
Subtotal surgical therapy for localized prostate cancer: a single-center precision prostatectomy experience in 25 patients, and SEER-registry data analysis
Introduction
Focal therapy has emerged as an integral part of a risk-stratified approach to prostate cancer (CaP) treatment in the recent years. Several techniques have now been described—all of them ablative (1,2).
The underlying reason for pursuing focal therapy in CaP patients is its promise of fewer functional side-effects without a compromise in oncological control (3). Accordingly, potency and continence results following focal therapy have been encouraging, with approximately ≥85% and ≥95% of the patients potent and continent by 12 months, respectively (4-8). Although oncological outcomes in the short-term appear reassuring (9), given the relative novelty of the focal therapy technology, long-term data on oncological efficacy are lacking. Further, a few limitations of focal ablative technologies have become apparent as experience with them has increased: (I) an inability or reluctance to treat a prostate gland >40 gram or apical cancers, (II) an inability to ablate >60% of the whole gland, (III) lack of pathological information on the ablated prostatic tissue, and (IV) a high positive biopsy rate [~50% clinical significant CaP (Gleason score 7 or above)] in the residual prostate tissue resulting in a high rate of redo procedures (~25% of the patients) within 3 years (6-8).
Building on the collective work of our peers in the field of focal therapy, while hoping to overcome the limitations of the focal ablative techniques, we recently described a novel surgical approach to CaP focal therapy—the precision prostatectomy. The robotic precision prostatectomy spares a 5–10 mm rim of prostate tissue unilaterally saving the ipsilateral neurovascular bundle in toto, while removing >90% of the gland along with the dominant cancer lesion. We recently reported on the short-term results in the first 8 patients that we had treated using this focal surgical approach (10,11). In this article, we sought to accomplish two goals: First, to provide an update on the safety, and functional and oncological outcomes in the first 25 consecutive men that have undergone precision prostatectomy and have greater than 2 years of follow-up, and second, to identify men within the Surveillance Epidemiology and End Results (SEER) data-registry that have undergone non-radical/focal treatment (surgical or ablative) for CaP over the years of registry subsistence, and assess long-term cancer survival outcomes in them. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1476).
Methods
Informed consent and study entry criteria for precision prostatectomy
Men meeting the criteria: (I) PSA ≤15 ng/mL, (II) stage ≤cT2, (III) dominant unilateral lesion with Gleason score ≤4+3 with any number of cores or percentage (%) cores involved ipsilaterally on TRUS prostate biopsy, (IV) no primary Gleason score ≥4 contralaterally on TRUS prostate biopsy, and (V) preoperatively potent with/without PDE-5 inhibitors were included in this IDEAL stage 2b prospective study (10,11).
The patients were apprised of the experimental nature of the intervention, and the fact that the risks could not be accurately estimated given the novelty of the procedure. Patients were required to review the informational material for at least seven days (11) before they would be considered for precision prostatectomy. Failing this in-home evaluation, patients were assigned to conventional radical prostatectomy. Informed consent was obtained from all patients. Data collection was done under an ongoing research protocol approved by the Henry Ford Hospital Institutional Review Board for the prospective trial of image-guided diagnosis and treatment of CaP (HFH-IRB#12507), and in compliance with HIPAA regulations.
Surgical technique of precision prostatectomy
Patient positioning, port placement, development of the space of Retzius, bladder neck incision and prostatic pedicle dissection were all performed in a manner similar to that of a traditional anterior approach of robotic radical prostatectomy (12,13). The difference was in the way the nerve-sparing was performed: A conventional nerve-sparing was performed on the side of the dominant cancer lesion, while, on the contralateral side (the precision side), the dissection was started anterior to the vas deferens/seminal vesicle complex, preserving all layers of Denonvilliers’ fascia, with the included erectogenic nerves (14,15). The dissection was then continued 5–10 mm into the prostatic capsule, deliberately attempting to leave behind a thin rim of prostatic capsule/peripheral tissue (5–10 mm) along with the seminal vesicle/ejaculatory duct complex (10,11). Systematic needle biopsies (via a suprapubic or transperineal approach) were taken from the remnant prostatic tissue, and sent for frozen section analysis. Completion prostatectomy was performed if the frozen biopsies showed residual cancer. Vesicourethral anastomosis was performed as previously described (12,13). The patients received a Foley or suprapubic tube per patient choice.
