
Pseudoangiosarcomatous squamous cell carcinoma: first case report on penis
Introduction
Penile squamous cell carcinoma is an uncommon neoplasia in developed countries, but has a high incidence in South America, Southeast Asia and Africa. In Brazil its incidence is 6.1/100 thousand inhabitants (1). This type of tumor is strongly associated with socioeconomic factors, genital hygiene, HPV infection and important developmental factors (2).
Penile lesions usually present as a vegetative lesion, and sometimes ulcerated. Histologically, penile squamous cell carcinoma is classified according to its histomorphological characteristics and its relation or not to HPV. Non-HPV-related variants include the usual variant, pseudohyperplastic, pseudoglandular, verrucous, papillary, adenosquamous, sarcomatoid, and mixed. HPV-related variants are basaloid, Warty, Warty-basaloid, clear cells tumor, and lymphoepithelioma-like. Histological grading is an important prognostic and predictive factor for metastasis to inguinal lymph nodes (2). The poorly differentiated pseudoangiosarcomatous variant of squamous cells carcinoma (SCC) exhibits proliferation of polygonal or flattened atypical keratinocytes that form interanastomosing channels that mimic vascular proliferation these pseudovessels have prominent neoplastic cells and red blood cells inside (3-6). These atypical cells are positive for cytokeratins and negative for vascular markers such as CD31 and CD34 (3,7,8). It is also positive for p16 in the presence of HPV (2). This report presents the first case of penile pseudoangiosarcomatous squamous cell carcinoma, describing its clinical, histological and immunohistochemical characteristics, as well as HPV status.
We present the following case in accordance with the CARE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1234).
Case presentation
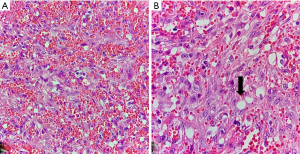
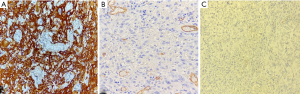
A 38-year-old patient was admitted to the urology outpatient clinic with a history of painful penile injury and urinary retention. Physical examination revealed extensive, vegetative and large lesion that compromised the urethral meatus, associated with bilateral lymphadenopathy. The patient underwent emasculation and bilateral orchiectomy surgery, and the material was sent for the pathology division. Macroscopy revealed two ulcerative vegetating lesions, the largest measuring 5.5 cm × 4.5 cm, circumferential, infiltrating the penis 13 cm deep and destroying the glans and part of the penile body, and extended to the scrotum skin. Microscopy showed poorly differentiated atypical squamous cells infiltrating the underlying stroma forming channels that simulated vessels containing neoplastic, inflammatory, and red blood cells (Figure 1A). Some neoplastic cells also contained large intracytoplasmic vacuoles, simulating a capillary (Figure 1B). The lesion infiltrated the corpus spongiosum, corpus cavernosum, urethra and scrotum, with free surgical margins. No angiolymphatic invasion was found, but perineural invasion was observed. These neoplastic squamous cells were positive for the cytokeratin marker and were negative for the CD31 and CD34 markers (Figure 2). Human papilloma virus (HPV) biomarkers, p16, E6 protein and polymerase chain reaction (PCR), were all negative. The histological diagnosis was undifferentiated squamous cell carcinoma (G4), angiosarcomatous type, occupying the entire glans and infiltrating the penile body. Testicles and epididymis showed no histological changes. The pathological staging was cT4 pNx pMx.
At follow-up, the patient underwent right iliac chain lymphadenectomy with histopathological report of metastasis to 7 lymph nodes, with confluence and extra-capsular extension. Then, a left iliac chain lymphadenectomy was performed, with histopathological report of metastasis to 2 lymph nodes, with extra-capsular extension in one of them. The patient was then re-staged for pT4 pN3 pMx and referred for radiotherapy, and treatment with 28 sessions of 5.040 cGy cobalt in the pelvis was indicated in the month following the last surgery. After 20 sessions, the patient was discharged with outpatient follow-up.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
The case described above is a rare variant of SCC in the penis, which mimics an angiosarcoma, requiring careful histological evaluation and immunohistochemical study for correct diagnosis. The microscopic findings of this case are similar to those described in a study in which complex anastomosed labyrinthine channels lined with polygonal or flattened cells were observed, and the pseudo lumen containing occasional tumor cells and red blood cells (9). Cells covering these channels were positive for AE1/AE3 and negative for CD31 and CD 34. In this case report it was observed that the degree of morbidity and mortality of angiosarcomatous SCC is higher than in other variants of SCC, probably due to its anaplastic nature, demonstrating high rates of early recurrence and metastases. These findings were also observed in a report of two cases of pseudoangiosarcomatous squamous cell carcinoma that affected the vulva (9). According to a literature review in which 12 studies were included, with a total of 19 patients, eight of these had regional lymph node metastasis, and 10 died of lung metastasis (8). The case presented in this report showed invasion of the corpus spongiosum, corpus cavernosum, urethra and scrotum, with lymph node metastasis, demonstrating the aggressive behavior of this tumor and the need for early diagnosis and radical treatment.
Although this is the first case described of penile angiosarcomatous squamous cell carcinoma, which limits the comparison of prognosis with other cases, it could be observed that this was an aggressive case, with scrotal sac skin invasion and lymph node metastases.
Conclusions
The above report is an atypical presentation of an HPV-negative advanced penile squamous cell carcinoma with aggressive lymph node metastasis. Further studies to assess the association of this finding with a worse prognosis are needed.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1234
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1234). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Coelho RWP, Pinho JD, Moreno JS, et al. Penile cancer in Maranhão, Northeast Brazil: the highest incidence globally? BMC Urol 2018;18:50. [Crossref] [PubMed]
- Cubilla AL, Amin MB, Ayala G, et al. Malignant epithelial tumours. In: Moch H, Humpphrey PA, Ulbright TM, et al. (eds) Who classification of Tumours of the urinary system and male genital organs. Lyon: International Agency for Research on Cancer, 2016, pp. 262-276.
- Banerjee SS, Eyden BP, Wells S, et al. Pseudoangiosarcomatous carcinoma: a clinicopathological study of seven cases. Histopathology 1992;21:13-23. [Crossref] [PubMed]
- Yamamoto O, Yasuda H. A case of pseudovascular adenoid squamous cell carcinoma of the skin with spindle cell pattern. J Dermatol 1997;24:587-94. [Crossref] [PubMed]
- Eusebi V, Lamovec J, Cattani MG, et al. Acantholytic variant of squamous-cell carcinoma of the breast. Am J Surg Pathol 1986;10:855-61. [Crossref] [PubMed]
- Nappi O, Wick MR, Pettinato G, et al. Pseudovascular adenoid squamous cell carcinoma of the skin. A neoplasm that may be mistaken for angiosarcoma. Am J Surg Pathol 1992;16:429-38. [Crossref] [PubMed]
- Koh SH, Oh SJ, Chun H, et al. Pseudoangiosarcomatous squamous cell carcinoma developing on a burn scar: a case report and review of the literature. Burns 2014;40:e47-52. [Crossref] [PubMed]
- Pitt MA, Morphopoulos G, Wells S, et al. Pseudoangiosarcomatous carcinoma of the genitourinary tract. J Clin Pathol 1995;48:1059-61. [Crossref] [PubMed]
- Santos LD, Krivanek MJ, Chan F, et al. Pseudoangiosarcomatous squamous cell carcinoma of the vulva. Pathology 2006;38:581-4. [Crossref] [PubMed]


