
Downregulation of miR-145 is associated with perineural invasion in penile carcinoma
Introduction
Penile cancer (PC) represents a public health problem, due to its high incidence, especially in countries such as Asia, Africa, and South America. Brazil has one of the highest incidence of PC in the world, with 2.9–6.8 per 100,000 inhabitants (1). The etiology of PC remains inconclusive in several aspects, but some risk factors have been associated with this malignant neoplasm. Among them, we highlight the lack of personal hygiene and infection by human papillomavirus (HPV), phimosis with chronic inflammation, smoking (2-4), and some epigenetic alterations, including histone methylation modification (5).
The main prognostic factors in PC are histological grade advanced, stage of the tumor (pT3, pT4), vascular and perineural invasion, and lymph node metastasis, reported in 20% to 65% of cases (6). The association of molecular markers with lymph node metastasis (7), recurrence, and perineural invasion (8,9) has been described in the PC. However, none of these markers that predict worse prognosis are used in clinical practice.
MicroRNAs are regulatory, noncoding RNAs about 8–25 nt. These biomolecules are widely studied due to their potential in mRNA silencing, in addition to having several biological functions (10). The expression profile of the microRNAs is dependent on the initiation and progression of human diseases, especially for various kinds of cancer, suggesting that miRNAs potentially represent prognostic markers (11).
MiR-145 is still involved in the regulation of important cell processes, such as p53-mediated cell-cycle arrest (10). Its deregulation was found in osteosarcoma (12), ovarian cancer (13), breast cancer (14), colorectal cancer (15). Reduced expression of miR-145 in colorectal cancer tissues is associated with lymph node metastasis and advanced clinical stage (15). Downregulation of miR-145 has been associated with higher HPV load and, consequently, overexpression of the E6 oncoprotein (16). MiR-145 is still involved in regulating critical cell processes, such as p53-mediated cell cycle arrest (17).
In PC miR-145 has already been addressed in two studies, but no significant relationship was found with factors with a worse prognosis and HPV. Here, we analyzed miR-145 in PC in penile tumors infected by HPV and to correlate it with the clinicopathological characteristics of the tumor and the protein expression of p53, mirR-145 may be potential biomarker to PC. To our knowledge, this is the first work that addresses the expression or miR-145 has reduced expression associated with cases of histological associated HPV, absence of p53 expression in positive HPV cases and perineural invasion.
We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1378).
Methods
Patients
Formalin-fixed paraffin-embedded samples from 52 patients with PC were obtained from the Aldenora Bello Cancer Hospital and the University Hospital of the Federal University of Maranhão in São Luís, Maranhão, Brazil, where the human development index is a low 0.768 (IBGE, 2010), and the incidence of PC is the highest recorded in the world (1). Tumor samples were obtained at the time of surgery, without neoadjuvant treatment, from January 2013 to June 2016.
The Scientific Commission approved the study-COMIC-HUUFMA with the opinion no. 2457/2014-60 and by the Research Ethics Committee of the University Hospital/CEP-HUUFMA with approval number: 1.093.435. To meet the regulations for research with human subjects, established by Resolution no. 466/12 of the National Health Council, all duly guided participants, provided a signed Free and Informed Consent Form. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All individuals or their legal guardians were educated about the study and signed an informed consent form.
This series is part of a previous study (7). For the classification of tumors the 8th edition American Joint Committee on Cancer, was used (18). The majority of tumors had histological subtype warty or basaloid (38.5%), histological grade 2 (63.5%) and primary tumor pT2 and pT3 (73.1%) prevailed (Table 1).