IDEAL stage 2b study of precision prostatectomy: variables, endpoints, follow-up and data analysis
For each patient, the following clinical characteristics were noted: age, race, body mass index, comorbidities, prior surgical history, preoperative prostate-specific antigen (PSA) level, clinical tumor stage, biopsy Gleason score, total number of cores on biopsy, number of positive cores on biopsy, percentage core positivity and urinary and sexual functional scores (see below). Preoperative patient preferences were recorded utilizing a previously validated 10-point clinical tool (Figure S1) (16). Operative characteristics collected included total operative time, console operative time, estimated blood loss, results of frozen section analysis, intraoperative complications and need to convert to radical prostatectomy. Pathological parameters collected included pathologic Gleason Score, pathological tumor stage and surgical margin status. The prostatectomy specimens were sectioned and processed according to the previously described whole-mount methodology by Ruijter et al. (17). Postoperative complications noted included need for blood transfusion, urinary tract infections, lymphoceles, deep venous thrombosis, pulmonary embolus, pneumonia, myocardial infections and death for 90 days after surgery. Preoperative and postoperative urinary and sexual function assessments were performed using the International Prostate Symptom Score (IPSS) and International Index of Erectile Function (IIEF-5 or SHIM) questionnaires. Postoperative PSA was collected at 4, 8 and 12 months. Three separate criteria were used to assess biochemical recurrence (BCR): (I) the American Urological Association (AUA) criteria for BCR following radical prostatectomy (i.e., a single value of PSA >0.4 or two consecutive values >0.2 ng/mL) (18) with remnant biopsy confirmation (the remnant biopsy confirmation was sought as a detectable PSA could be the result of residual benign or malignant cells, and histologic verification was required to diagnose BCR from cancer), (II) Phoenix criteria (19), and lastly (III) Huber criteria for focal therapy (20). Kaplan-Meier (KM) analysis was used to generate 3-year BCR, clinically-significant CaP (ISUP grade group ≥2) in the remnant, metastasis, cancer-specific mortality and overall mortality estimates. All patients were followed for at least 24 months.
SEER registry based assessment of non-radical CaP treatment
The SEER program covers approximately 35% of the US population, and is one of the most comprehensive source of population-based information in the US that includes stage of cancer at the time of diagnosis, treatment performed, and patient survival data (21). For the current study, data were abstracted from the 18-registry SEER dataset [2004–2015]. Only patients that had a pre-treatment biopsy-proven diagnosis of localized CaP (ICD-O code 61.9, histologic code 8140), and subsequently underwent a non-radical treatment for it were included. After excluding patients with missing data, a final sample size of 4,116 patients was obtained. Patients were grouped according to treatment type: hyperthermia, cryotherapy or segmental prostatectomy (22). The outcome of interest was CaP specific mortality. KM analysis was utilized to assess differences in CaP specific mortality among the groups and generate 10-year survival estimates. Cox proportional hazards modeling was performed to identify predictors of CaP specific mortality. The SEER study was approved by the Henry Ford Hospital Institutional Review Board (HFH-IRB# 13342) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
All statistical analyses were performed using SAS 9.3 (SAS Institute, Cary, NC). A two-sided statistical significance was defined as a P value <0.05.
