changes in feline interstitial cystitis: a human model. Am J Physiol Renal Physiol 2000;278:F540-53. [PubMed]
- Birder LA, Barrick SR, Roppolo JR, et al. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol 2003;285:F423-9. [PubMed]
- Birder LA, Wolf-Johnston A, Buffington CA, et al. Altered inducible nitric oxide synthase expression and nitric oxide production in the bladder of cats with feline interstitial cystitis. J Urol 2005;173:625-9. [PubMed]
- Reche Júnior A, Buffington CA. Increased tyrosine hydroxylase immunoreactivity in the locus coeruleus of cats with interstitial cystitis. J Urol 1998;159:1045-8. [PubMed]
- Buffington CA, Pacak K. Increased plasma norepinephrine concentration in cats with interstitial cystitis. J Urol 2001;165:2051-4. [PubMed]
- Qin C, Foreman RD. Viscerovisceral convergence of urinary bladder and colorectal inputs to lumbosacral spinal neurons in rats. Neuroreport 2004;15:467-71. [PubMed]
- Peng HY, Chen GD, Lai CY, et al. PI3K modulates estrogen-dependent facilitation of colon-to-urethra cross-organ reflex sensitization in ovariectomized female rats. J Neurochem 2010;113:54-66. [PubMed]
- Ustinova EE, Fraser MO, Pezzone MA. Cross-talk and sensitization of bladder afferent nerves. Neurourol Urodyn 2010;29:77-81. [PubMed]
- Rudick CN, Chen MC, Mongiu AK, et al. Organ cross talk modulates pelvic pain. Am J Physiol Regul Integr Comp Physiol 2007;293:R1191-8. [PubMed]
- Qin C, Malykhina AP, Akbarali HI, et al. Cross-organ sensitization of lumbosacral spinal neurons receiving urinary bladder input in rats with inflamed colon. Gastroenterology 2005;129:1967-78. [PubMed]
- Pezzone MA, Liang R, Fraser MO. A model of neural cross-talk and irritation in the pelvis: implications for the overlap of chronic pelvic pain disorders. Gastroenterology 2005;128:1953-64. [PubMed]
- Whitmore KE. Self-care regimens for patients with interstitial cystitis. Urol Clin North Am 1994;21:121-30. [PubMed]
- Levy RL, Cain KC, Jarrett M, et al. The relationship between daily life stress and gastrointestinal symptoms in women with irritable bowel syndrome. J Behav Med 1997;20:177-93. [PubMed]
- Lutgendorf SK, Kreder KJ, Rothrock NE, et al. Stress and symptomatology in patients with interstitial cystitis: a laboratory stress model. J Urol 2000;164:1265-9. [PubMed]
- Rothrock NE, Lutgendorf SK, Kreder KJ, et al. Stress and symptoms in patients with interstitial cystitis: a life stress model. Urology 2001;57:422-7. [PubMed]
- Peng HY, Hsieh MC, Lai CY, et al. Glucocorticoid mediates water avoidance stress-sensitized colon-bladder cross-talk via RSK2/PSD-95/NR2B in rats. Am J Physiol Endocrinol Metab 2012;303:E1094-106. [PubMed]
- Winston JH, Sarna SK. Developmental origins of functional dyspepsia-like gastric hypersensitivity in rats. Gastroenterology 2013;144: 570-579.e3.
- Spanos C, Pang X, Ligris K, et al. Stress-induced bladder mast cell activation: implications for interstitial cystitis. J Urol 1997;157:669-72. [PubMed]
- Cikler E, Ercan F, Cetinel S, et al. The protective effects of melatonin against water avoidance stress-induced mast cell degranulation in dermis. Acta Histochem 2005;106:467-75. [PubMed]
- Ercan F, Oktay S, Erin N. Role of afferent neurons in stress induced degenerative changes of the bladder. J Urol 2001;165:235-9. [PubMed]
- Cetinel S, Ercan F, Cikler E, et al. Protective effect of melatonin on water avoidance stress induced degeneration of the bladder. J Urol 2005;173:267-70. [PubMed]
- Zeybek A, Sağlam B, Cikler E, et al. Taurine ameliorates stress-induced degeneration of the urinary bladder. Acta Histochem 2007;109:208-14. [PubMed]
- Sato Y, Ando K, Fujita T. Role of sympathetic nervous system in hypotensive action of taurine in DOCA-salt rats. Hypertension 1987;9:81-7. [PubMed]
- Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol 1996;91:906-13. [PubMed]
- Burr RL, Jarrett ME, Cain KC, et al. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil 2009;21:1148-e97. [PubMed]
- Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology 2010;138:294-304.e3.
- Mazur M, Furgała A, Jabłoński K, et al. Autonomic nervous system activity in constipation-predominant irritable bowel syndrome patients. Med Sci Monit 2012;18:CR493-499. [PubMed]
- Anderberg UM, Liu Z, Berglund L, et al. Elevated plasma levels of neuropeptide Y in female fibromyalgia patients. Eur J Pain 1999;3:19-30. [PubMed]
- Yunus MB, Dailey JW, Aldag JC, et al. Plasma and urinary catecholamines in primary fibromyalgia: a controlled study. J Rheumatol 1992;19:95-7. [PubMed]
- Martinez-Lavin M, Vidal M, Barbosa RE, et al. Norepinephrine-evoked pain in fibromyalgia. A randomized pilot study BMC Musculoskelet Disord 2002;3:2. [ISRCTN70707830]. [PubMed]
- Zamunér AR, Barbic F, Dipaola F, et al. Relationship between sympathetic activity and pain intensity in fibromyalgia. Clin Exp Rheumatol 2015;33:S53-7. [PubMed]
- Birklein F, O'Neill D, Schlereth T. Complex regional pain syndrome: An optimistic perspective. Neurology 2015;84:89-96. [PubMed]
- Wasner G. Vasomotor disturbances in complex regional pain syndrome--a review. Pain Med 2010;11:1267-73. [PubMed]
- Groeneweg G, Huygen FJ, Coderre TJ, et al. Regulation of peripheral blood flow in complex regional pain syndrome: clinical implication for symptomatic relief and pain management. BMC Musculoskelet Disord 2009;10:116. [PubMed]
- Albazaz R, Wong YT, Homer-Vanniasinkam S. Complex regional pain syndrome: a review. Ann Vasc Surg 2008;22:297-306. [PubMed]
- Chaturvedi A, Dash HH. Sympathetic blockade for the relief of chronic pain. J Indian Med Assoc 2001;99:698-703. [PubMed]
- Mailis-Gagnon A, Bennett GJ. Abnormal contralateral pain responses from an intradermal injection of phenylephrine in a subset of patients with complex regional pain syndrome (CRPS). Pain 2004;111:378-84. [PubMed]


