
Combination therapy with curcumin plus tamsulosin and finasteride in the treatment of men with benign prostatic hyperplasia: a single center, randomized control study
Introduction
Benign prostatic hyperplasia (BPH) is a common disorder that occurs mainly in older men. Recent studies have shown that prostatic inflammation contributes to the pathogenesis and disease progression of BPH, which is also correlated with the severity of lower urinary tract symptoms (LUTS) and erectile dysfunction (ED) (1,2). The most common medical treatments for BPH are alpha-blockers (e.g., tamsulosin) and 5-alpha reductase inhibitors (e.g., finasteride), both of which lack direct anti-inflammatory effects and have a possible side impact on sexual and erectile functions (3,4). As a result, combination therapy with anti-inflammatory drugs seems to be a more effective strategy for BPH treatment (5). However, non-steroidal anti-inflammatory drugs (NSAIDS) commonly used in clinical practice have many side effects such as gastrointestinal inflammation, upper gastrointestinal ulcers and perforation (6). Moreover, NSAIDS has shown adverse reactions to the central nervous, circulatory, and respiratory systems, limiting their clinical applications (6). Therefore, there is a need to explore a novel anti-inflammatory agent with high efficacy and lower toxicity, which may elevate compliance, alleviate the LUTS, and improve BPH patients’ quality of life (QoL).
In recent years, phytotherapeutic agents have attracted considerable attention because of their comprehensive anti-inflammatory and anti-oxidative stress properties, and, more importantly, they have the advantages of being safe and non-toxic (7,8). Curcumin is a natural plant polyphenol extracted from the root of plants of Zingiberaceae and Araceae. Much evidence is available to show that curcumin possesses anti-inflammatory, antioxidant, anti-cancer, anti-fibrosis, lipid-modifying, and antiarthritic effects without obvious adverse effects (9). Curcumin has also shown a potential function of suppressing the progression of BPH. It has been reported that the incidence of BPH in India, especially in southern India, was significantly lower than that in western countries, with reduced LUTS symptoms and a lower incidence of acute urinary retention (10). Residents in these areas have a high curcumin intake due to their preference for curry. Moreover, data from a prospective controlled clinical trial revealed that the supplementation of curcumin with finasteride leads to significant improvements in the International Prostate Symptom Score (IPSS) and QoL scores and controls urinary infections in BPH patients (11). Cosentino et al. (12) also found that curcumin could significantly reduce the inflammatory symptoms in BPH patients who underwent transurethral resection of the prostate (TURP), with fewer side effects than NSAIDS.
Periprostatic fat (PPF), which surrounds the prostate, can produce several hormones and cytokines involving autocrine, paracrine, and endocrine signals, including vascular endothelial growth factor, interleukin-1 beta, interleukin-6, leptin, and adiponectin (13). Our previous study found that periprostatic fat thickness (PPFT) was significantly correlated with prostate volume (PV), LUTS symptoms, and ED degree in BPH patients. PPFT can better identify high-risk BPH patients with disease progression (14). Some clinical studies have also suggested a positive impact of curcumin supplementation on body weight, body mass index (BMI), and waist circumference (WC) (15,16). However, to the best of our knowledge, there has been no research targeting the effect of curcumin on PPF in BHP patients. To the best of our knowledge, this is the first study evaluating the effects of curcumin combined with best standard management (BSM) on BMI, body weight, and PPF in BPH patients.
Therefore, this study evaluates the efficacy of combined use of curcumin and the standard treatment strategy (tamsulosin and finasteride) to improve WC, visceral fat (PPF), LUTS, and sexual function in BPH patients. Moreover, this prospective study also aims to assess the efficacy and safety of curcumin. We present the following article in accordance with the CONSORT reporting checklist (available at https://dx.doi.org/10.21037/tau-21-567).
