TGF-β mediated DNA methylation in prostate cancer
Introduction
The underlying mechanism promoting tumor progression has been elusive. Almost all tumors harbor a defective negative feedback loop of signaling by transforming growth factor-β (TGF-β). TGF-β signaling consists of Smad and non-Smad pathways (1). In advanced cancer cells, the non-Smad pathways predominate and progress leading to deregulated signaling cascades (2). This deregulation creates a unique TGF-β mediated tumor microenvironment that sets off a vicious cycle and promotes many of the hallmarks of tumor progression, including sustained angiogenesis, immune system evasion, proliferation, loss of the apoptotic response, epithelial-to-mesenchymal transition (EMT) and metastasis. These combined effects lead to uncontrolled tumor growth and spread, for which we coin the term “TGF-β mediated vicious cycle in tumor progression”. Recent evidence demonstrated that TGF-β mediates aggressive cancer including auto-induction of TGF-β1 and increased expression of DNA methyltransferases (DNMTs) (2,3). This latter observation suggests that the expression of these methylated genes may be an important event in TGF-β mediated tumor progression.
DNA methylation in cancer
Epigenetic changes are characteristic of nearly all malignancies and include changes in DNA methylation, histone modification and altered expression of microRNAs. DNA methylation plays a critical role in cancer development and progression. Alteration of DNA methylation patterns leads to deregulation of gene expression, in the absence of mutation. In the past few years, there has been an explosion in the number of publications in DNA methylation in all types of cancers (900 papers as of March 2012), including representative publications in prostate cancer (4-7), bladder cancer (8), renal cell carcinoma (9), breast cancer (10), lung cancer (11), ovarian cancer (12), oral cancer (13), pancreatic cancer (14), and other cancers. All tumors that have been examined show changes in DNA methylation, suggesting that this may represent a basic element of cancer biology, which has a significant impact on tumor pathology. Readers are referred to many excellent reviews on the biology of DNA methylation (15-17). This increased interest in the study of DNA methylation has created an opportunity for us to query the relationship between TGF-β signaling and DNA methylation in cancer, which has not been appreciated to date.
Biology of TGF-β signaling
TGF-β is a potent pleiotropic cytokine that regulates mammalian development, differentiation, and homeostasis in essentially all cell types and tissues. Its signaling is mediated through Smad and non-Smad pathways to regulate transcription, translation, microRNA biogenesis, protein synthesis and post-translational modifications (1,18,19). TGF-β binds to the type II TGF-β receptor (TβRII) which recruits and transphosphorylates the type I TGF-β receptor (TβRI) (20). The activated TβRI then phosphorylates Smad2 and Smad3 at the c-terminus. Activated Smad2/3 forms heterooligomers with Smad4 and migrates to the nucleus to regulate transcription. The Smad complexes interact with a myriad of transcriptional co-regulators and other factors to mediate target gene expression or repression (21,22). Smad2/3 also interacts with and regulates microRNA processing. TGF-β also signals through a number of non-Samd pathways, including m-TOR, RhoA, Ras, MAPK, PI3K/AKT, PP2A/p70s6K, and JNK (1,23,24). Finally, a direct action of the activated TβRI can interact with eEF1A1 to block protein synthesis (19). Dysregulation of both Smad and non-Smad pathways is implicated in aberrant TGF-β signaling and its pro-tumorigenic events in advanced cancer (3).
TGF-β signaling and DNA methylation
TGF-β is a key regulator for DNA methylation through an increase in DNMTs expression, especially in cancer (3,12). There exists a differential effect of TGF-β mediated DNMT activities between benign and malignant cells. In benign cells, TGF-β inhibits DNMT expression (25,26). In cancer cells, TGF-β stimulates DNMT expression (3,12). It should be noted that, in light of the importance of both TGF-β signaling and DNA methylation in tumor progression, the majority of the methylated genes in cancer are relevant to TGF-β signaling (12). This is consistent with our observation that over-expression of TGF-β and/or DNMTs is associated with aggressiveness and poor prognosis in prostate cancer (3,27).
Review of literature
In this review, we will focus our discussion in prostate cancer as an example, because the pattern of DNA methylation is organ specific. We surveyed the recent literature to identify the existing methylated genes in prostate cancer and attempt to determine which ones are mediated by TGF-β signaling. We have identified over 80 genes in which promoters are methylated in prostate cancer. This is a significant increase from 2006, when only 30 genes had been identified (28). Interestingly, the non-Smad pathways of known relevance to TGF-β are more often associated with de novo gene methylation (3,29). In contrast, the Smad-mediated pathways often lead to promoter de-methylation of genes (see below). In Table 1, we summarize the known TGF-β relevant genes in which the promoter becomes methylated in prostate cancer. We also identified those which have been known to be induced by TGF-β. Since, in advanced cancer cells, TGF-β induces the activation of Erk, JNK, AKT, and NF-κB (1,3), the above methylated gene have been documented in the literature to be related with one of the above transcription factors, thus are considered as TGF-β relevant.

