
Fluoroscopy-free minimally invasive ureteral stricture balloon dilatation: a retrospective safety and efficacy cohort study
Introduction
Ureteral stricture, a structural change of the ureter causing obstructive uropathy, if left untreated, consequentially leads to irreversible renal failure. Surgical repair is considered the gold standard treatment. However, despite the relatively lower success rate compared to the operative interventions, endourological management is always performed as the primary treatment due to its simplicity and minimal invasiveness (1). Balloon dilation, one of most frequently used endourological method, can achieve a high success rate for patients with benign, short, and with intact vascular supply (2,3). Thus, balloon dilation remains an ideal option for carefully selected patients and provides a choice for those who cannot tolerate open surgery.
Traditionally, balloon dilation is conducted via X-ray supervision because the involvement of an X-ray can minimize the invasiveness and make the procedure more precise. With the help of a C-arm, the ureter is visualized by injection of contrast material, and the disappearance of the “waist sign” is considered as a mark of success (4). However, the C-arm occupies a large space in the operation room, requires an additional specialist for manipulation and emits substantial radiation as a hazard to both the patient and surgeons (5). With the development of the ureteroscope, especially with the decreasing lens size, non-X-ray assisted direct vision balloon dilation operations have been made possible.
In our study, we try to explore a safe, effective and X-ray free balloon dilation operation protocol to avoid radiation hazards during the surgical procedure. We elaborate the details of the procedure and demonstrate its safety and efficacy by reporting surgical complications and follow-up data. We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tau-21-203).
Methods
Patient population
Clinical data of patients undergoing minimally invasive endourological treatment for ureteral stricture at Peking University First Hospital between February 2015 and November 2019 were retrospectively analyzed. We identified 109 patients who received endourological treatment for ureteral stricture in our hospital’s medical database. After excluding patients undergone simple catheter dilation, endoureterotomy and other treatment method, 76 patients received balloon dilation without intraoperative fluoroscopic guidance were included in our study (Figure 1). Preoperative clinical assessment included the clinical evaluation of symptoms, routine blood and urine tests, computed tomography urography (CTU) examination to roughly determine the location and length of the stricture, and diuretic renal scan for kidney function. Patients whose strictures were longer than 2 cm or who had evidence of malignancy were recommended for other treatment modality. The safety of the procedure was evaluated by perioperative and postoperative complication rate, which were reported according to the Clavien-Dindo modified classification. Successful outcome was defined as disappearance of preoperative symptoms (including removal of preoperative catheter), relief of hydronephrosis (compared to preoperative ultrasound or CTU) and stable of renal function (indicated by serum creatine level or glomerular filtration rate (GFR) of diuretic renal scan). The study was approved by the Institutional Review Board of Peking University First Hospital (IRB No. 2018-250).
Follow-up was accomplished during a scheduled clinical assessment after surgery. Basic clinical symptoms were evaluated each time. Urinary ultrasound, CTU and a diuretic renal scan was performed one month after ureteral stent removal and set as the postoperative baseline. Urinary ultrasound and CT were repeated and compared to the baseline to determine the evolution of hydronephrosis at 3 months and every 6 months thereafter. For patients who failed to attend routine postoperative visits in our hospital, a consultative phone call was made to alert patients, and the results were collected. This study was approved by the Biomedical Research Ethics Committee of Peking University First Hospital (IRB No. 2018-250) and all procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Operation procedure (Video 1)
Assessment of ureteral stricture
The patient was placed in the lithotomy position, and general anesthesia was suggested for all patients. The procedure usually started with cystoscopy to inspect the bladder and bilateral ureteral orifices. A guidewire was inserted in the Wolf F4.5/6.5 ureteroscope. Under the guidance of the guidewire, the scope was retrogradely placed in the ureter and, upon reaching the stricture, carefully inserted to pass through the stricture under direct vision. Then, following the guidewire, we pushed the ureteroscope forward to pass through the stricture to examine the upper ureter and the stricture as follows: (I) Examine the stricture, and ensure that the ureteral mucosa above it was intact and that there was no stenosis in the upper lumen. In this step, we usually used the F6.5 scope body to initially expand the stenosis to ensure that the original unexpanded balloon could pass the stricture in the balloon placement step. (II) Measure the length of the stricture. We placed the lens to reach the uppermost end of the stenosis, and at the same time, the surgeon fixed his fingers on the ureteroscope at the urethral orifice. The ureteroscope was withdrawn until the stenosis was completely passed. At this time, the distance between the finger and the orifice was used to estimate the length of the stenosis. Then, we retracted the ureteroscope and left the guidewire as a safety wire in the ureter.