Results
Baseline characteristics, preferences and operative outcomes in patients undergoing precision prostatectomy
Table 1 provides details on the baseline characteristics. Twenty-five patients underwent precision prostatectomy successfully during the study-period. One patient, not part of this report, was excluded due to positive intraoperative biopsy necessitating conversion to radical prostatectomy. Median (IQR) age and PSA were 56.5 (53.1–62.3) years and 4.2 (3.8–5.9) ng/mL, respectively. All patients were potent preoperatively with a median SHIM score of 23, however, 5 (20%) patients were on phosphodiesterase type 5 inhibitors (PDE-5i). Only 1 of the 25 patients (4%) met the Epstein criteria (23) for active surveillance.
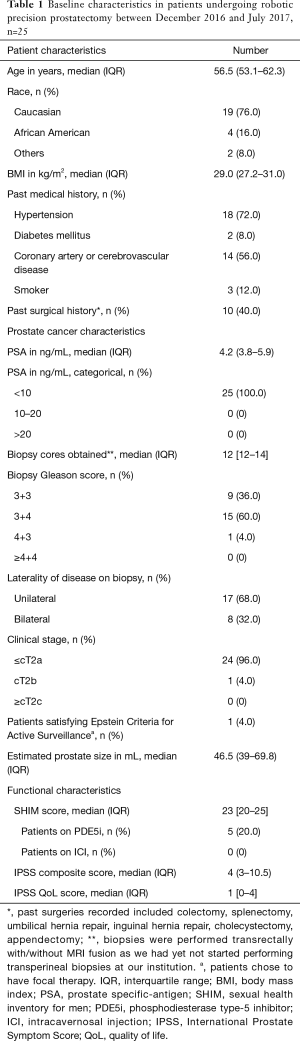
Full table
Median (IQR) console time was 133 min (125–141 min). There were no complications intraoperatively but one patient experienced a lymphocele postoperatively (Table 2).
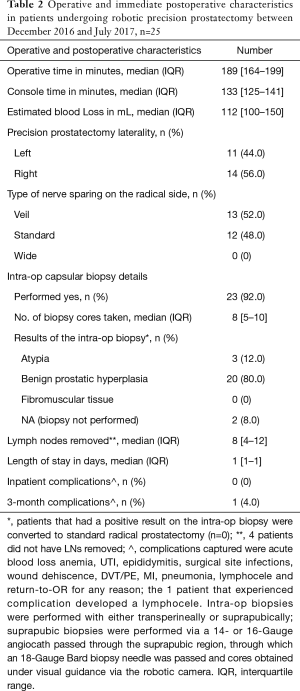
Full table
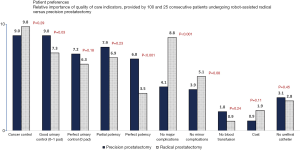
Figure 1 provides the breakdown of the preoperative patient preferences. Cancer control was the top priority for patients in both the radical and precision prostatectomy groups, however, the preferences for functional quality-of-life, especially potency preservation, varied significantly among the two groups.
Oncological and functional outcomes in patients undergoing precision prostatectomy
All patients were followed for a minimum of 2 years. At 12 months, all (100%) patients were continent (0–1 pads), with 92% (n=23 of 25) of the patients using 0 pads. The median (IQR) time to urinary continence was 1 month (1–4 months). Twenty-three (92%) of the 25 patients were potent at 12 months. The median SHIM score was 21 at 12 months with 76% of the patients using PDE-5i on an as-needed basis (Table 3). The median time to sexual potency was 4 months (4–12 months).