Full table
In addition, there are a few genes that are de-methylated and are mediated through Smad2/3 activation, such as α2 [1] collagen (113), CD133 (26), and maspin (or SFN, 14-3-3 sigma) (41,59,67,114,115). However, a reversal of the methylation status in these genes can be observed in cancer cells when the TGF-β signaling events switched from the Smad pathways to the non-Smad pathways in cancer cells as in the case for maspin (116) and CD133 (117).
Table 2 lists genes that are not currently documented in the literature as TGF-β relevant. However, TGF-β mediates an over-expression of DNMTs in cancer cells, which is responsible for promoter methylation of these genes and. in non-cancer cells, TGF-β down-regulates the expression of DNMTs (25,26).
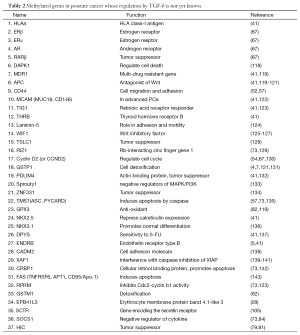
Full table
DNA methylation associated with tumor initiation and progression
A characteristic of DNA methylation in cancer is its heterogeneity. Despite of this variation, some trends can be discerned. We rationalize that genes that are wildly methylated are likely involved during early stages of tumor development, such as GSTP-1 (4), which may be used for the early detection of prostate cancer. Many investigators have used specific methylation pattern for prediction of cancer progression. However, during progression of prostate cancer, the tumor becomes increasingly heterogeneous, it will be difficult to pinpoint which genes are methylated that can be used as a prognostic marker and such efforts have been met with mixed results (144). It is reasonable to assume that as tumors progress, there will be more genes that undergo promoter methylation and demethylation. Therefore, the development of a rapid analysis of DNA methylation profile make it possible to follow the methylation patterns which may be used as an indication of disease progression.
Conclusions and future directions
Based on the present review, it is apparent that TGF-β signaling and DNA methylation are two important events in prostate cancer development and progression. In tumor progression, the deregulated TGF-β signaling mediates an increase in the number of genes undergoing DNA hypermethylation. These genes are generally associated with prevention of apoptosis, promotion of proliferation, facilitation of cell migration and evasion of the immune surveillance, resulting in tumor progression. In the era of personalized medicine, it becomes more important that we clearly define which genes are affected by TGF-β signaling and which genes are promoter hypermethylated during prostate cancer progression. Recent reports point out that some dietary and lifestyle interventions in cancer patients are mainly mediated through a reduction in DNA methylation (125,145,146), while others may lead to both gains and losses (147). It is possible that these dietary and lifestyle factors may be mediated at least partly through a normalization of the vicious cycle of TGF-β signaling in cancer microenvironment (148).
Acknowledgements
Funding: Studies in this report are supported by research grants from the National Cancer Institute (U01 CA152738 EDRN, UO1 CA114810 SPECS, P50 CA90386 SPORE) and the Department of Defense (W81XWH-09-1-0311 and W81XWH-08-1-0720).
Footnote
Conflicts of Interest: M. McClelland and D. Mercola are cofounders Proveri Inc., which is engaged in translational development of aspects of the subject matter. The other authors have no conflicts of interest to declare.
References
- Mu Y, Gudey SK, Landström M. Non-Smad signaling pathways. Cell Tissue Res 2012;347:11-20. [PubMed]
- Yu N, Kozlowski JM, Park II, et al. Over-expression of transforming growth factor 1 in malignant prostate cells is partly caused by a runaway of TGF-1 auto-induction mediated through a defective recruitment of protein phosphatase 2A by TGF-type I receptor. Urology 2010;76:1519.e8-13.
