Phytotherapy: emerging therapeutic option in urologic disease
Introduction
Complementary and alternative medicine (CAM) is defined as a group of diverse medical and health care systems, practices, and products that are not generally considered part of conventional medicine by the National Institutes of Health in North America. CAM practices are often grouped into broad categories, such as natural products, mind-body medicine, and manipulative and body-based practices. Phytotherapy belongs to the area of CAM and the definition of phytotherapy is the use of plants or plant extracts for medicinal uses. Phytotherapy is greatly differentiated from the pharmacological treatment for which various substances are made into drugs. Herbal phytotherapy is a natural therapy to prevent or treat diseases by analyzing the medical actions of certain plants and using the ingredients excellent in medical effect. Regardless of east or west, the herbal medicine has been used to keep health and cure diseases for a very long time through traditional principles on life. In spite of the remarkable development of modern medicine, the most of Asians are still favorably disposed toward herb medicines, also now is the time when it is required to develop natural remedies using the medicinal herbs that are helpful for preventing and treating the chronic diseases.
Prevalence
CAM is commonly used in the United States (1-3). Almost half of patients use alternative therapies for chronic medical conditions in conjunction with prescription medication (4). Kaufman et al. (5) reported the results of a survey showing that 81% of the population had used at least one medication (prescription, vitamin/mineral, or herbal supplement) during the preceding week. 14% of the population used phytotherapy and 16% of the regular prescription user also used herbal supplements. A growing number of the world’s population in different communities are turning to plant derived remedies for many reasons. A survey revealed that 71% of Canadians and 19% of the adult population in the United States were using natural health products (6). Most users of herbal products decide to consume herbal remedies in the belief that these natural products are safer than synthetic drugs, and this perception is mainly based on personal sentiment, information coming from friends or the media.
History of the phytotherapy: from traditional to modern usage of herbs
Phytotherapies are used all over the world as a rich source of therapeutic agents for the prevention of diseases. Countries with ancient civilizations such as China, India, South America, Egypt, etc. are still using several plant remedies for various conditions. The reason for the popularity and acceptability is belief that all natural products are safe. The demand for plant based medicines, health products, pharmaceuticals, food supplement, cosmetics, etc. are increasing in both developing and developed countries, due to the growing recognition that the natural products are non-toxic, have less side effects and easily available (7). Nowadays, there is a revival of interest with herbal-based medicine due to the increasing understanding of the hazards associated with the indiscriminate use of modern medicine and it is now very fast growing in the international market. However, there are healing things of the nature and there are also plants that can poison people and so the scientific approach is necessary.
What kind of form shows effectiveness: whole herbal extracts, ingredients, or combination?
Many of effective phytotherapies are on the drug market as whole extracts, and doctors have believed that synergistic interactions between the components of individual or mixtures of herbs are a vital part of their therapeutic efficacy. Until recently there has been little clinical evidence to demonstrate conclusively that this is the case, and it very often it is argued that the dose of supposed active constituents is too low to exert any therapeutically significant effect at all. In fact the mechanism of action is still unknown and there are several cases of a whole herbal extract showing a better effect than an equivalent dose of an isolated compound. In the past, it was difficult to meet this requirement because of the lack of analytical methods. Many drug preparations at that time were not yet appropriate for controlled clinical studies. Synergistic interactions are of importance, to explain difficulties in always isolating a single active ingredient, and explain the efficacy of apparently low doses of active components in a herbal product.
For example, Ginkgo biloba, has been assessed using an in vitro platelet aggregation test. The ginkgolides are known to be platelet activating factor (PAF) antagonists, which is one of their mechanisms of anti-inflammatory activity. A mixture of ginkgolides A, B and C, at a dose of 100-240 mg, can generate a PAF-antagonizing effect in humans (8). However a dose of 120 mg of a standardized Ginkgo extract containing only 6-7 mg of ginkgolides, together with bilobalide and flavonol glycosides, has an equivalent effect (9). The implications of these results are of course that an isolated ginkgolide would be less effective than a mixture, despite the fact that ginkgolide B is known to be a specific PAF antagonist and has been the subject of many pharmacological experiments.