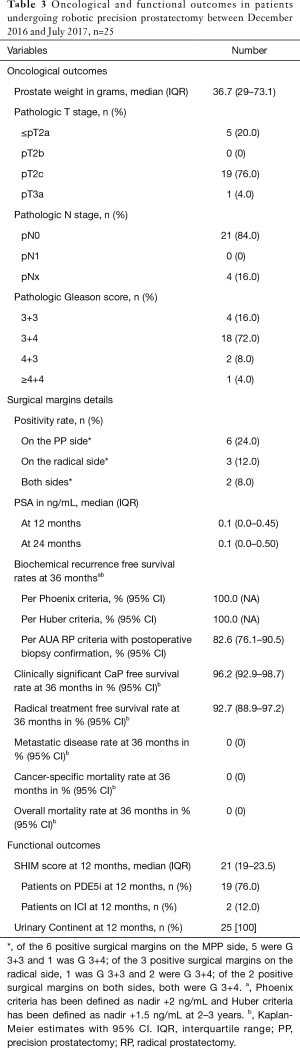
Full table
From an oncological standpoint, none (0%) of the patients had BCR per the Phoenix or Huber criteria for focal therapy at 36 months (19,20). The median PSA was 0.1 ng/mL post-surgery at 12 and 24 months follow-up. Given the novelty of our procedure and the lack of guidelines regarding oncological follow-up, we also assessed BCR in these patients using the AUA criteria for whole-gland radical prostatectomy coupled with remnant biopsy confirmation: 4 patients (36-month KM-estimate: 17.4%) had BCR per this criterion. Three patients had Gleason 3+3 disease while 1 patient had Gleason 3+4 disease in the remnant. Two patients including the patient with Gleason 3+4 disease had remnant removal surgery (36-month KM-estimate: 7.3%), while the other 2 chose active surveillance. It is interesting to note that surgical margin status was not associated with BCR (P=0.661)—of the 4 patients who experienced BCR, 1 had a positive margin on both sides, 1 only on the precision prostatectomy side, and 2 had negative margins. Of the 21 patients who did not experience BCR per the AUA criteria, 5 had undetectable PSA and declined a protocol biopsy, while the remaining 16 patients had no evidence of cancer on the biopsy of the remnant (median cores taken, 7). At 36 months, all patients were alive and free of metastatic disease (Table 3).
Baseline and long-term oncological data from the SEER data-registry on focal therapies
Table 4 provides details on the baseline characteristics. Of the 4,116 patients that underwent non-radical treatment of CaP during the 12-year study-period, 4.1% (n=169) underwent hyperthermia, 86.8% (n=3,571) cryotherapy, and the remaining 9.1% (n=376) segmental prostatectomy. Patients undergoing segmental prostatectomy were of younger age compared to other groups (P<0.001). Patients treated with segmental prostatectomy had higher pre-treatment PSA levels, with 12.2% of patients having a value >20 ng/mL, compared to 4.7% and 5.7% of the patients treated with hyperthermia and cryotherapy, respectively (P<0.001). On the other hand, segmental prostatectomy patients had lower biopsy Gleason scores, with only 9.6% of patients having a Gleason score ≥8, compared to 13.6% and 14.3% of the patients in hyperthermia and cryotherapy groups, respectively (P<0.001). Overall, 28.9%, 40.4% and 39.1% of the patients undergoing hyperthermia, cryotherapy and segmental prostatectomy were D’Amico high-risk (P=0.003).
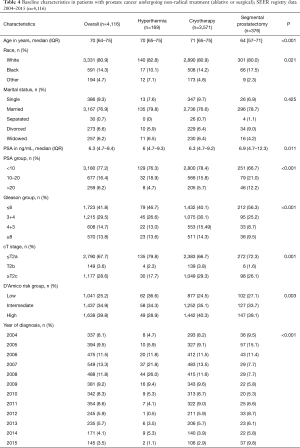
Full table
The median follow-up in the 3 treatment groups—hyperthermia, cryotherapy and segmental prostatectomy—was 7.4, 6.2 and 5.4 years, respectively. Figure 2 provides 10-year CaP-specific mortality estimates for the 3 treatment groups: overall, and stratified by D’Amico risk categories. In the overall cohort, at 10 years, 2.3%, 8.4% and 4.6% had died of CaP in hyperthermia, cryotherapy and segmental prostatectomy groups, respectively. These results did not attain statistical significance, Log-rank P=0.298 (Figure 2A). In the D’Amico risk based sub-group analysis: similarly, no differences were seen in 10-year CaP death rates among the treatment groups.