Methods
Patients
A total of 143 untreated adult outpatients with a previous history of BPH or new cases, who visited the Department of Urology, Xiangya Hospital, Central South University (Changsha, Hunan) between December 2018 and January 2020 were identified, and 122 patients were included in the prospective, randomized, single center, two-parallel, 6-month clinical study with a case-to-control ratio of 1:1. The inclusion criteria were as follows: (I) age between 50 and 70 years; (II) total prostate-specific antigen (TPSA) <4 ng/mL; (III) IPSS ≥8; (IV) PV ≥30 mL; (V) maximum urine flow rate (Qmax) <5 mL/s and urine output ≥100 mL. Besides, subjects who had taken alpha-blockers, 5-alpha reductase inhibitors, proprietary Chinese medicine, or botanically derived drugs to treat BPH within the last 4 weeks could also be included in the study after 4 weeks of drug withdrawal under the supervision of the researchers. The exclusion criteria were as follows: (I) allergic to curcumin; (II) LUTS and ED medication history; (III) history of prostate surgery, prostate cancer, urethral stricture, urinary tract infection, urinary bladder stones, bladder cancer, or neurogenic bladder dysfunction; (IV) history of serious heart disease, renal dysfunction, hepatic dysfunction, diabetes, sexually transmitted diseases, malignant tumor, peptic ulcer, hemorrhagic disease, or mental illness. All patients signed the consent form before study entry. The study was approved by the Institutional Review Board of the Xiangya Hospital of Central South University with ethics clearance number 201703544. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Patients were allocated randomly to either the BSM or curcumin + BSM groups using computer randomization. In the BSM group, tamsulosin 0.2 mg, finasteride 5 mg, and 3 placebo tablets were administered once every night (n=61) for 6 months, while tamsulosin 0.2 mg, finasteride 5 mg, and 3 Naturewise® tablets (curcumin 2,250 mg) were administered once every night (n=61) in the curcumin + BSM group. Curcumin tablets and placebo tablets were indistinguishable in appearance. Among the enrolled 122 patients, 116 patients completed the study (Figure S1).
Assessment parameters
The following data were collected from all patients: age, BMI values, WC, PPFT, prostate-specific antigen (PSA) value, PV, Qmax, IPSS, and QoL, and the 5-item version of the International Index of Erectile Function (IIEF-5) scores, which are the primary outcome. PPFT was defined as the shortest vertical distance from the midsagittal symphysis pubis to the prostate. Magnetic resonance imaging (MRI) of patients was obtained using the 3.0T MAGNETIC resonance scanner (Intera Archieva, Philips Medical System, Amsterdam, The Netherlands) in our hospital, including axial T1-weighted images, multi-plane T2-weighted images, axial diffusion-weighted images, and dynamic contrast-enhanced prostate images. Analysis of these images was performed by two radiologists with experience in prostate radiology using an image archiving and communication system who were blinded to participants’ group allocation. The radiologists agreed on the results. We compared basic data of the two patient groups, including the clinical indicators of the two groups before and after the treatment and the changes of the clinical indicators of the two groups after the treatment.
Statistical analysis
Data were analyzed using SPSS version 20.0 statistical software (SPSS Inc., Chicago, IL, USA). Continuous variables were described as mean ± standard deviation (SD), and categorical variables were expressed as frequencies and percentages. Records were statistically analyzed using the Student’s t-test. In addition, intergroup differences and intragroup differences in clinical indicators were analyzed by the Student’s t-test and chi-square test. All statistical assessments were two-sided and considered significant at P<0.05.
Results
Patient characteristics
The demographic and baseline characteristics of the clinical indicators studied are shown in Table 1. One hundred and twenty-two patients were enrolled in this study, of whom 116 completed the whole procedure (116/122, 95.1%). Six patients withdrew from the survey; of these 4 were in the BSM group (2 patients were lost to follow-up, 1 patient withdrew due to gastrointestinal adverse reactions, 1 patient had prostate surgery due to acute urinary retention), and 2 patients were in the curcumin + BSM group and were lost to follow-up (Figure S1). There were no significant differences in demographic and baseline characteristics between the two groups.