Therefore, we need to know whether there are interactions between active ingredients in natural product.
Why should urologists know phytotherapy?
Interest in phytotherapy grows in Asian and western countries for the prevention and management of disease, the improvement of general health and antiaging. And also, there are several studies about the efficacy of phytotherapy in urologic disease like benign prostatic hyperplasia (BPH), erectile dysfunction (ED), late-onset hypogonadism (LOH) and infertility in male. There are several reasons why urologists should improve their knowledge about CAM. CAM is increasingly popular and is used by many of their patients. Usually, patients do not reveal this information, unless directly asked. The urologist should be able to advise patients about the benefits and risks of CAM, especially as natural products may eventually interact with prescribed medications, or increase the risk of complications such as bleeding during surgery. Therefore, review of the phytotherapy used in these urologic diseases is necessary.
Phytotherapy in BPH
A large number of different plants are used for the preparation of extracts, such as Hypoxis rooperi (South African star grass), Serenoa repens/Sabal serrulata (saw palmetto berry), Pygeum africanum (African plum), Seceale cereale (rye pollen) and Cucurbita pep (pumpkin seeds) and so on (Table 1). Some of these stem from the roots, seeds, bark or fruits of a single plant only, while others are combination preparations of two or more plants (10). The number of components identified is constantly increasing; the most important ones seem to be phytosterols (beta-sitosterol, campestrol, stigmasterol, delta-5-sterol, delta-7-sterols), phytoestrogens (lignins, flavonoids, isoflavonoids), fatty acids (lauric and myristic acid), lectins, plant oils, polysaccharides, lupenone, lupeol, terpenoids.
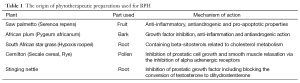
Full table
Proposed mechanism of action
Many biological mechanisms of plant extracts such as 5 alpha-reductase inhibition, aromatase activity, androgen blockade, alpha 1-blockade, inhibition of prostaglandin synthesis, and anti-inflammatory activity among others have been proposed based on in-vitro data. Although these mechanisms have been documented in vitro, there is no convincing evidence that any of these mechanisms acts in vivo. For example, a reduction of prostate volume or serum prostate specific antigen (PSA) has not been shown in any clinical trial.
Saw palmetto
The most popular extract in terms of sales revenue and use in clinical trials is that from the dried berry of the American dwarf palm, S. repens (botanical name Sabal serrulata) or saw palmetto. Numerous mechanisms of action for saw palmetto have been proposed based on the results of in vitro and in vivo animal and human studies. Currently 3 mechanisms of action have been investigated in the laboratory setting, including anti-inflammatory, antiandrogenic and pro-apoptotic properties. There are several studies about the effect of saw palmetto on lower urinary tract symptoms (LUTS) in the patients with BPH. This product has been tested in two large-scale prospective randomized trials against standard therapy. Carraro et al. (11) demonstrated that improvements in the International Prostate Symptom Scores (IPSS) and quality of life were identical in both saw palmetto and finasteride groups. The rise in Qmax was slightly higher in the finasteride group. Reduction of PSA and prostate volume were demonstrable for the finasteride group only. This study has weaknesses due to the short study period and the lack of a placebo arm. Debruyne et al. (12) studied this extract against tamsulosin, and they concluded that both products are equally effective. This study has also been criticized for the lack of a placebo arm. A 12-month, placebo-controlled trial with a saw palmetto product has been reported by Bent et al. (13). Although this was probably a well-designed phytotherapy trial lasting 12 months, changes in the AUA symptom score, Qmax, prostate volume, and postvoid residual volume (PVR) were identical between saw palmetto and placebo. Recently Anceschi et al. (14) studied to evaluate the effect of saw palmetto in reducing intraoperative and postoperative complications of men undergoing surgical treatment for BPH. No intraoperative complications occurred and no blood transfusions were required in the pretreatment group. Additionally, the postoperative course was significantly more favorable in the pretreatment group. This study suggests that pretreatment with saw palmetto before surgery is effective for decreasing potential intraoperative and postoperative complications.