- Zhang Q, Chen L, Helfand BT, et al. Transforming Growth Factor-â-induced DNA methyltransferase contributes to aggressive prostate cancer phenotypes and predicts biochemical recurrence after radical prostatectomy. PLoS ONE 2011;6:e25168. [PubMed]
- Nelson WG, De Marzo AM, Yegnasubramanian S. Epigenetic alterations in human prostate cancers. Endocrinology 2009;150:3991-4002. [PubMed]
- Phé V, Cussenot O, Rouprêt M. Methylated genes as potential biomarkers in prostate cancer. BJU Int 2010;105:1364-70. [PubMed]
- Park JY. Promoter hypermethylation in prostate cancer. Cancer Control 2010;17:245-55. [PubMed]
- Goering W, Kloth M, Schulz WA. DNA methylation changes in prostate cancer. Methods Mol Biol 2012;863:47-66. [PubMed]
- Sánchez-Carbayo M. Hypermethylation in bladder cancer: biological pathways and translational applications. Tumour Biol 2012;33:347-61. [PubMed]
- Morris MR, Maher ER. Epigenetics of renal cell carcinoma: the path towards new diagnostics and therapeutics. Genome Med 2010;2:59. [PubMed]
- Huang Y, Nayak S, Jankowitz R, et al. Epigenetics in breast cancer: what’s new? Breast Cancer Res 2011;13:225. [PubMed]
- Rauch TA, Wang Z, Wu X, et al. DNA methylation biomarkers for lung cancer. Tumour Biol 2012;33:287-96. [PubMed]
- Matsumura N, Huang Z, Mori S, et al. Epigenetic suppression of the TGF-beta pathway revealed by transcriptome profiling in ovarian cancer. Genome Res 2011;21:74-82. [PubMed]
- González-Ramírez I, García-Cuellar C, Sánchez-Pérez Y, et al. DNA methylation in oral squamous cell carcinoma: molecular mechanisms and clinical implications. Oral Dis 2011;17:771-8. [PubMed]
- Delpu Y, Hanoun N, Lulka H, et al. Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr Genomics 2011;12:15-24. [PubMed]
- Cedar H, Bergman Y. Programming of DNA Methylation Patterns. Annu Rev Biochem 2012;81:97-117. [PubMed]
- Chiam K, Ricciardelli C, Bianco-Miotto T. Epigenetic biomarkers in prostate cancer: Current and future uses. Cancer Lett 2014;342:248-56. [PubMed]
- Sandoval J, Esteller M. Cancer epigenomics: beyond genomics. Curr Opin Genet Dev 2012;22:50-5. [PubMed]
- Hata A, Davis BN. Control of microRNA biogenesis by TGFbeta signaling pathway-A novel role of Smads in the nucleus. Cytokine Growth Factor Rev 2009;20:517-21. [PubMed]
- Hussey GS, Chaudhury A, Dawson AE, et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell 2011;41:419-31. [PubMed]
- Shi Y, Massagué J. Mechanisms of TGF-beta Signaling from Cell Membrane to the Nucleus. Cell 2003;113:685-700. [PubMed]
- Sontag E, Sontag JM, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J 1997;16:5662-71. [PubMed]
- Vogelmann R, Nguyen-tat MD, Giehl K, et al. TGFbeta-induced downregulation of E-cadherin-based cell-cell adhesion depends on PI3-kinase and PTEN. J Cell Sci 2005;118:4901-12. [PubMed]
- Kang JS, Liu C, Derynck R. New regulatory mechanisms of TGF-beta receptor function. Trends Cell Biol 2009;19:385-94. [PubMed]
- Hong M, Wilkes MC, Penheiter SG, et al. Non-Smad transforming growth factor-β signaling regulated by focal adhesion kinase binding the p85 subunit of phosphatidylinositol 3-kinase. J Biol Chem 2011;286:17841-50. [PubMed]
- Luo X, Zhang Q, Liu V, et al. Cutting Edge: TGF- Induced expression of Foxp3 in T cells is mediated through inactivation of ERK. J Immunol 2008;180:2757-61. [PubMed]
- You H, Ding W, Rountree CB. Epigenetic regulation of cancer stem cell marker CD133 by transforming growth factor-beta. Hepatology 2010;51:1635-44. [PubMed]
- Zhang Q, Helfand BT, Jang TL, et al. NF-kappaB-Mediated Transforming Growth Factor--Induced Expression of Vimentin is an Independent Predictor of Biochemical Recurrence After Radical Prostatectomy. Clin Cancer Res 2009;15:3557-67. [PubMed]
- Schulz WA, Ingenwerth M, Djuidje CE, et al. Changes in cortical cytoskeletal and extracellular matrix gene expression in prostate cancer are related to oncogenic ERG deregulation. BMC Cancer 2010;10:505. [PubMed]
- Lu R, Wang X, Chen ZF, et al. Inhibition of the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway decreases DNA methylation in colon cancer cells. J Biol Chem 2007;282:12249-59. [PubMed]
- Kim IY, Ahn HJ, Zelner DJ, et al. Loss of expression of transforming growth factor type I and type II receptors correlates with tumor grade in human prostate cancer tissues. Clin Cancer Res 1996;2:1255-61. [PubMed]