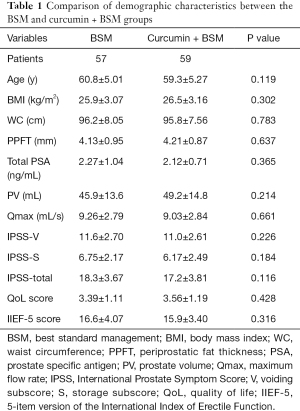
Full table
Clinical evaluations after treatment
At the end of the 6-month treatment, no statistically significant differences were observed in BMI, WC, and PPFT in the BSM group compared with baseline. In the curcumin + BSM group, there was also no significant difference in BMI compared with baseline, while both WC and PPFT decreased significantly compared to those at baseline (P<0.05). Additionally, there were significant improvements in PV, Qmax, IPSS-total, IPSS-voiding subscore (IPSS-V), IPSS-storage subscore (IPSS-S), and QoL from baseline after treatment in both groups (P<0.05). The IIEF-5 score also decreased slightly in the BSM group but increased moderately in the curcumin + BSM group; however, neither of these changes were statistically significant (Table 2).
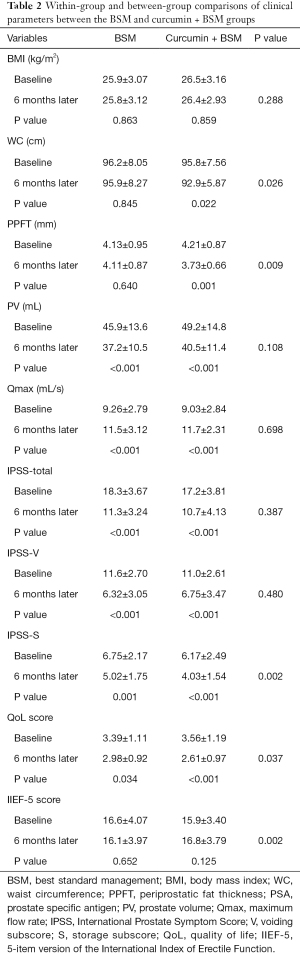
Full table
No significant difference was observed in BMI, PV, Qmax, IPSS-total and IPSS-V after 6-month treatment in between-group analysis. However, WC and PPFT in the curcumin + BSM group were significantly lower than those in the BSM group (P<0.05). Although IPSS-total and IPSS-V revealed no significant differences between the two groups after treatment, IPSS-S and QoL scores significantly improved the curcumin + BSM group (P<0.05). Interestingly, IIEF-5 in the curcumin + BSM group improved significantly compared with that in the BSM group (P<0.05), suggesting that curcumin may possess a protective effect on erectile function (Table 2).
Discussion
BPH is a common diagnosis in older men. However, the pathogenesis of BPH is far from being completely understood. In recent years, growing evidence has become available to show that BPH is associated with chronic inflammation (1,2). Besides, it has been proven that obesity is correlated with the LUTS of BPH and ED (17,18). Treatments of BPH include medications and surgery. Surgical treatments are indicated for patients who fail to respond or respond poorly to drug therapy, including TURP, holmium laser resection of the prostate (HoLEP), prostate laser vaporization, etc. However, surgery is traumatic and has many complications, such as bleeding, urethral stricture, retrograde ejaculation, and urinary incontinence (19,20). The standard medical treatments for BPH are alpha-blockers and 5-alpha reductase inhibitors, both of which lack direct anti-inflammatory effects and may have potential adverse effects on sexual function and erection (3,4).
Curcumin is the main bioactive component of turmeric. Curcumin has a long history of being used as a spice in diet, and also its use as traditional medicine can be traced to more than 2,000 years ago in India and China (9). Previous experimental studies have demonstrated that curcumin possesses anti-inflammatory, antioxidant, anti-cancer, anti-fibrosis, lipid-modifying, and antiarthritic properties (12). In addition, curcumin has been reported to effectively inhibit the occurrence and development of BPH in male Wistar rats and reduce the expression of TGF-1, an inflammatory cytokine, in prostate tissues (21). A prospective clinical comparative study showed that compared with finasteride alone, the additional use of curcumin was superior in improving patients’ IPSS and QoL scores and controlling urinary tract infection (11). However, the effect of curcumin on sexual function in BPH patients was not investigated in the former study.