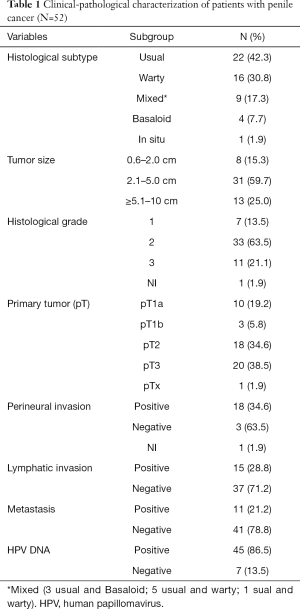
Full table
Tumor samples
FFPE samples were used, a pathologist selected two H&E stained slides containing paired samples from each case. One slide presented neoplastic tissue, and the other presented adjacent tissue, 2 cm distant from the primary tumor and comprising at least 70% of malignant cells. The adjacent tissue did not contain neoplastic tissue, fibrosis, adipose tissue, or any other contaminant. RNA and DNA were extracted from histological slides containing 10 micrometers of each tissue.
HPV detection
For identification DNA HPV, extraction of the genomic DNA was performed using the protocol of Sambrook et al. [1989]. HPV DNA was performed using the nested PCR technique using primers PGMY09/11 and GP5+/6+ (19), which produce a fragment referring to the L1 region of the HPV viral capsid.
The identification of the subtypes HPV of the samples HPV-positive, was performed the sequencing reaction with the DYEnamic ET Terminator Cycle Sequencing Kit, according to the protocol suggested by the manufacturer (GE Healthcare, Little Chalfont, UK).
qRT-PCR
Total RNA was extracted using the High Pure miRNA Isolation Kit. Transcription reaction was performed with the MicroRNA TaqMan® RT kit according to the manufacturer’s instructions. The reactions were performed in triplicate and miR-45 normalized with an endogenous control (RNU6B), and the relative expression of miR-145 was calculated using the 2−ΔΔCt method (20). The primer sequences used were: miR-145, 5'-AAG CTT CAG AGG GTT TCC GGT ACTT-3' (forward) and 5'-CTC GAG AGC CTC ACA GGG ATG TTA TG-3' (reverse); RNU6B, 5'-CGC TTC GGC AGC ACA TAT AC-3' (forward) and 5'-TTC ACG AAT TTG CGT GTC AT-3' (reverse).
Immunohistochemical analysis
Immunohistochemistry of p53 was performed using the monoclonal the anti-p53 monoclonal antibody clone DO-7 (Dako), com marcação nuclear was used the expression patterns were previously defined by Lopes et al. (21). Ki-67 immunohistochemistry was performed using the Ki-67 Clone SP6 (Spring, Bioscience, USA) primary antibody diluted in a ratio of 1:200 µL. As negative control, we used amygdala samples. Ki-67 scores were used according to the intensity of staining and percentage of positive cells: negative (score 0), low (score 1), moderate (score 2, 25–50%), and high expression (score 3, >50%), as described by Muñoz et al. (22).
Statistical analysis
The χ2 test was used to determine the association among clinicopathological, protein expression, and HPV identification. Student’s t-test was used to compare microRNA expression levels with clinical-pathological parameters and protein expression. Survival analysis was performed using the Kaplan-Meier method to determine disease-free survival and overall survival compared with the level of microRNA expression and clinicopathological characteristics. The log-rank test was used to compare survival curves. All statistical analyses were performed using SPSS software (version 23.0, Chicago, IL, USA). For all analyses, P values ≤0.05 were considered statistically significant.
Results
miR-145 expression profile in PC
All the 52 patients were diagnosed with squamous cell penile carcinoma, and the average time between the emergence of symptoms until the search for treatment was 21.8 months, with mean follow-up was 17.3 months. They presented an average age of 61.8 years, consisting mainly of farmers (45%); most were married (73.8%), with little or no educational background (73.8%). Most of these patients underwent partial penectomy (73.1%), with lesions located on the gland (96.1%).