- Zhang Q, Rubenstein JN, Jang TL, et al. Insensitivity to transforming growth factor-signaling is resulted from promoter methylation of cognate receptors in human prostate cancer cells (LNCaP). Mol Endocrinol 2005;19:2390-9. [PubMed]
- Yamashita S, Takahashi S, McDonell N, et al. Methylation silencing of transforming growth factor-beta receptor type II in rat prostate cancers. Cancer Res 2008;68:2112-21. [PubMed]
- Dumont N, Wilson MB, Crawford YG, et al. Sustained induction of epithelial to mesenchymal transition activates DNA methylation of genes silenced in basal-like breast cancers. Proc Natl Acad Sci USA 2008;105:14867-72. [PubMed]
- Yang X, Pursell B, Lu S, et al. Regulation of beta 4-integrin expression by epigenetic modifications in the mammary gland and during the epithelial-to-mesenchymal transition. J Cell Sci 2009;122:2473-80. [PubMed]
- Sohn BH, Park IY, Lee JJ, et al. Functional switching of TGF-beta1 signaling in liver cancer via epigenetic modulation of a single CpG site in TTP promoter. Gastroenterology 2010;138:1898-908. [PubMed]
- Yu J, Cao Q, Yu J, et al. The neuronal repellent SLIT2 is a target for repression by EZH2 in prostate cancer. Oncogene 2010;29:5370-80. [PubMed]
- Skonier J, Neubauer M, Madisen L, et al. cDNA cloning and sequence analysis of beta ig-h3, a novel gene induced in a human adenocarcinoma cell line after treatment with transforming growth factor-beta. DNA Cell Biol 1992;11:511-22. [PubMed]
- Shah JN, Shao G, Hei TK, et al. Methylation screening of the TGFBI promoter in human lung and prostate cancer by methylation-specific PCR. BMC Cancer 2008;8:284. [PubMed]
- Kawasaki T, Nosho K, Ohnishi M, et al. IGFBP3 promoter methylation in colorectal cancer: relationship with microsatellite instability, CpG island methylator phenotype, and p53. Neoplasia 2007;9:1091-8. [PubMed]
- Yang YA, Zhang GM, Feigenbaum L, et al. Smad3 reduces susceptibility to hepatocarcinoma by sensitizing hepatocytes to apoptosis through downregulation of Bcl-2. Cancer Cell 2006;9:445-57. [PubMed]
- Vasiljević N, Wu K, Brentnall AR, et al. Absolute quantitation of DNA methylation of 28 candidate genes in prostate cancer using pyrosequencing. Dis Markers 2011;30:151-61. [PubMed]
- Kojima T, Takasawa A, Kyuno D, et al. Downregulation of tight junction-associated MARVEL protein marvelD3 during epithelial-mesenchymal transition in human pancreatic cancer cells. Exp Cell Res 2011;317:2288-98. [PubMed]
- Zhou W, Yu W, Xie W, et al. The role of SLIT-ROBO signaling in proliferative diabetic retinopathy and retinal pigment epithelial cells. Mol Vis 2011;17:1526-36. [PubMed]
- Ying TH, Yang SF, Tsai SJ, et al. Fisetin induces apoptosis in human cervical cancer HeLa cells through ERK1/2-mediated activation of caspase-8-/caspase-3-dependent pathway. Arch Toxicol 2012;86:263-73. [PubMed]
- Batlle E, Bacani J, Begthel H, et al. EphB receptor activity suppresses colorectal cancer progression. Nature 2005;435:1126-30. [PubMed]
- Nakanishi H, Nakamura T, Canaani E, et al. ALL1 fusion proteins induce deregulation of EphA7 and ERK phosphorylation in human acute leukemias. Proc Natl Acad Sci USA 2007;104:14442-7. [PubMed]
- Guan M, Xu C, Zhang F, et al. Aberrant methylation of EphA7 in human prostate cancer and its relation to clinicopathologic features. Int J Cancer 2009;124:88-94. [PubMed]
- Majid S, Dar AA, Shahryari V, et al. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer 2010;116:66-76. [PubMed]
- Lin TY, Cheng YC, Yang HC, et al. Loss of the candidate tumor suppressor BTG3 triggers acute cellular senescence via the ERK-JMJD3-p16(INK4a) signaling axis. Oncogene 2012;31:3287-97. [PubMed]
- Fosslien E. Review: molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann Clin Lab Sci 2001;31:325-48. [PubMed]
- Bastian PJ, Ellinger J, Wellmann A, et al. Diagnostic and prognostic information in prostate cancer with the help of a small set of hypermethylated gene loci. Clin Cancer Res 2005;11:4097-106. [PubMed]
- Woodson K, O’Reilly KJ, Ward DE, et al. CD44 and PTGS2 methylation are independent prognostic markers for biochemical recurrence among prostate cancer patients with clinically localized disease. Epigenetics 2006;1:183-6. [PubMed]
- Krop I, Parker MT, Bloushtain-Qimron N, et al. HIN-1, an inhibitor of cell growth, invasion, and AKT activation. Cancer Res 2005;65:9659-69. [PubMed]