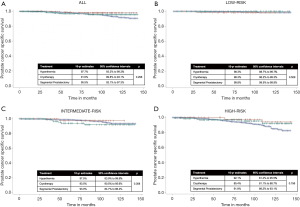
Cox regression analysis revealed older age, advanced clinical stage, and higher pre-treatment PSA levels and biopsy scores to be associated with worse survival. The type of focal therapy was not associated with CaP-specific mortality (Table 5).
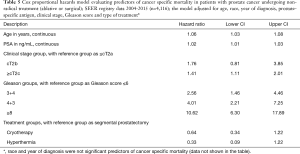
Full table
Conclusions
Whole-gland treatment of localized CaP is associated with significant functional side-effects, in particular erectile dysfunction (24,25). In an attempt to minimize this, focal CaP ablative techniques have recently been developed (1,2) following the well-established organ-preserving treatment paradigms in other malignancies (26-29).
Reports on focal ablative therapies have demonstrated promising functional outcomes, however, concerns regarding high secondary procedure rates and clinically-significant residual cancer have hampered the adoption (6-8). A study of 150 men (n=145 ≥Gleason 7) undergoing partial gland HIFU (high-intensity focused ultrasound) by Bass et al showed residual cancer in 70.5% of the men who underwent confirmatory biopsy and a 25% re-treatment rate, despite an attempt to ablate 5-10 fold the lesion volume detected on mp-MRI (6). It should be further noted that over 50% of these positive biopsies were in the areas that had been ‘treated’. Similarly, a study of focal HIFU by Mortezavi et al. demonstrated a residual cancer rate of 55.9%, with 75% of these patients harboring clinically-significant CaP (7). The median follow-up in these studies was limited, between 6 and 14 months. We have previously reasoned and demonstrated via our IDEAL stage 0 studies (10,11) that the reason for high residual cancer rates after focal, partial or hemigland HIFU or other ablative treatments is the inherent multifocality of cancer foci within the prostate, coupled with the fact that that mp-MRI technology cannot detect lesions smaller than 0.5 cc (whether high or low Gleason grade) (30) and focal ablative techniques spare 5–10 mm of peripheral tissue on the treatment side and do not treat the contralateral peripheral zone (4,6-8). It seems logical that by maximizing prostatic extirpation, the failure rates may be minimized [see Figure 1 of (10)], and a more acceptable balance may be achieved between the functional quality-of-life preservation and oncological control.
Accordingly, in the present study, we demonstrated that at 12 months, all patients were continent and 92% of the patients were potent, and at 36 months, 96.2% and 92.7% of the patients were free from clinically-significant CaP and radical treatment, respectively. All patients were alive and free of metastatic disease at study conclusion. However this evaluation of our technique is not devoid of limitations, within the bounds of which our results should be interpreted. These limitations include single-center design, and lack of long-term follow-up. However, the goal of this paper was to report on early results of the technique especially the functional results. Another limitation of our study is that patients selected for precision prostatectomy did not routinely undergo mp-MRI imaging, PSMA-PET imaging, or saturation biopsies to better characterize the burden and topography of CaP within the gland. Although this lack of standardization is a drawback of our current study, it is also an opportunity for further improvement of our oncological outcomes in the future.