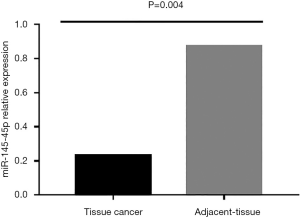
Regarding the expression of mir-145 (Figure 1), miR-145 expression was significantly lower in the primary tumor compared to adjacent tissue (median 0.23±0.87; fc =−17.5).
Clinical-pathological features vs. miR-145
The association of clinical-pathological features with miR-145 expression is summarized in Table 2. It was also observed that miR-145 expression was significantly reduced in patients with perineural invasion (P=0.033) and HPV-associated histological subtypes (P=0.024), pT3, and lymph node metastasis cases showed lower expression levels, although no statistical difference.

Full table
Phimosis was present in 65.8% (27/41) of the evaluated patients, and in these, miR-145 expression was reduced (0.379±0.065; P=0.09) compared to patients without this condition. Regarding the other clinical-pathological characteristics, 34.6% (18/52) of the patients had undergone lymphadenectomy, 33.9% (7/18) had lymphadenitis, 66.1% (11/18) were diagnosed with lymph node metastasis, the latter showed lower miR-145 expression (0.127±0.0562; P=0.8). Similarly, the mean expression of miR-145 in patients with lymph node enlargement was lower (66.6%; 12/18; −0.760±0.433; P=0.09) compared to patients without lymph node enlargement.
Five patients died from cancer during the treatment period, 43 stayed disease-free, and four abandoned treatment. Among the 52 patients, relapses episodes were observed in seven patients. Besides the surgical treatment, two patients were subjected to chemotherapy and seven to radiotherapy.
miR-145 and HPV
HPV infection was detected in 86.5% (45/52) of the primary tumor samples. Most of these cases were also detected through histological diagnosis (39/52). The most frequent subtypes were HPV 16 (21/45; 46,6%), followed by 59, 74, 73, 11, 30, 56, 58, 66, 6, 44, 51, 53 and 63 (Table 3). The presence of HPV infection was associated with the absence of metastasis (P=0.029; Table S1).

Full table
Considering all the groups of tumors in the sample, it was observed that the expression of miR-145 was significantly lower in p53- tumors (P=0.045; Table 3). The same was found in the p53-HPV+ tumor sample group (−1.268±−0.30; P=0.0044). There was no statistical difference between the expression of p53 and Ki67 with the clinicopathological characteristics and the presence of HPV (Table S1).
Survival analysis
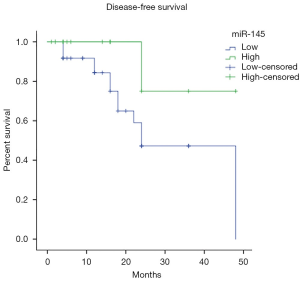
Our results showed that patients with perineural invasion had a lower shorter disease-free survival time (log-rank P=0.012) (Figure 2). Patients with reduced expression of miR-145, showed disease-free survival period of 31.3 months, while those had high expression presented a disease-free survival period of months 42, there was no significant difference (log-rank P=0.072) (Figure 3).
Discussion
Mir-145 is a tumor suppressor (Tsmir) that is differentially expressed in some cancers, capable of regulating multiple oncogenes’ expression in several biological pathways (23). Previously, Barzon et al. (24) studied this microRNA and its correlation with some histological characteristics and HPV in PC but did not find any relevant results. These results were probably because comparisons were not made between neoplastic and non-neoplastic samples, and there was a much lower prevalence of positive HPV cases. Kuasne et al. (25) have detected, by microarray, changes in miRNA-145 expression in PC samples. The two studies described above were the only ones to provide clues about the potential role of miRNA-145 in penile carcinogenesis.
Despite the lack of information on the aberrant expression of microRNAs associated with the worst prognosis in PC, some studies have already presented important data. In 24 PC samples, Hartz et al. (26) observed hsa-miR-1, hsa-miR-101 and hsa-miR-204 was significantly associated with lymph node metastasis and unfavorable prognosis. Recently our group found that elevated expression and miR-107 and miR-223-p were associated with the lowest on disease-free life and lymph node metastasis, respectively (7).