Ensure proper placement of the balloon
Along the safety wire (outside of the lens), the ureteroscope was replaced and reached the stenosis. Under the supervision of the ureteroscope, a ureteral catheter (F6, the same as the original unexpanded balloon) was pushed to pass through the stenosis along the guidewire. After withdrawing the inner guidewire, we should see that the urine flows out of the tube, which is a definitive indication that the same size balloon catheter (F6, 6 cm, BARD X-FORCE U30) would pass through the stricture. The guidewire was re-indwelled through the ureteral catheter, and the catheter was withdrawn. Under the direct observation of ureteroscopy, we pushed the balloon through the stricture along with the guidewire, ensuring the balloon cover all the stricture and left 2 cm below the distal end of the stricture according to the length we measured above. In this step, not all patients need a catheter test, but for patients with stenosis close to 2 cm or multiple stenosis, if the test could not be passed, the X-ray free surgery was no longer recommended.
Dilate the stricture
Under the direct observation of ureteroscopy, we set the pressure to 30 atmosphere (ATM) and kept it constant for three minutes. The stricture was observed to be immediately inflated after pressurization. In general, during the expansion process, the pressure would decrease due to the stricture opening, and we would maintain pressure at 30 ATM until three minutes had elapsed. After the dilation, we withdrew the balloon and left the guidewire in place.
Re-examine the ureter and indwell two double-J stents
After the dilation, we pushed the ureteroscope forward to the pelvis and carefully explored the ureteral stricture, ensuring that it is dilated and spread apart. Finally, we replaced the F8/9.8 ureteroscope, examined the whole upper tract and the two indwelling guidewires under direct vision and finally replaced them with two double-J stents and then an ultrasound was used to make sure the upper ends of stents are in the renal pelvis.
Statistical analysis
SPSS v.26 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Normally distributed continuous variables are expressed as mean values with standard deviations, and were compared using Student’s t-test. Nonnormally distributed continuous variables are expressed as median and interquartile ranges, and were compared using the Mann-Whitney U-test. Categorical variables were compared using the Chi-square test or Fisher’s exact test. A univariable analysis was performed to compare variable between successful and failed patients, and a multivariable survival analysis (Cox regression model) was performed to investigate potential variables related to time-dependent success rate.
Results
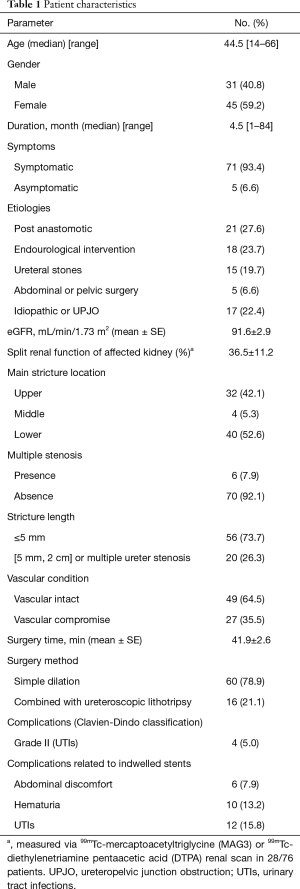
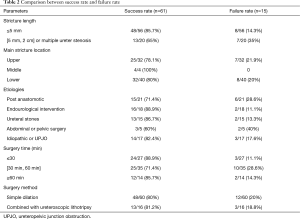
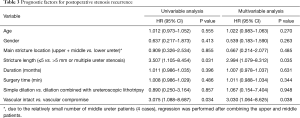
In our study, seventy-six patients with ureteral stricture underwent ureteral balloon dilation without fluoroscopic assistance. The patients’ characteristics are summarized in Table 1. All patients were discharged after receiving a Kidney-Ureter-Bladder plain film examination to determine the position of double-J stents, and all the positions presented well in our study. All stents were removed 3 months postoperatively. We considered the disappearance of preoperative symptoms, relief of hydronephrosis and stable renal function as the main criterion for success (59/76 patients). For patients with no significant changes in hydronephrosis, we also considered the surgery successful if there was no evidence of ureteral stricture on CTU (2/76 patients). In the 22.5 months median follow-up time, the one-year cumulative success rate and the two-year cumulative success rate were 85.9%±4.1% and 80.2%±5.0% (61/76), respectively, and the majority (13/15, 86.7%) recurrence of ureteral stricture or hydronephrosis deterioration were found within 2 years after surgery. The comparison of success and failure rate related to the variables that are key parameters of balloon dilation procedure is summarized in Table 2. The univariable and multivariable analyses for prognostic significance of postoperative stenosis recurrence are summarized in Table 3, the longer stenotic lesion length (>5 mm) or multiple ureter stenosis (P=0.035, HR =2.994) and compromised vascular supply of ureter (P=0.038, HR =3.030, the vascular supply status was determined by patients’ history, and the patients with a prior surgery involved the mobilization of ureter or periureteral tissue were defined as compromised blood supply (3) were significantly associated with postoperative stenosis recurrence.