We supplemented our IDEAL stage 2b study results with an assessment of 10-year CaP-specific survival data from the SEER data-registry. We postulated that this examination will help provide answers regarding long-term oncological efficacy of focal therapy treatments, while follow-up data from ongoing prospective studies of focal therapy mature. The findings were rather surprising, and demonstrated good long-term oncological efficacy for focal therapy techniques, with 10-year CaP-specific mortality rates of 2.5% to 7% for intermediate-risk CaP. It is important to point out here that the CaP patients included in the SEER study were diagnosed with CaP pre-treatment via prostate biopsy, and do not represent men with incidentally detected CaP post-treatment (such as after segmental prostatectomy). This suggests that these procedures were undertaken with a curative intent. Why a non-radical treatment was sought can only be speculated, but it does not impair the relevance of findings. While care must be taken when interpreting the results of this study, as the SEER registry does not provide information on additional treatments that these patients may have undergone subsequent to the initial treatment, it is unlikely that patients in the low and intermediate risk categories would have undergone subsequent salvage treatments given the fact that these patients chose to have non-radical treatment in the first place. These findings are further supported by a recent report evaluating short-term outcomes of HIFU—the study noted a 5-year CaP-specific survival rate of 100% for men with low and intermediate risk CaP (9). It thus seems plausible that focal treatment of the dominant lesion or the index lesion (31-33) may be all that is needed in some CaP patients, and a risk-stratified surgical approach to CaP treatment may be reasonable, especially in men who place a high emphasis on their sexual function. The key is to identify these patients accurately, and to maximize prostatic tissue extirpation without compromising the integrity of the neurovascular tissue.
In the end, only the long-term data will demonstrate the true efficacy of focal therapy. As Yogi Berra said “It’s hazardous to make predictions, especially about the future”. However, if focal therapy does deliver on its promise in the long-term, it will be a major advance in how care is delivered to CaP patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Badrinath R. Konety, Daniel W. Lin) for the series “Current and Future Topics on Prostate Cancer” published in Translational Andrology and Urology. The article has undergone external peer review.
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1476
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-1476
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1476). The series “Current and Future Topics on Prostate Cancer” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from all patients. The SEER study was approved by the Henry Ford Hospital Institutional Review Board (HFH-IRB# 13342). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- van der Poel HG, van den Bergh RCN, Briers E, et al. Focal Therapy in Primary Localised Prostate Cancer: The European Association of Urology Position in 2018. Eur Urol 2018;74:84-91. [Crossref] [PubMed]
- Valerio M, Ahmed HU, Emberton M, et al. The role of focal therapy in the management of localised prostate cancer: a systematic review. Eur Urol 2014;66:732-51. [Crossref] [PubMed]
- Ahmed HU. The index lesion and the origin of prostate cancer. N Engl J Med 2009;361:1704-6. [Crossref] [PubMed]
- Ahmed HU, Hindley RG, Dickinson L, et al. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol 2012;13:622-32. [Crossref] [PubMed]
- Golan R, Bernstein AN, McClure TD, et al. Partial Gland Treatment of Prostate Cancer Using High-Intensity Focused Ultrasound in the Primary and Salvage Settings: A Systematic Review. J Urol 2017;198:1000-9. [Crossref] [PubMed]
- Bass R, Fleshner N, Finelli A, et al. Oncologic and Functional Outcomes of Partial Gland Ablation with High Intensity Focused Ultrasound for Localized Prostate Cancer. J Urol 2019;201:113-9. [Crossref]
- Mortezavi A, Krauter J, Gu A, et al. Extensive Histological Sampling following Focal Therapy of Clinically Significant Prostate Cancer with High Intensity Focused Ultrasound. J Urol 2019;202:717-24. [Crossref] [PubMed]