Cernilton
Cernilton, the most popular preparation of S. cereale, contains various amounts of water soluble and acetone soluble pollen extract fractions. It has seen widespread popularity in Japan, Argentina and parts of Western Europe for treating LUTS secondary to BPH (15). Several studies demonstrated various in vitro mechanisms of action for S. cereale, including the inhibition of prostatic cell growth and smooth muscle relaxation via the inhibition of alpha adrenergic receptors (16,17). A more recent study provided in vivo evidence for interference with rat androgen metabolism as the mechanism of action (18). A systematic review of Cernilton included 4 trials, of which 2 were placebo controlled and 2 were comparative (15). The trials were of short duration and they included a total of 444 men. The investigators mentioned that differences in the control agents and methods of reporting results did not permit all studies to be combined in a meta-analysis. Across the board Cernilton appeared to decrease nocturia compared with placebo but it did not improve Qmax, PVR or prostate volume compared with placebo or the comparative study agents. Aoki et al. (19) reported that the patients treated with tamsulosin and cernilton showed greater improvement of LUTS compared with the patients treated with tamsulosin only.
African Plum
An extract from the bark of the African prune tree, Pygeum africanum, has been used in Europe for more than 35 years to treat mild to moderate LUTS (20). Most clinical studies have used a bark extract of P. africanum that has the trade name of Tadenan. P. africanum has a poorly defined mechanism of action. The proposed mechanisms of P. africanum closely resemble those of saw palmetto. Currently there exists evidence of variable quality for the 3 primary mechanisms, including growth factor inhibition, anti-inflammation and antiandrogenic action. P. africanum inhibits basal growth of cultured human prostatic myofibroblasts and fibroblasts (21) as well as the stimulatory effects of growth factors, including epidermal growth factor and basic fibroblast growth factor (22). The strongest data supporting its antiandrogenic activity involve its interaction with dihydrotestosterone (DHT). Pretreatment was shown to significantly decrease the obstructive effects of DHT on micturition and reduce prostate weight (23). In a meta-analysis by Ishani et al. (20), 18 trials in a total of 1,562 men were analyzed. The 4 primary end points in this meta-analysis were physician reported symptom improvement, nocturia, Qmax and PVR. P. africanum decreased nocturia by 19% compared with placebo in a total of 325 patients in 3 studies, although this was not significantly different, and increased Qmax compared with placebo by 23% in a total of 363 men in 4 studies. Additionally, P. africanum decreased PVR by 24% in a total of 264 men in 2 studies. Additionally, experimental studies have shown that P. africanum exerts an antiinflammatory effect, and decreases hypersensitivity of the detrusor muscle of the urinary bladder (24).
Nettle Root
Previous studies have shown that nettle root has antiinflammatory effects, binds to sex hormone-binding globulin, and inhibits cellular proliferation. It also inhibits sodium potassium ATPase action (Na-K ATPase) (25). Lopatkin et al. (26) investigated the safety and efficacy of nettle root over a 96-week period in 219 men with moderate/severe LUTS. Patients were randomized to receive a placebo or a fixed dose of 160 mg Sabal fruit extract combined with 120 mg nettle root extract over 24 weeks, followed by another 24-week control period during which all patients received medication. IPSS, urinary flow rates, and residual urine volumes improved significantly in the treatment group, thus providing evidence of a clinically relevant benefit over a period of 96 weeks.