Full table
Full table
Full table
Among the fifteen unsuccessful patients, one patient found renal atrophy one year after surgery and received conservative observation. The other fourteen patients underwent surgical intervention: two patients underwent another balloon dilation, two patients underwent a scheduled double-J stent replacement, one patient underwent nephrectomy and nine patients underwent ureteral reconstruction surgeries.
Discussion
Balloon dilation is a technique first developed for the treatment of coronary artery disease in 1979 (6). Four years later, Banner et al reported the use of balloon dilation in ureteral strictures with a 48% success rate in 44 patients (7). A recent meta-analysis summarized a total of 19 studies from 1983 to 2016 and showed a short-term success rate of 60%±10% and a long-term success rate of 54%±14% with a random effects model (8). The outcome of balloon dilation is influenced by several parameters, such as stricture etiology, stricture length, stricture location, renal function, and stent duration (9). Traditionally, all procedures of balloon dilation are carried out under fluoroscopic control. There are a few obvious benefits to X-ray guidance. First, the stenotic segment is clearly visualized while a guidewire and balloon catheter are passed through the segment. Second, the waist sign, the abolition of waist and the extravasation of contrast around the stricture site are considered to be a sign of success of the operation. However, during traditional balloon dilation, both patient and surgeon would receive a large amount of hazardous radiation, and this ionizing radiation may cause DNA damage that increases the risk for radiation related diseases. Therefore, it is of great significance to explore a surgical procedure that retains the advantages of X-ray assisted balloon dilation and avoids intraoperative radiation damage.
The key to the success of this X-ray free surgical procedure is that the position of the balloon should be accurate. In our protocol, while without fluoroscopic assistance, the whole image of the stricture was not very clear. We took several steps to compensate. First, we pushed the ureteroscope (F 4.5/6.5) to pass through the stenosis directly with a guidewire, observed the ureter above the stricture under direct vision, and retracted and measured the length of stenosis. This method is an accurate way to examine the stricture directly. Second, after initially expanding the stenosis using a hard F 6.5 ureteroscope body, we replaced the ureteroscope with an F 6.0 ureteral catheter and observed the drainage of upper urine to predict whether the balloon (F 6.0) could pass through the stenosis smoothly. Third, we usually left the balloon more than 2 cm below the distal end of the stricture to ensure the expansion effect. Fourth, since the waist sign is not visible without the help of X-ray imaging, under the surveillance of the ureteroscope (F 4.5), we directly used the maximum pressure of 30 ATM to ensure the complete expansion of the stricture because, fundamentally, the divulsion of the ureteral stricture under the balloon pressure caused the waist to disappear.
One of the crucial difficulties with balloon dilation is that for some patients, the stricture is so narrow that even the guidewire could not easily pass through. When this situation occurs, as in the traditional surgical procedure, we try to replace the guidewire with hydrophilic guidewire, finer guidewire or guidewire with a different hardness to pass the stenosis. Traditionally, after the guidewire passes through, the surgeon will expand the stenosis using a ureteral catheter from small to large to F 6.0 under X-ray assistance to ensure that the original unexpanded balloon (F 6.0) can pass through the narrow segment. In our protocol, we used an F 4.5/6.5 ureteroscope to directly expand and pass through the stenosis along the guidewire. While directly observing the stenosis and upper ureteral conditions, we pushed the scope body up. Because the ureteroscope is an F 4.5/6.5 specification, the scope body could be used to expand the stenosis to F 6.5 under direct visualization to ensure the safety and the passing of the original balloon (F 6.0) through the stricture. Obviously, this procedure is a simpler, more accurate and safer way to get through the stricture and expand it to the appropriate size.
The etiologies of ureteral strictures are divided into benign and malignant pathologies. Our focus is on benign causes, which may occur for intrinsic genitourinary processes or be iatrogenic in nature resulting from trauma, ureteral injury after endoscopic or percutaneous procedures, stone passage, radiation therapy, and open or laparoscopic surgery. The most prevalent risk factors are the presence of ureteral calculi and associated endourological treatment. Roberts et al retrospectively evaluated the records of 21 patients and found prolonged ureteral stone impaction greater than 2 months in duration is associated with a high incidence of stricture formation (24%) (10). In addition, according to Fam et al, in their prospective study, 5 out of 77 (7.8%) patients developed ureteral strictures following routine KUB (Kidney-Ureter-Bladder plain film) or ultrasound examination 3 and 6 months after ureteroscope treatment (11). Our statistics are consistent with the literature. A proportion of 18/76 (23.7%) patients had previously undergone urological procedures, and 15 patients (19.7%) were concomitantly diagnosed with stones. We performed a holmium laser lithotripsy operation immediately after balloon dilation.