- Henrique R, Costa VL, Cerveira N, et al. Hypermethylation of Cyclin D2 is associated with loss of mRNA expression and tumor development in prostate cancer. J Mol Med (Berl) 2006;84:911-8. [PubMed]
- Hesson LB, Cooper WN, Latif F. The role of RASSF1A methylation in cancer. Dis Markers 2007;23:73-87. [PubMed]
- Lee SJ, Lee MH, Kim DW, et al. Cross-Regulation between Oncogenic BRAFV600E Kinase and the MST1 Pathway in Papillary Thyroid Carcinoma. PLoS One 2011;6:e16180. [PubMed]
- Alumkal JJ, Zhang Z, Humphreys EB, et al. Effect of DNA methylation on identification of aggressive prostate cancer. Urology 2008;72:1234-9. [PubMed]
- Rodrigues RF, Roque L, Krug T, et al. Poorly differentiated and anaplastic thyroid carcinomas: chromosomal and oligo-array profile of five new cell lines. Br J Cancer 2007;96:1237-45. [PubMed]
- Wang SE, Narasanna A, Whitell CW, et al. Convergence of p53 and transforming growth factor beta (TGFbeta) signaling on activating expression of the tumor suppressor gene maspin in mammary epithelial cells. J Biol Chem 2007;282:5661-9. [PubMed]
- Hinshelwood RA, Huschtscha LI, Melki J, et al. Concordant epigenetic silencing of transforming growth factor-beta signaling pathway genes occurs early in breast carcinogenesis. Cancer Res 2007;67:11517-27. [PubMed]
- Wang X, Sun DF, Lu R, et al. RAF may induce cell proliferation through hypermethylation of tumor suppressor gene promoter in gastric epithelial cells. Cancer Sci 2009;100:117-25. [PubMed]
- Bhaskaran N, Souchelnytskyi S. Systemic analysis of TGFbeta proteomics revealed involvement of Plag1/CNK1/RASSF1A/Src network in TGFbeta1-dependent activation of Erk1/2 and cell proliferation. Proteomics 2008;8:4507-20. [PubMed]
- Mishra DK, Chen Z, Wu Y, et al. Global methylation pattern of genes in androgen-sensitive and androgen-independent prostate cancer cells. Mol Cancer Ther 2010;9:33-45. [PubMed]
- Kelley K, Berberich SJ. FHIT gene expression is repressed by mitogenic signaling through the PI3K/AKT/FOXO pathway. Am J Cancer Res 2011;1:62-70. [PubMed]
- Qin H, Wang L, Feng T, et al. TGF-beta promotes Th17 cell development through inhibition of SOCS3. J Immunol 2009;183:97-105. [PubMed]
- Pierconti F, Martini M, Pinto F, et al. Epigenetic silencing of SOCS3 identifies a subset of prostate cancer with an aggressive behavior. Prostate 2011;71:318-25. [PubMed]
- Diaw L, Woodson K, Gillespie JW. Prostate cancer epigenetics: a review on gene regulation. Gene Regul Syst Bio 2007;1:313-25. [PubMed]
- Rao ZY, Cai MY, Yang GF, et al. EZH2 supports ovarian carcinoma cell invasion and/or metastasis via regulation of TGF-beta1 and is a predictor of outcome in ovarian carcinoma patients. Carcinogenesis 2010;31:1576-83. [PubMed]
- Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS ONE 2012;7:e30393. [PubMed]
- Bamforth SD, Bragança J, Farthing CR, et al. Cited2 controls left-right patterning and heart development through a Nodal-Pitx2c pathway. Nat Genet 2004;36:1189-96. [PubMed]
- Bañez LL, Sun L, van Leenders GJ, et al. Multicenter clinical validation of PITX2 methylation as a prostate specific antigen recurrence predictor in patients with post-radical prostatectomy prostate cancer. J Urol 2010;184:149-156. [PubMed]
- Vinarskaja A, Schulz WA, Ingenwerth M, et al. Association of PITX2 mRNA down-regulation in prostate cancer with promoter hypermethylation and poor prognosis. Urol Oncol 2013;31:622-7. [PubMed]
- Suzuki M, Shigematsu H, Shivapurkar N, et al. Methylation of apoptosis related genes in the pathogenesis and prognosis of prostate cancer. Cancer Lett 2006;242:222-30. [PubMed]
- Lunghi P, Giuliani N, Mazzera L, et al. Targeting MEK/MAPK signal transduction module potentiates ATO-induced apoptosis in multiple myeloma cells through multiple signaling pathways. Blood 2008;112:2450-62. [PubMed]
- Ren C, Li L, Yang G, et al. RTVP-1, a tumor suppressor inactivated by methylation in prostate cancer. Cancer Res 2004;64:969-76. [PubMed]
- Hameetman L, Rozeman LB, Lombaerts M, et al. Peripheral chondrosarcoma progression is accompanied by decreased Indian Hedgehog signalling. J Pathol 2006;209:501-11. [PubMed]