The data presented in this work demonstrate that miR-145 is a potential biomarker of perineural invasion (PNI). In previous studies, PNI has been recognized as a prognostic indicator of poor survival in patients with PC (27). The downregulation of miR-145 is associated with poor PFS (progression-free survival) in patients with lung cancer, colorectal cancer, esophageal cancer, breast cancer, and glioma (28). This can be explained by the fact that miR-145 targets multiple oncogenes that act in metastatic and epithelial-mesenchymal transition processes, such as Vimentin, Cadherin, Fibronectin, SMAD3, MMP11, Snail1, ZEB1/2, HIF-1α and Rock-1 (29). Interestingly, miR-145 has been observed in extracellular vesicles isolated from body fluids (30). In addition, it acts in several tumorigenic functions, including the regulation of cell proliferation, differentiation, apoptosis, invasion and metastasis (23). This distinct role of miR-145 can introduce this biomolecule as a potential candidate for the control of cancer metastasis by miRNA replacement therapy (30).
Therefore our data demonstrate that this microRNA could promote cancer cells to invade the nerve bundle and spread out. PNI represents a possible way of spreading tumor cells, being able to promote metastasis, representing, therefore, a histological characteristic of worse prognosis (31). Our study has also shown that cases with downregulation of miR-145 have shorter disease-free survival and a worse prognosis, such as perineural invasion. This implies that the study expression of miR-145 can be a useful tool in the prognostic evaluation of this subset of patients
Concerning the downregulation of miR-145 in cases of histological associated HPV and absence of p53 expression in positive HPV cases. In neoplasms associated with HPV infection, such as head/neck and cervical neoplasms, the negative regulation of miR-145 was related to viral virus infection and loss of p53 (32,33). This is probably because the wild-type p53 gene is a tumor suppressor that regulates the transcription of miR-145 positively. However, with the activation of HPV E6 (HPV-E6) oncoprotein, suppression of p53 expression occurs because the HPV E6 oncoprotein can degrade the p53 protein via ubiquitination, resulting in reduction (33). This may be a possible explanation for the low expression of miR-145 in PC observed in the present study.
MiR-145 acts regulating a series of oncogenes such as EGFR, C-MYC, KLF4, E2F3 e CDK6 (23). Among these, EGFR and C-MYC have been widely studied for PC. Studies have demonstrated that EGFR is often overexpressed its protein and gene dysregulation, has already been associated with advanced stage, lower overall survival and metastatic lymph node status (34). Downregulation of miR-145 may be one of the mechanisms for high expression of EGFR, especially in HPV positive tumors (35). Additionally, this microRNA can regulate pathways such as Wnt, TGF-beta, Calcium which are involved in cell growth, progression, survival, angiogenesis, and apoptosis (23).
One limitation in our study is the use of an adjacent sample as a control, but it is necessary to consider the difficulty in obtaining normal samples for the study of patients with PC. Furthermore, this methodology has been accepted in studies with this type of tumor as reported in the work of Macedo et al. (35) and Pinho et al. (7).
Conclusions
In summary, our results showed that miR-145 is less expressed in PC samples compared to the region adjacent to the tumor. Also, the reduction of this microRNA may be triggered by HPV, which decreases p53 protein expression. Our data also hint that miR-145 expression may be an indicator of PNI- positive. To our knowledge, this is the first study to address the relationship of miR-145 with HPV and p53 in PC and perineural invasion. Deregulation of miR-145 appears to be a factor for promoting tumorigenesis, being, therefore, a potential biomarker.