Phytotherapy in ED
The placebo effect of all medical treatments is well known and also demonstrable in the treatment of ED; placebo-controlled trials with newer oral chemical drugs showed placebo responses of 25-41%. A comparable placebo effect was shown in trials of LUTS and BPH. Thus, small studies with supplements of androstenedione, dehydroepiandrosterone (DHEA), zinc supplements, as well as other herbal preparations, have provided no evidence of superiority over placebo (10). Whether any of these dietary supplements have merit is questionable at this time, but still numerous supplements seem to be promoted in alternative medicine (Table 2). The dropout rates for the use of current therapies are high and the overall satisfaction is below expectation. Many of these patients resort to CAM, which is often phytotherapy. In this respect, knowledge of the various herbal and traditional medicines, which has been well documented for thousands of years, is important to the urologists. Ginkgo biloba, Korean red ginseng, Horny goat weed, L-arginine and Schizandra chinensis are some of more popular dietary supplements for ED.
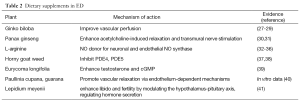
Full table
Ginko biloba
Ginkgo biloba for the treatment of ED has attracted interest in alternative medicine circles for several reasons including its apparent ability to improve vascular perfusion. Although ginkgo has been used frequently to improve cerebrovascular perfusion in patients with dementia, its central effects have also been postulated to improve antidepressant-induced ED. One study treated 60 patients who did not respond to papaverine injections with an extract of Ginkgo biloba for 12 to 18 months. There was improvement of blood perfusion in some men after 6 to 8 weeks (27). And 50% of the patients regained erectile function after 6 months. Sikora et al. (28) presented the results of a placebo-controlled, double-blind, randomized, follow-up study using an extract of ginkgo, 240 mg/d for 24 weeks on treatment of vasculogenic ED. No significant difference was found between the two groups, which highlights the continuing need for high-quality randomized studies before recommendations can be made. Other recent ginkgo studies using a placebo for other non-ED conditions have failed to observe significant benefits (29). Thus, any data proposing ginkgo supplements for sexual function or ED improvement are suspect until larger randomized trials are completed.
Korean red ginseng (Panax ginseng)
Korean red ginseng is one of the most commonly used ginseng products in the United States; many brands are marketed under the name Panax (42). Among the compounds found in root extracts of ginseng are steroids, peptides, and triterpenoidal Dglucosides (specifically panaxsapogenin) (43). Active ingredients of ginseng are tetracyclic triterpenoid saponins (ginsenosides) (44). Korean red ginseng has been investigated to reduce severe climacteric symptoms or improve mood in postmenopausal women with limited positive results compared with placebo (45,46). It also may improve vascular endothelial abnormalities in hypertensive patients by increasing the concentration of NO (47). A laboratory investigation of Korean red ginseng on rabbit corpus cavernosal smooth muscle found that it can cause a dose-dependent relaxation by increasing the release of NO from corporal sinusoids and may increase intracellular sequestration of calcium (30). From the result of a double-blind, placebo-controlled, preliminary trial of Korean red ginseng in patients with ED without previous treatment, mean IIEF scores were significantly higher in the ginseng-treated patients compared with placebo (31). When analyzed individually, scores for erectile function, sexual desire, and intercourse satisfaction were improved significantly with ginseng. Approximately 60% of treated patients experienced an improvement in erection compared with 20% with placebo. The authors commented that the mechanism of action for ginseng probably was not related to testosterone levels, which did not change significantly during the study, although serum testosterone normalized after ginseng in four of the seven patients with a decreased baseline level. More research is needed to resolve this issue.
Horny goat weed (Epimedium Herbs)
There are about 50 Epimedium (Berberidaceae) species distributed in Asia, Europe, and the Middle and Far East. Epimedium herbs have been used to treat infertility and impotence for over 2,000 years (48). Icariin, an amorphous yellow powder with a melting point between 231 and 233 °C, has been isolated from the aerial parts of Epimedium herbs. It is able to inhibit phosphodiesterase-5 (PDE5) and PDE4 (37). It also inhibits all 3 PDE5 isoforms (A1, A2, and A3) and enhances cGMP levels in sodium nitroprusside-treated corpus cavernosum muscles in rats. Effects of Icariin on PDE5 activity in vitro and cGMP level in cavernous smooth muscle cells have been well elucidated (38). It also potentiates PDE5 inhibitor action in the relaxation of phenylephrine-precontracted strips of rabbits. E. brevicornum maxim extract relaxes rabbit corpus cavernosum through multitargets on NO/cGMP signalling pathway (49).