- Abreu AL, Peretsman S, Iwata A, et al. High Intensity Focused Ultrasound Hemigland Ablation for Prostate Cancer: Initial Outcomes of a United States Series. J Urol 2020;204:741-7. [Crossref] [PubMed]
- Guillaumier S, Peters M, Arya M, et al. A Multicentre Study of 5-year Outcomes Following Focal Therapy in Treating Clinically Significant Nonmetastatic Prostate Cancer. Eur Urol 2018;74:422-9. [Crossref] [PubMed]
- Sood A, Jeong W, Taneja K, et al. The Precision Prostatectomy: an IDEAL Stage 0, 1 and 2a Study. BMJ Surg Interv Health Technologies 2019;1:e000002 [Crossref]
- Sood A, Abdollah F, Jeong W, et al. The Precision Prostatectomy: "Waiting for Godot Eur Urol Focus 2020;6:227-30. [Crossref] [PubMed]
- Menon M, Shrivastava A, Kaul S, et al. Vattikuti Institute prostatectomy: contemporary technique and analysis of results. Eur Urol 2007;51:648-57; discussion 657-8. [Crossref] [PubMed]
- Ghani KR, Trinh QD, Menon M. Vattikuti Institute Prostatectomy-Technique in 2012. J Endourol 2012;26:1558-65. [Crossref] [PubMed]
- Clarebrough EE, Challacombe BJ, Briggs C, et al. Cadaveric analysis of periprostatic nerve distribution: an anatomical basis for high anterior release during radical prostatectomy? J Urol 2011;185:1519-25. [Crossref] [PubMed]
- Costello AJ, Brooks M, Cole OJ. Anatomical studies of the neurovascular bundle and cavernosal nerves. BJU Int 2004;94:1071-6. [Crossref] [PubMed]
- Sammon J, Trinh QD, Menon M. Robotic radical prostatectomy: a critical analysis of surgical quality. Curr Opin Urol 2011;21:195-9. [Crossref] [PubMed]
- Ruijter E, van Leenders G, Miller G, et al. Errors in histological grading by prostatic needle biopsy specimens: frequency and predisposing factors. J Pathol 2000;192:229-33. [Crossref] [PubMed]
- Thompson IM, Valicenti RK, Albertsen P, et al. Adjuvant and salvage radiotherapy after prostatectomy: AUA/ASTRO Guideline. J Urol 2013;190:441-9. [Crossref] [PubMed]
- Roach M 3rd, Hanks G, Thames H Jr, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965-74. [Crossref] [PubMed]
- Huber PM, Afzal N, Arya M, et al. Prostate Specific Antigen Criteria to Diagnose Failure of Cancer Control following Focal Therapy of Nonmetastatic Prostate Cancer Using High Intensity Focused Ultrasound. J Urol 2020;203:734-42. [Crossref] [PubMed]
- NCI. Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2018 Sub (1975-2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969-2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released April 2019, based on the November 2018 submission. 2019.
- Roy S, Morgan SC. Who Dies From Prostate Cancer? An Analysis of the Surveillance, Epidemiology and End Results Database. Clin Oncol (R Coll Radiol) 2019;31:630-6. [Crossref] [PubMed]
- Dall'Era MA, Albertsen PC, Bangma C, et al. Active surveillance for prostate cancer: a systematic review of the literature. Eur Urol 2012;62:976-83. [Crossref] [PubMed]
- Barocas DA, Alvarez J, Resnick MJ, et al. Association Between Radiation Therapy, Surgery, or Observation for Localized Prostate Cancer and Patient-Reported Outcomes After 3 Years. JAMA 2017;317:1126-40. [Crossref] [PubMed]
- Capogrosso P, Vertosick EA, Benfante NE, et al. Are We Improving Erectile Function Recovery After Radical Prostatectomy? Analysis of Patients Treated over the Last Decade. Eur Urol 2019;75:221-8. [Crossref] [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [Crossref] [PubMed]
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91-102. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [Crossref] [PubMed]
- Bissada NK, Yakout HH, Fahmy WE, et al. Multi-institutional long-term experience with conservative surgery for invasive penile carcinoma. J Urol 2003;169:500-2. [Crossref] [PubMed]
- Bratan F, Melodelima C, Souchon R, et al. How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 2015;275:144-54. [Crossref] [PubMed]
- Liu W, Laitinen S, Khan S, et al. Copy number analysis indicates monoclonal origin of lethal metastatic prostate cancer. Nat Med 2009;15:559-65. [Crossref] [PubMed]
- Guo CC, Wang Y, Xiao L, et al. The relationship of TMPRSS2-ERG gene fusion between primary and metastatic prostate cancers. Hum Pathol 2012;43:644-9. [Crossref] [PubMed]
- Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nat Genet 2015;47:367-72. [Crossref] [PubMed]