The findings of our prospective trial indicate that the IIEF-5 score decreased from baseline in the BSM (tamsulosin and finasteride) group after 6-month standard medical treatment. The adverse effect of finasteride may be the main cause (22). Interestingly, the IIEF-5 score increased moderately from baseline in the curcumin + BSM group. Moreover, there was a statistically significant improvement of the IIEF-5 score in the curcumin + BSM group compared with that in the BSM group, indicating that curcumin may have a protective and promoting effect on sexual function. It has been proven that curcumin can enhance erectile function in a rat model of type-2 diabetes by increasing the expression of heme oxygenase-1 and decreasing the expression of NF-κβ-activating proteins (23). Furthermore, Abdel Aziz et al. (24) previously reported that curcumin combined with tadalafil showed obvious superiority in improving erectile function compared to tadalafil alone in a diabetic model of ED. These animal experimental results were consistent with the findings from the current clinical study.
The IPSS questionnaire has been applied to evaluate the severity of LUTS and disease progression in BPH patients. The IPSS is comprised of seven items. The IPSS-S score mainly describes urinary storage symptoms, including frequency, urgency, and nocturia, making patients’ lives difficult and affecting patients’ daily work and social life. The IPSS-V score mainly describes urination obstruction symptoms, including incomplete emptying, intermittency, weak stream, and straining to void, which can also be very painful for patients. In this study, although there were significant improvements in PV, Qmax, IPSS-total, IPSS-V, IPSS-S, and QoL scores from baseline after treatment in both groups, the IPSS-S and QoL scores showed a better and significantly increased improvement in the curcumin + BSM group when compared with the BSM group. These results support the hypothesis that the IPSS-S score is mainly affected by prostatitis and that curcumin has a significant anti-inflammatory effect on prostatitis (25-27).
PPF consists of highly active adipocytes, which can secrete many growth factors, chemokines, and inflammation-modifying molecules as paracrine cells, and it may play a predominant role in the occurrence and progression of prostate diseases (13). Preliminary studies by our group have shown that PPFT was significantly associated with PV, LUTS, and severity of ED in BPH patients, and PPFT could better identify high-risk patients with clinical progression of BPH (14). Besides, we found that the lipo-factor leptin was able to aggravate BPH by down-regulating BMP and activin membrane-bound inhibitor homolog (Xenopus laevis) (BAMBI) to activate the TGF-β/EMT signaling pathway in a rat model of testosterone BPH (28). In addition, Jazayeri-Tehrani et al. (15) stated that nano-curcumin could significantly reduce WC, blood sugar, and serum lipid levels in patients with non-alcoholic fatty liver disease (NAFLD), without significant reduction in body weight and BMI. However, another study on NAFLD showed that curcumin could significantly reduce body weight and BMI (16). Moreover, curcumin has been shown to significantly decrease serum leptin levels and increase serum adiponectin levels in NAFLD patients (29). However, no studies have been carried out to explore the effect of curcumin on PPF in BPH patients.
To the best of our knowledge, this is the first study evaluating the effects of curcumin combined with BSM treatment on BMI, body weight, and PPF in BPH patients. In the present study, no statistically significant differences were observed in BMI, WC, and PPFT in the BSM group compared with baseline, while significant differences were observed in WC and PPFT in the curcumin + BSM group without a significant difference in BMI. The present study suggests a significant benefit of curcumin in improving abdominal fat distribution and reducing PPF in BPH patients. Consistent with the findings of our previous studies, curcumin may indirectly affect disease progression in BPH patients by affecting the secretion of adipocytokines in PPF, a hypothesis that needs to be tested by further in vitro studies.
Overall, the treatment of curcumin was well-tolerated. In our study, curcumin combined with BSM treatment for 6 months significantly improved PPFT, erectile function, and urinary retention symptoms and QoL scores. Thus pharmacological treatment of BPH involved anti-inflammatory treatment such as curcumin may provide a better treatment option for BPH patients. The current study has several limitations. First, this prospective study was designed as a single-center study with a relatively small number of patients enrolled and limited clinical data. Second, a follow-up period of 6 months is relatively short when studying a chronic disease. Ideally, a comprehensive evaluation would include assessments at multiple time points throughout the follow-up procedure. Third, a standard dosage of curcumin was used, and future studies should assess different dosages. Fourth, this study was not a randomized controlled trial to study the pharmacokinetic gradients to determine the optimal dose; such trials should be carried out in the future. Furthermore, although this study was designed to explore the effect of curcumin combined with BSM on PPF, serum lipids, leptin, and adiponectin, other adipocytes were not included in this study due to cost consideration.