L-arginine
Nitric oxide plays a major role in mediating an erection through vasorelaxation by way of increased levels of cyclic guanosine monophosphate and decreased calcium levels in vascular smooth muscle cells (32). The amino acid L-arginine acts as an NO donor for neuronal and endothelial NO synthase (33) and has been shown to increase NO production when consumed in supraphysiologic doses of more than 3 g per day. A placebo-controlled clinical trial that used L-arginine, 2,800 mg/d, for 2 weeks, found that 40% of patients had improvement in their erections (34). Responders were younger and had better overall vascular function by hemodynamic investigation than the nonresponders. However, low dosage of L-arginine (1,500 mg/d) did not demonstrate a benefit compared with placebo for men with mixed ED (35). In another trial involving high dosage of L-arginine (5 g/d) for men with organic ED that was mostly the result of diabetic or arteriogenic etiologies, approximately 31% of the men in the L-arginine group showed self-reported improvement compared with 12% in the placebo group (36).
Schizandra chinensis
Schisandra chinensis is a deciduous woody vine native to the forests of Korea, China and Russia. It has five tastes, sweet, sour, bittier, astringent, and salty. According to the types of taste, sweet may have a good effect on stomach, sour and salty on liver and testis, bitter and astringent on heart and lung. Its berries have been used in oriental herbal medicine for ED, enuresis, frequency, diarrhoea, spontaneous sweating, night sweating, cough, asthma, sputum, wheezing, jaundice, and diabetes (50). Sohn et al. (51) created a new herbal formulation that mainly consists of the seeds of Schizandra chinensis and investigated the effects of this herbal formulation on the penile erection and corpus cavernosum of spontaneous hypertensive male rats. The herbal formulation was made with 5 natural substances, including Schizandra chinensis, Cornus officinalis, Lycium chinense, Rubus coreanus and Cuscuta chinensis. This study showed that the herbal formulation enhances intracavernous pressure and NO-cGMP activity in penile tissues of spontaneous hypertensive male rats. Pharmacological studies have shown that Schisandra chinensis extract has a dose-dependent relaxation effect on vascular smooth muscle and that vascular relaxation is mediated by an endothelium-dependent NO pathway and a direct effect on vascular smooth muscle cells via dephosphorylation of the myosin light chain (52). Han et al. (53) evaluated the relaxant effects of Schisandra chinensis extract on corporal tissue in the penis and the mechanism of action and its constituents on corporal smooth muscle cells. It induced relaxation of corporal smooth muscle that occurred primarily via an endothelium-independent pathway. Also, the relaxation effects on corporal smooth muscle were, in part, due to the activation of potassium channels and inhibition of TRPC6 channels.
Phytotherapy in LOH
According to the recommendation of the International Society for the Study of the Aging Male (ISSAM), LOH is defined as a clinical and biochemical syndrome associated with advancing age characterized by typical symptoms and deficiency in serum testosterone levels (54). These patients usually show decreased libido, erectile dysfunction, obesity and other bothersome symptoms related with low testosterone level. Therefore, the basic essential treatment for LOH involves testosterone replacement therapy (TRT) (55). However, TRT should be applied only after clarification of serum testosterone levels. Some patients who visit outpatient clinics want relief from symptoms as quickly as possible. In addition, other patients cannot undergo TRT due to high PSA levels, liver dysfunction, etc. In such cases, palliative treatment to relieve these symptoms should be considered. Moreover, as interest in alternative medicine for anti-aging have been growing, studies on the preventive effect of natural products on anti-aging have also increased. Therefore, several natural products which might have effects on aging have been introduced.