- Yamada H, Vijayachandra K, Penner C, et al. Increased sensitivity of transforming growth factor (TGF) beta 1 null cells to alkylating agents reveals a novel link between TGFbeta signaling and O(6)-methylguanine methyltransferase promoter hypermethylation. J Biol Chem 2001;276:19052-8. [PubMed]
- Konishi N, Nakamura M, Kishi M, et al. DNA hypermethylation status of multiple genes in prostate adenocarcinomas. Jpn J Cancer Res 2002;93:767-73. [PubMed]
- Yamanaka M, Watanabe M, Yamada Y, et al. Altered methylation of multiple genes in carcinogenesis of the prostate. Int J Cancer 2003;106:382-7. [PubMed]
- Kang GH, Lee S, Lee HJ, et al. Aberrant CpG island hypermethylation of multiple genes in prostate cancer and prostatic intraepithelial neoplasia. J Pathol 2004;202:233-40. [PubMed]
- Yegnasubramanian S, Kowalski J, Gonzalgo ML, et al. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res 2004;64:1975-86. [PubMed]
- Lodygin D, Epanchintsev A, Menssen A, et al. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res 2005;65:4218-27. [PubMed]
- Noordhuis MG, Fehrmann RS, Wisman GB, et al. Involvement of the TGF-beta and beta-catenin pathways in pelvic lymph node metastasis in early-stage cervical cancer. Clin Cancer Res 2011;17:1317-30. [PubMed]
- Suzuki M, Shigematsu H, Shames DS, et al. DNA methylation-associated inactivation of TGFbeta-related genes DRM/Gremlin, RUNX3, and HPP1 in human cancers. Br J Cancer 2005;93:1029-37. [PubMed]
- Hasegawa K, Yazumi S, Wada M, et al. Restoration of RUNX3 enhances transforming growth factor-beta-dependent p21 expression in a biliary tract cancer cell line. Cancer Sci 2007;98:838-43. [PubMed]
- Richiardi L, Fiano V, Vizzini L, et al. Promoter methylation in APC, RUNX3, and GSTP1 and mortality in prostate cancer patients. J Clin Oncol 2009;27:3161-8. [PubMed]
- Cui J, Rohr LR, Swanson G, et al. Hypermethylation of the caveolin-1 gene promoter in prostate cancer. Prostate 2001;46:249-56. [PubMed]
- Razani B, Zhang XL, Bitzer M, et al. Caveolin-1 regulates transforming growth factor (TGF)-beta/SMAD signaling through an interaction with the TGF-beta type I receptor J Biol Chem 2001;276:6727-38. [PubMed]
- Rosemblit N, Chen CL. Regulators for the rat clusterin gene: DNA methylation and cis-acting regulatory elements. J Mol Endocrinol 1994;13:69-76. [PubMed]
- Rauhala HE, Porkka KP, Saramäki OR, et al. Clusterin is epigenetically regulated in prostate cancer. Int J Cancer 2008;123:1601-9. [PubMed]
- Rizzi F, Bettuzzi S. Clusterin (CLU) and prostate cancer. Adv Cancer Res 2009;105:1-19. [PubMed]
- Becker J, Volland S, Noskova I, et al. Keratoepithelin reverts the suppression of tissue factor pathway inhibitor 2 by MYCN in human neuroblastoma: a mechanism to inhibit invasion. Int J Oncol 2008;32:235-40. [PubMed]
- Ribarska T, Ingenwerth M, Goering W, et al. Epigenetic inactivation of the placentally imprinted tumor suppressor gene TFPI2 in prostate carcinoma. Cancer Genomics Proteomics 2010;7:51-60. [PubMed]
- Zhang C, Basta T, Fawcett SR, et al. SOX7 is an immediate-early target of VegT and regulates Nodal-related gene expression in Xenopus. Dev Biol 2005;278:526-41. [PubMed]
- Guo L, Zhong D, Lau S, et al. Sox7 Is an independent checkpoint for beta-catenin function in prostate and colon epithelial cells. Mol Cancer Res 2008;6:1421-30. [PubMed]
- Park JY, Zheng W, Kim D, et al. Candidate tumor suppressor gene SLC5A8 is frequently down-regulated by promoter hypermethylation in prostate tumor. Cancer Detect Prev 2007;31:359-65. [PubMed]
- Bennett KL, Romigh T, Eng C. Disruption of transforming growth factor-beta signaling by five frequently methylated genes leads to head and neck squamous cell carcinoma pathogenesis. Cancer Res 2009;69:9301-5. [PubMed]
- Sørensen KD, Wild PJ, Mortezavi A, et al. Genetic and epigenetic SLC18A2 silencing in prostate cancer is an independent adverse predictor of biochemical recurrence after radical prostatectomy. Clin Cancer Res 2009;15:1400-10. [PubMed]
- Bourque M, Liu B, Dluzen DE, et al. Sex differences in methamphetamine toxicity in mice: effect on brain dopamine signaling pathways. Psychoneuroendocrinology 2011;36:955-69. [PubMed]
- Irvine SA, Foka P, Rogers SA, et al. A critical role for the Sp1-binding sites in the transforming growth factor-beta-mediated inhibition of lipoprotein lipase gene expression in macrophages. Nucleic Acids Res 2005;33:1423-34. [PubMed]