Conclusions
Our results demonstrate that curcumin combined with tamsulosin and finasteride can effectively improve LUTS and the QoL in BPH patients, with good safety and tolerance characteristics. In addition, curcumin combined with tamsulosin and finasteride has more beneficial effects in reducing PPFT, protecting erectile function, and improving urinary retention symptoms and QoL scores in BPH patients compared with tamsulosin and finasteride alone. However, further larger multicenter clinical studies are warranted to confirm the efficacy and beneficial effects of curcumin in BPH patients.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundations of China (81902606 to YG, 81700663 to YH); and the Natural Science Foundations of Hunan Province (2020JJ5891 to YG, 2020JJ5893 to YH).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-567
Trial Protocol: Available at https://dx.doi.org/10.21037/tau-21-567
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tau-21-567
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-567). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Xiangya Hospital of the Central South University with ethics clearance number 201703544. All the participates signed a consent form, and all personal information has been anonymized before publication. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torkko KC, Wilson RS, Smith EE, et al. Prostate biopsy markers of inflammation are associated with risk of clinical progression of benign prostatic hyperplasia: findings from the MTOPS study. J Urol 2015;194:454-61. [Crossref] [PubMed]
- Lokeshwar SD, Harper BT, Webb E, et al. Epidemiology and treatment modalities for the management of benign prostatic hyperplasia. Transl Androl Urol 2019;8:529-39. [Crossref] [PubMed]
- Rosen RC, Wei JT, Althof SE, et al. Association of sexual dysfunction with lower urinary tract symptoms of BPH and BPH medical therapies: results from the BPH Registry. Urology 2009;73:562-6. [Crossref] [PubMed]
- Kaplan SA, Chung DE, Lee RK, et al. A 5-year retrospective analysis of 5α-reductase inhibitors in men with benign prostatic hyperplasia: finasteride has comparable urinary symptom efficacy and prostate volume reduction, but less sexual side effects and breast complications than dutasteride. Int J Clin Pract 2012;66:1052-5. [Crossref] [PubMed]
- Dimitropoulos K, Gravas S. New therapeutic strategies for the treatment of male lower urinary tract symptoms. Res Rep Urol 2016;8:51-9. [PubMed]
- Geusens P, Emans PJ, de Jong JJ, et al. NSAIDs and fracture healing. Curr Opin Rheumatol 2013;25:524-31. [Crossref] [PubMed]
- Asare GA, Afriyie D, Ngala RA, et al. Shrinkage of prostate and improved quality of life: management of BPH patients with croton membranaceus ethanolic root extract. Evid Based Complement Alternat Med 2015;2015:365205 [Crossref] [PubMed]
- Ye Z, Huang J, Zhou L, et al. Efficacy and safety of serenoa repens extract among patients with benign prostatic hyperplasia in China: a multicenter, randomized, double-blind, placebo-controlled trial. Urology 2019;129:172-9. [Crossref] [PubMed]
- Yang Y, Duan W, Liang Z, et al. Curcumin attenuates endothelial cell oxidative stress injury through Notch signaling inhibition. Cell Signal 2013;25:615-29. [Crossref] [PubMed]
- Sinha R, Anderson DE, McDonald SS, et al. Cancer risk and diet in India. J Postgrad Med 2003;49:222-8. [PubMed]
- Ledda A, Belcaro G, Dugall M, et al. Meriva®, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: a pilot, product evaluation registry study. Panminerva Med 2012;54:17-22. [PubMed]
- Cosentino V, Fratter A, Cosentino M. Anti-inflammatory effects exerted by Killox®, an innovative formulation of food supplement with curcumin, in urology. Eur Rev Med Pharmacol Sci 2016;20:1390-8. [PubMed]
- Dahran N, Szewczyk-Bieda M, Vinnicombe S, et al. Periprostatic fat adipokine expression is correlated with prostate cancer aggressiveness in men undergoing radical prostatectomy for clinically localized disease. BJU Int 2019;123:985-94. [Crossref] [PubMed]