Tribulus terrestris
Tribulus terrestris is a plant that grows in many countries around the world. It contains many unique compounds that have steroid-like or steroid saponin activity (56-58). A compound called protodioscin can be extracted from some of these plants under appropriate conditions and apparently transformed into DHEA (59). Some investigations have found this compound potentially improves erectile function in vitro (60,61). It has failed in initial studies to change body composition, enhance exercise, or impact testosterone levels in young men, but increases levels of androstenedione or estradiol when combined with other DHEA-like products (62). Gauthaman et al. (63) showed that Tribulus terrestris extract is able to increase androgen but is not as effective as testosterone. Oral treatment, which contains 45% protodioscin, increased the prostate weight of castrated male rats by 29.85%, while testosterone increased the prostate weight of castrated male rats by 43.28% compared to nontreated castrated male rats. Results similar to those observed with androstendione/DHEA could be expected, but adequate clinical trials addressing its individual impact or other unique effects on hormonal levels in hypogonadal men or those with ED have not been published.
Kampo
A Japanese traditional herbal medicine, Kampo, which originated from traditional Chinese herbal medicine, developed during the 17-19th centuries in Japan. Female climacteric disorders improved significantly following treatment with Kampo in many cases (64). Kampo is a Japanese traditional herbal medicine and has been widely applied for female climacteric disorders. Amano et al. (65) demonstrated that nearly 70% of patients with LOH experienced relief from various symptoms after 4 weeks of Japanese traditional herbal medicine administration; moreover, no serious adverse reactions were evident. However, data about the androgen level after Kamp administration is lacking. Therefore, further study is necessary to support the effect of Kampo on LOH.
Phytotherapy in infertility
The lack of available specific therapies for men with infertility demands the exploration of alternative therapies. Given the lack of knowledge about etiologic factors, sometimes general therapy may yield good results in a subcategory of patients. The rationale for the use of these therapies is based on the supposition that some forms of male infertility are caused by oxidative insult and hormonal imbalance, and the use of alternative therapies may improve male fertility potential and semen quality (66). Aerobic metabolism of human sperm produces different reactive oxygen species (ROS), which are essential for sperm capacitation, acrosome reaction, and oocyte fusion (67). To counteract the toxic effects of ROS, seminal plasma and spermatozoa act as an array of antioxidant mechanisms.
Withania somnifera
Withania somnifera, also known as Indian ginseng, has been described in folk medicine as an aphrodisiac and geriatric tonic. Different investigators have reported that W. somnifera possesses antiserotogenic, anticancer, and anabolic activity and is beneficial in the treatment of arthritis, geriatric problems, stress, and male sexual dysfunction. W. somnifera has been shown to inhibit lipid peroxidation in stress-induced animals (68) and induced testicular development and spermatogenesis in immature Wistar rats by directly affecting the seminiferous tubules (69). Histological examination showed that the testes of immature male rats treated with W. somnifera leaf aqueous extract contained closely packed and larger seminiferous tubules compared to control. Apparent spermatogenesis was observed in some of the tubules. Dense spermatid nuclei and sperm heads, which indicate spermiogenesis, can be easily seen in the seminiferous epithelia. Testicular weight was found to be increased in treated rats. However, serum testosterone and follicle-stimulating hormone levels were decreased compared to control while the luteinizing hormone level remained unchanged. Shukla et al. (70) demonstrated that treatment with W. somnifera significantly reduced apoptosis and ROS concentrations and improved metal ion concentrations in infertile men. It was concluded that W. somnifera improved semen quality by reducing oxidative stress and cell death and improving essential metal ion concentrations.