- Kim JW, Cheng Y, Liu W, et al. Genetic and epigenetic inactivation of LPL gene in human prostate cancer. Int J Cancer 2009;124:734-8. [PubMed]
- Higuchi T, Nakamura M, Shimada K, et al. HRK inactivation associated with promoter methylation and LOH in prostate cancer. Prostate 2008;68:105-13. [PubMed]
- Ribas VT, Arruda-Carvalho M, Linden R, et al. Early c-Jun N-terminal kinase-dependent phosphorylation of activating transcription factor-2 is associated with degeneration of retinal ganglion cells. Neuroscience 2011;180:64-74. [PubMed]
- Dragovic RA, Ritter LJ, Schulz SJ, et al. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod 2007;76:848-57. [PubMed]
- Devaney J, Stirzaker C, Qu W, et al. Epigenetic deregulation across chromosome 2q14.2 differentiates normal from prostate cancer and provides a regional panel of novel DNA methylation cancer biomarkers. Cancer Epidemiol Biomarkers Prev 2011;20:148-59. [PubMed]
- Carey JP, Asirvatham AJ, Galm O, et al. Inhibitor of differentiation 4 (Id4) is a potential tumor suppressor in prostate cancer. BMC Cancer 2009;9:173. [PubMed]
- Hogg K, Etherington SL, Young JM, et al. Inhibitor of differentiation (Id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-beta family. Endocrinology 2010;151:1247-56. [PubMed]
- Vinarskaja A, Goering W, Ingenwerth M, et al. ID4 is frequently downregulated and partially hypermethylated in prostate cancer. World J Urol 2012;30:319-25. [PubMed]
- Sørensen KD, Borre M, Ørntoft TF, et al. Chromosomal deletion, promoter hypermethylation and downregulation of FYN in prostate cancer. Int J Cancer 2008;122:509-19. [PubMed]
- Kim AN, Jeon WK, Lim KH, et al. Fyn mediates transforming growth factor-beta1-induced down-regulation of E-cadherin in human A549 lung cancer cells. Biochem Biophys Res Commun 2011;407:181-4. [PubMed]
- Kannangai R, Diehl AM, Sicklick J, et al. Hepatic angiomyolipoma and hepatic stellate cells share a similar gene expression profile. Hum Pathol 2005;36:341-7. [PubMed]
- Suzuki M, Shigematsu H, Shames DS, et al. Methylation and gene silencing of the Ras-related GTPase gene in lung and breast cancers. Ann Surg Oncol 2007;14:1397-404. [PubMed]
- Yamane K, Suzuki H, Ihn H, et al. Cell type-specific regulation of the TGF-beta-responsive alpha2(I) collagen gene by CpG methylation. J Cell Physiol 2005;202:822-30. [PubMed]
- Hurtubise A, Momparler RL. Evaluation of antineoplastic action of 5-aza-2’-deoxycytidine (Dacogen) and docetaxel (Taxotere) on human breast, lung and prostate carcinoma cell lines. Anticancer Drugs 2004;15:161-7. [PubMed]
- Li X, Kaplun A, Lonardo F, et al. HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase pi in prostate carcinoma cells. Mol Cancer Res 2011;9:733-45. [PubMed]
- Rivenbark AG, Stolzenburg S, Beltran AS, et al. Epigenetic reprogramming of cancer cells via targeted DNA methylation. Epigenetics 2012;7:350-60. [PubMed]
- Pellacani D, Packer RJ, Frame FM, et al. Regulation of the stem cell marker CD133 is independent of promoter hypermethylation in human epithelial differentiation and cancer. Mol Cancer 2011;10:94. [PubMed]
- Liu WB, Ao L, Zhou ZY, et al. CpG island hypermethylation of multiple tumor suppressor genes associated with loss of their protein expression during rat lung carcinogenesis induced by 3-methylcholanthrene and diethylnitrosamine. Biochem Biophys Res Commun 2010;402:507-14. [PubMed]
- Dobosy JR, Roberts JL, Fu VX, et al. The expanding role of epigenetics in the development, diagnosis and treatment of prostate cancer and benign prostatic hyperplasia. J Urol 2007;177:822-31. [PubMed]
- Henrique R, Ribeiro FR, Fonseca D, et al. High promoter methylation levels of APC predict poor prognosis in sextant biopsies from prostate cancer patients. Clin Cancer Res 2007;13:6122-9. [PubMed]
- Tilandyová P, Kajo K, Kliment J, et al. Detection of DNA hypermethylation as a potential biomarker for prostate cancer. Klin Onkol 2010;23:293-9. [PubMed]
- Liu JW, Nagpal JK, Jeronimo C, et al. Hypermethylation of MCAM gene is associated with advanced tumor stage in prostate cancer. Prostate 2008;68:418-26. [PubMed]
- Ellinger J, Bastian PJ, Jurgan T, et al. CpG island hypermethylation at multiple gene sites in diagnosis and prognosis of prostate cancer. Urology 2008;71:161-7. [PubMed]