The outcome of this X-ray free surgical procedure is similar to the traditional balloon dilation. During a median follow up of 22.5 months, the one-year cumulative success rate and the two-year cumulative success rate were 85.9%±4.1% and 80.2%±5.0%, respectively. The longer stenotic lesion length (>5 mm) or multiple ureter stenosis (P=0.035, HR =2.994) and compromised vascular supply of ureter (P=0.038, HR =3.030) were significantly associated with postoperative stenosis recurrence. These factors showed that the severity of stenosis as well as the devascularized status of ureter were associated with poor outcomes from a different perspective. Therefore, a detailed preoperative assessment of stenosis and individualized surgical strategy selection is the key to the success of balloon dilation.
This study has certain limitations and constraints. First, for patients whose stenosis is too narrow for the guidewire to pass, the operation procedure cannot be successfully performed. Second, the research data represent a retrospective review of findings at a single center. Third, limited by the small number of patients, the results of this study might have some statistical bias.
Conclusions
In carefully selected patients with short, benign and uncomplicated strictures, the fluoroscopy-less minimal ureteral balloon dilatation can be a safe and effective alternative, with less radiation hazards for both patient and surgeon. This procedure is safe and easy to learn and has significant clinical meaning for urologists and patients.
Acknowledgments
The authors thank Wenke Han, Jian Lin, Cheng Shen, Zhijun Xi and Xiang Chen for their contributions on this study. The authors thank the entire staff of the Department of Urology, Peking University First Hospital. This study was supported by Scientific Research Seed Fund of Peking University First Hospital (2019SF11) and Beijing Municipal Science and Technology Project Z2011000054200.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tau-21-203
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tau-21-203
Peer Review File: Available at https://dx.doi.org/10.21037/tau-21-203
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tau-21-203). XL serves as an unpaid editorial board member of Translational Andrology and Urology. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Biomedical Research Ethics Committee of Peking University First Hospital (IRB No. 2018-250) and all procedures performed in this study were in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tyritzis SI, Wiklund NP. Ureteral strictures revisited…trying to see the light at the end of the tunnel: a comprehensive review. J Endourol 2015;29:124-36. [Crossref] [PubMed]
- Reus C, Brehmer M. Minimally invasive management of ureteral strictures: a 5-year retrospective study. World J Urol 2019;37:1733-8. [Crossref] [PubMed]
- Richter F, Irwin RJ, Watson RA, et al. Endourologic management of benign ureteral strictures with and without compromised vascular supply. Urology 2000;55:652-7. [Crossref] [PubMed]
- Banner MP, Pollack HM, Ring EJ, et al. Catheter dilatation of benign ureteral strictures. Radiology 1983;147:427-33. [Crossref] [PubMed]
- Harris AM. Radiation Exposure to the Urologist Using an Overcouch Radiation Source Compared With an Undercouch Radiation Source in Contemporary Urology Practice. Urology 2018;114:45-8. [Crossref] [PubMed]
- Grüntzig A, Kumpe DA. Technique of percutaneous transluminal angioplasty with the Grüntzig ballon catheter. AJR Am J Roentgenol 1979;132:547-52. [Crossref] [PubMed]
- Banner MP, Pollack HM. Dilatation of ureteral stenoses: techniques and experience in 44 patients. AJR Am J Roentgenol 1984;143:789-93. [Crossref] [PubMed]
- Lu C, Zhang W, Peng Y, et al. Endoscopic Balloon Dilatation in the Treatment of Benign Ureteral Strictures: A Meta-Analysis and Systematic Review. J Endourol 2019;33:255-62. [Crossref] [PubMed]
- Lojanapiwat B, Soonthonpun S, Wudhikarn S. Endoscopic treatment of benign ureteral strictures. Asian J Surg 2002;25:130-3. [Crossref] [PubMed]
- Roberts WW, Cadeddu JA, Micali S, et al. Ureteral stricture formation after removal of impacted calculi. J Urol 1998;159:723-6. [Crossref] [PubMed]
- Fam XI, Singam P, Ho CC, et al. Ureteral stricture formation after ureteroscope treatment of impacted calculi: a prospective study. Korean J Urol 2015;56:63-7. [Crossref] [PubMed]