- Zhang B, Chen X, Liu YH, et al. Periprostatic fat thickness measured on MRI correlates with lower urinary tract symptoms, erectile function, and benign prostatic hyperplasia progression. Asian J Androl 2021;23:80-4. [Crossref] [PubMed]
- Jazayeri-Tehrani SA, Rezayat SM, Mansouri S, et al. Nano-curcumin improves glucose indices, lipids, inflammation, and Nesfatin in overweight and obese patients with non-alcoholic fatty liver disease (NAFLD): a double-blind randomized placebo-controlled clinical trial. Nutr Metab (Lond) 2019;16:8. [Crossref] [PubMed]
- Panahi Y, Kianpour P, Mohtashami R, et al. Efficacy and safety of phytosomal curcumin in non-alcoholic fatty liver disease: a randomized controlled trial. Drug Res (Stuttg) 2017;67:244-51. [Crossref] [PubMed]
- Gacci M, Sebastianelli A, Salvi M, et al. Central obesity is predictive of persistent storage lower urinary tract symptoms (LUTS) after surgery for benign prostatic enlargement: results of a multicentre prospective study. BJU Int 2015;116:271-7. [Crossref] [PubMed]
- Calogero AE, Burgio G, Condorelli RA, et al. Epidemiology and risk factors of lower urinary tract symptoms/benign prostatic hyperplasia and erectile dysfunction. Aging Male 2019;22:12-9. [Crossref] [PubMed]
- Ray AF, Powell J, Speakman MJ, et al. Efficacy and safety of prostate artery embolization for benign prostatic hyperplasia: an observational study and propensity-matched comparison with transurethral resection of the prostate (the UK-ROPE study). BJU Int 2018;122:270-82. [Crossref] [PubMed]
- Wang B, Zhang S, Sun C, et al. Comparison between a transurethral prostate split and transurethral prostate resection for benign prostatic hyperplasia treatment in a small prostate volume: a prospective controlled study. Ann Transl Med 2020;8:1016. [Crossref] [PubMed]
- Kim SK, Seok H, Park HJ, et al. Inhibitory effect of curcumin on testosterone induced benign prostatic hyperplasia rat model. BMC Complement Altern Med 2015;15:380. [Crossref] [PubMed]
- Traish AM, Haider KS, Doros G, et al. Finasteride, not tamsulosin, increases severity of erectile dysfunction and decreases testosterone levels in men with benign prostatic hyperplasia. Horm Mol Biol Clin Investig 2015;23:85-96. [Crossref] [PubMed]
- Draganski A, Tar MT, Villegas G, et al. Topically applied curcumin-loaded nanoparticles treat erectile dysfunction in a rat model of type-2 diabetes. J Sex Med 2018;15:645-53. [Crossref] [PubMed]
- Abdel Aziz MT, Rezq AM, Atta HM, et al. Molecular signalling of a novel curcumin derivative versus Tadalafil in erectile dysfunction. Andrologia 2015;47:616-25. [Crossref] [PubMed]
- Morgia G, Russo GI, Urzì D, et al. A phase II, randomized, single-blinded, placebo-controlled clinical trial on the efficacy of Curcumina and Calendula suppositories for the treatment of patients with chronic prostatitis/chronic pelvic pain syndrome type III. Arch Ital Urol Androl 2017;89:110-3. [Crossref] [PubMed]
- Vicari E, Arancio A, Catania VE, et al. Resveratrol reduces inflammation-related prostate fibrosis. Int J Med Sci 2020;17:1864-70. [Crossref] [PubMed]
- Cai T, Mazzoli S, Bechi A, et al. Serenoa repens associated with Urtica dioica (ProstaMEV) and curcumin and quercitin (FlogMEV) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: results from a prospective randomised study. Int J Antimicrob Agents 2009;33:549-53. [Crossref] [PubMed]
- Zhang B, Chen X, Xie C, et al. Leptin promotes epithelial-mesenchymal transition in benign prostatic hyperplasia through downregulation of BAMBI. Exp Cell Res 2020;387:111754 [Crossref] [PubMed]
- Mirhafez SR, Farimani AR, Dehhabe M, et al. Effect of phytosomal curcumin on circulating levels of adiponectin and leptin in patients with non-alcoholic fatty liver disease: a randomized, double-blind, placebo-controlled clinical trial. J Gastrointestin Liver Dis 2019;28:183-9. [Crossref] [PubMed]
(English Language Editors: B. Meiser and J. Chapnick)