Acknowledgments
Funding: The study was supported by the Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão (FAPEMA) under grant number 00696/14 and coordination the author GEBS and Nucleus of Oncology Research (NPO/ HUJBB).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1378
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1378
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1378). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Scientific Commission-COMIC-HUUFMA with the opinion no. 2457/2014-60 and by the Research Ethics Committee of the University Hospital/CEP-HUUFMA with approval number: 1.093.435. All individuals or their legal guardians were educated about the study and signed an informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coelho RWP, Pinho JD, Moreno JS, et al. Penile cancer in Maranhão, Northeast Brazil: the highest incidence globally? BMC Urol 2018;18:50. [Crossref] [PubMed]
- Christodoulidou M, Sahdev V, Houssein S, et al. Epidemiology of penile cancer. Curr Probl Cancer 2015;39:126-36. [Crossref] [PubMed]
- Ferrándiz-Pulido C, Torres I, García-Patos V. Penile squamous cell carcinoma. Actas Dermosifiliogr 2012;103:478-87. [PubMed]
- Vuichoud C, Klap J, Loughlin KR. The Emerging Role and Promise of Biomarkers in Penile Cancer. Urol Clin North Am 2016;43:135-43. [Crossref] [PubMed]
- Zhang L, Wei P, Shen X, et al. MicroRNA Expression Profile in Penile Cancer Revealed by Next-Generation SmallRNA Sequencing. PLoS One 2015;10:e0131336 [Crossref] [PubMed]
- Protzel C, Alcaraz A, Horenblas S, et al. Lymphadenectomy in the surgical management of Penile cancer. Eur Urol 2009;55:1075-88. [Crossref] [PubMed]
- Pinho JD, Silva GEB, Teixeira Júnior AAL, et al. MIR-107, MIR-223 and MIR-21-5P reveals potential Biomarkers in penile cancer. Asian Pac J Cancer Prev 2020;21:391-7. [Crossref] [PubMed]
- Silva Amâncio AM, Cunha IW, Neves JI, et al. Epidermal growth factor receptor as an adverse survival predictor in squamous cell carcinoma of the penis. Hum Pathol 2017;61:97-104. [Crossref] [PubMed]
- da Cunha IW, Souza MJ, da Costa WH, et al. Epithelial-mesenchymal transition (EMT) phenotype at invasion front of squamous cell carcinoma of the penis influences oncological outcomes. Urol Oncol 2016;34:433.e19-26. [Crossref] [PubMed]
- Shen J, Sanford A, Feng Jaing S. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett 2013;329:125-36. [Crossref] [PubMed]
- Babaei K, Shams S, Keymoradzadeh A, et al. An insight of microRNAs performance in carcinogenesis and tumorigenesis;an overviews of cancer therapy. Life Sci 2020;240:117077 [Crossref] [PubMed]
- Zhang Z, Zhang M, Chen Q, et al. Downregulation of microRNA-145 promotes epithelial-mesenchymal transition via regulating Snail in osteosarcoma. Cancer Gene Ther 2017;24:83-8. [Crossref] [PubMed]
- Wu H, Xiao Z, Wang K, et al. MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys Res Commun 2013;441:693-700. [Crossref] [PubMed]
- Zheng M, Sun X, Li Y, et al. MicroRNA-145 inhibits growth and migration of breast cancer cells through targeting oncoprotein ROCK1. Tumour Biol 2016;37:8189-96. [Crossref] [PubMed]
- Qin J, Wang F, Jiang H, et al. MicroRNA-145 suppresses cell migration and invasion by targeting paxillin in human colorectal cancer cells. Int J Clin Exp Pathol 2015;8:1328-40. [PubMed]
- Gunasekharan V, Laimins LA. Human Papillomaviruses modulate MicroRNA 145 expression to directly control genome amplification. J Virol 2013;87:6037-43. [Crossref] [PubMed]
- Teng Y, Yam GH, Li N, et al. MicroRNA regulation of MDM2-p53 loop in pterygium. Exp Eye Res 2018;169:149-56. [Crossref] [PubMed]
- Amin. American joint committee on cancer. AJCC cancer staging manual. 8th ed.