- Sathyanarayana UG, Padar A, Suzuki M, et al. Aberrant promoter methylation of laminin-5-encoding genes in prostate cancers and its relationship to clinicopathological features. Clin Cancer Res 2003;9:6395-400. [PubMed]
- Yee DS, Tang Y, Li X, et al. The Wnt inhibitory factor 1 restoration in prostate cancer cells was associated with reduced tumor growth, decreased capacity of cell migration and invasion and a reversal of epithelial to mesenchymal transition. Mol Cancer 2010;9:162. [PubMed]
- Wissmann C, Wild PJ, Kaiser S, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 2003;201:204-12. [PubMed]
- Gudjonsson JE, Johnston A, Stoll SW, et al. Evidence for altered Wnt signaling in psoriatic skin. J Invest Dermatol 2010;130:1849-59. [PubMed]
- Fukuhara H, Kuramochi M, Fukami T, et al. Promoter methylation of TSLC1 and tumor suppression by its gene product in human prostate cancer. Jpn J Cancer Res 2002;93:605-9. [PubMed]
- Dong SW, Cui YT, Zhong RR, et al. Decreased expression of retinoblastoma protein-interacting zinc-finger gene 1 in human esophageal squamous cell cancer by DNA methylation. Clin Lab 2012;58:41-51. [PubMed]
- Padar A, Sathyanarayana UG, Suzuki M, et al. Inactivation of cyclin D2 gene in prostate cancers by aberrant promoter methylation. Clin Cancer Res 2003;9:4730-4. [PubMed]
- Meiers I, Shanks JH, Bostwick DG. Glutathione S-transferase pi (GSTP1) hypermethylation in prostate cancer: review 2007. Pathology 2007;39:299-304. [PubMed]
- Vanaja DK, Ballman KV, Morlan BW, et al. PDLIM4 repression by hypermethylation as a potential biomarker for prostate cancer. Clin Cancer Res 2006;12:1128-36. [PubMed]
- Kwabi-Addo B, Ren C, Ittmann M. DNA methylation and aberrant expression of Sprouty1 in human prostate cancer. Epigenetics 2009;4:54-61. [PubMed]
- Yu J, Liang QY, Wang J, et al. Zinc-finger protein 331, a novel putative tumor suppressor, suppresses growth and invasiveness of gastric cancer. Oncogene 2013;32:307-17. [PubMed]
- Das PM, Ramachandran K, Vanwert J, et al. Methylation mediated silencing of TMS1/ASC gene in prostate cancer. Mol Cancer 2006;5:28. [PubMed]
- Schulz WA, Hatina J. Epigenetics of prostate cancer: beyond DNA methylation. J Cell Mol Med 2006;10:100-25. [PubMed]
- Chung W, Kwabi-Addo B, Ittmann M, et al. Identification of novel tumor markers in prostate, colon and breast cancer by unbiased methylation profiling. PLoS ONE 2008;3:e2079. [PubMed]
- Chang G, Xu S, Dhir R, et al. Hypoexpression and epigenetic regulation of candidate tumor suppressor gene CADM-2 in human prostate cancer. Clin Cancer Res 2010;16:5390-401. [PubMed]
- Fang X, Liu Z, Fan Y, et al. Switch to full-length of XAF1 mRNA expression in prostate cancer cells by the DNA methylation inhibitor. Int J Cancer 2006;118:2485-9. [PubMed]
- Lee MG, Huh JS, Chung SK, et al. Promoter CpG hypermethylation and downregulation of XAF1 expression in human urogenital malignancies: implication for attenuated p53 response to apoptotic stresses. Oncogene 2006;25:5807-22. [PubMed]
- Murphy TM, Perry AS, Lawler M. The emergence of DNA methylation as a key modulator of aberrant cell death in prostate cancer. Endocr Relat Cancer 2008;15:11-25. [PubMed]
- Jerónimo C, Henrique R, Oliveira J, et al. Aberrant cellular retinol binding protein 1 (CRBP1) gene expression and promoter methylation in prostate cancer. J Clin Pathol 2004;57:872-6. [PubMed]
- Santourlidis S, Warskulat U, Florl AR, et al. Hypermethylation of the tumor necrosis factor receptor superfamily 6 (APT1, Fas, CD95/Apo-1) gene promoter at rel/nuclear factor kappaB sites in prostatic carcinoma. Mol Carcinog 2001;32:36-43. [PubMed]
- Jerónimo C, Bastian PJ, Bjartell A, et al. Epigenetics in prostate cancer: biologic and clinical relevance. Eur Urol 2011;60:753-66. [PubMed]
- Ho E, Beaver LM, Williams DE, et al. Dietary factors and epigenetic regulation for prostate cancer prevention. Adv Nutr 2011;2:497-510. [PubMed]
- Lim U, Song MA. Dietary and lifestyle factors of DNA methylation. Methods Mol Biol 2012;863:359-76. [PubMed]
- Ornish D, Magbanua MJ, Weidner G, et al. Changes in prostate gene expression in men undergoing an intensive nutrition and lifestyle intervention. Proc Natl Acad Sci USA 2008;105:8369-74. [PubMed]
- Lee C, Zhang Q, Kozlowski J, et al. Natural products and transforming growth factor-beta (TGF-β) signaling in cancer development and progression. Curr Cancer Drug Targets 2013;13:500-5. [PubMed]