- Gravitt PE, Peyton C, Alessi T, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol 2000;38:357-61. [PubMed]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc 2008;3:1101-8. [Crossref] [PubMed]
- Lopes A, Bezerra ALR, Pinto CAL, et al. p53 as a new prognostic factor for lymph node metastasis in penile carcinoma: analysis of 82 patients treated with amputation and bilateral lymphadenectomy. J Urol 2002;168:81-6. [Crossref] [PubMed]
- Muñoz JJ, Drigo SA, Barros-Filho MC, et al. Down-regulation of SLC8A1 as a putative apoptosis evasion mechanism by modulation of calcium levels in Penile carcinoma. J Urol 2015;194:245-51. [Crossref] [PubMed]
- Cui SY, Wang R, Chen L. MicroRNA-145: a potent tumour suppressor that regulates multiple cellular pathways. J Cell Mol Med 2014;18:1913-26. [Crossref] [PubMed]
- Barzon L, Cappelesso R, Peta E, et al. Profiling of expression of human papillomavirus-related cancer miRNAs in penile squamous cell carcinomas. Am J Pathol 2014;184:3376-83. [Crossref] [PubMed]
- Kuasne H, Barros-Filho MC, Busso-Lopes A, et al. Integrative miRNA and mRNA analysis in penile carcinomas reveals markers and pathways with potential clinical impact. Oncotarget 2017;8:15294-306. [Crossref] [PubMed]
- Hartz JM, Engelmann D, Furst K, et al. Integrated Loss of miR-1/miR-101/miR-204 Discriminates Metastatic from Nonmetastatic Penile Carcinomas and Can Predict Patient Outcome. J Urol 2016;196:570-8. [Crossref] [PubMed]
- Zhou X, Qi F, Zhou R, et al. The role of perineural invasion in penile cancer;a meta-analysis and systematic review. Biosci Rep 2018;38:BSR20180333 [Crossref] [PubMed]
- Xu L, Zhang Y, Tang J, et al. The Prognostic Value and Regulatory Mechanisms of microRNA-145 in Various Tumors: A Systematic Review and Meta-analysis of 50 Studies. Cancer Epidemiol Biomarkers Prev 2019;28:867-81. [Crossref] [PubMed]
- Zeinali T, Mansoori B, Mohammadi A, et al. Regulatory mechanisms of miR-145 expression and the importance of its function in cancer metastasis. Biomed Pharmacother 2019;109:195-207. [Crossref] [PubMed]
- Wang J, Zhang KY, Liu SM, et al. Tumor- associated circulating microRNAs as biomarker of cancer. Molecules 2014;19:1912-38. [Crossref] [PubMed]
- Liebig C, Ayala G, Wilks JA, et al. Perineural Invasion in cancer. Cancer 2009;115:3379-91. [Crossref] [PubMed]
- Lajer CB, Garnaes E, Friis-Hansen L, et al. The role of miRNAs in human papillomavirus (HPV)-associated cancers: bridging between HPV-related head and neck cancer and cervical cancer. Brit J Cancer 2012;106:1526-34. [Crossref] [PubMed]
- Wang Q, Qin J, Chen A, et al. Downregulation of microRNA-145 is associated with aggressive progression and poor prognosis in human cervical cancer. Tumour Biol 2015;36:3703-8. [Crossref] [PubMed]
- Ali SM, Pal SK, Wang K, et al. Comprehensive genomic profiling of advanced penile carcinoma suggests a high frequency of clinically relevant genomic alterations. Oncologist 2016;21:33-9. [Crossref] [PubMed]
- Macedo J, Silva E, Nogueira L, et al. Genomic profiling reveals the pivotal role of hrHPV driving copy number and gene expression alterations, including mRNA downregulation of TP53 and RB1 in penile cancer. Mol Carcinog 2020;59:604-17. [Crossref] [PubMed]