Escin
Recently, escin, the main ingredient of which is extract of Aesculus hippocastanum seed (71,72) has been shown to be effective in treating chronic venous malfunction, such as hemorrhoids, deep venous varicocele of lower extremities, valvular insufficiency and postoperative edema (73). Fang Y et al. (74) evaluated the efficacy of escin to improve sperm quality in Chinese male patients with varicocele-associated infertility. In response to treatment, the improvement rates in sperm density in the control, the surgery and the escin group, were 38.5%, 68.8%, and 57.5%, respectively. This study demonstrated that escin is very effective in improving sperm quality. Only very low frequencies of mild adverse effects were observed during treatment, most of which resolved without further symptomatic drug therapy.
Anthocyanin
Anthocyanin is a water-soluble natural pigment that appears as red, purple, and blue in plants and belongs to the flavonoid parent class of molecules. It has been shown to mediate antioxidant reactions by stabilizing or inactivating free radicals and preventing cellular oxidative stress (75,76).
Varicocele is detected in approximately 15% of young males. In adults, it is asymptomatic in most cases. Nonetheless, it has been reported to account for 35% of primary and 81% of secondary male infertility (77), and is the most common cause of infertility. Infertility has been associated with reactive oxygen species (ROS). Aitken et al. (78) reported that ROS levels were high in infertile patients, particularly those with varicocele, in whom the production of oxygen free radicals was elevated to such an extent that it was associated with the development of infertility. Jang et al. (79) evaluated the mechanism of development of male infertility caused by varicocele and assessed the possible therapeutic effects of anthocyanin. They suggest that the antioxidant effect of anthocyanin prevented the damage caused by varicocele induced reactive oxygen species. Thus, anthocyanin may be effective in producing healthy sperm in patients with varicocele.
Conclusions
Phytotherapy may have an effect on urologic disease such as BPH, ED, LOH and male infertility. Patients with certain types of urologic disease may benefit from phytotherapy. However, there are several potential risks for people using phytotherapies. One is that appropriate diagnoses may be missed in some patients preventing the treatment of patients with established, evidence-based conventional standard therapy, and another is that patients often do not reveal to their doctor that they are using phytotherapy, and may thus risk complications from drug interaction or toxicity. Urologists have to inform their patients that the belief that all natural products are safe and without toxicity is incorrect. Therefore, more evidence-based studies that prove the action of mechanism of these therapies are essential in the clinical application of phytotherapy.
Acknowledgements
Funding: This research was supported by High Value-added Food Technology Development Program (111016-3), Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Eisenberg DM, Davis RB, Ettner SL, et al. Trends in alternative medicine use in the United States, 1990–1997: results of a follow up national survey. JAMA 1998;280:1569-75. [PubMed]
- Elder NC, Gillcrist A, Minz R. Use of alternative health care by family practice patients. Arch Fam Med 1997;6:181-4. [PubMed]
- Gulla J, Singer AJ, Gaspari R. Herbal use in emergency department patients. Ann Emerg Med 2000;35:226-8. [PubMed]
- Fairfield KM, Eisenberg DM, Davis RB, et al. Patterns of use, expenditure, and perceived efficacy of complimentary and alternative therapies in HIV-infected patients. Arch Intern Med 1998;158:2257-64. [PubMed]
- Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States. The Slone survey. JAMA 2002;287:337-44. [PubMed]
- Jordan SA, Cunningham DG, Marles RJ. Assessment of herbal medicinal products: challenges, and opportunities to increase the knowledge base for safety assessment. Toxicol Appl Pharmacol 2010;243:198-216. [PubMed]
- Kalia, AN. Text Book of Industrial Pharmacognosy. London: Oscar publication. 2005.
- Chung KF, McCusker M, Page P, et al. Effect of a ginkgolide mixture (BN 52063) in antagonising skin and platelet responses to platelet activating factor in man. Lancet 1987;1:248-51. [PubMed]
- Wagner H. New targets in the Phytopharmacology of Plants. In: Herbal medicine, a concise overview for healthcare professionals. Butterworth-Heinemann 1999:34-42.
- Dreikorn K. Complementary and alternative medicine in urology. BJU Int 2005;96:1177-84. [PubMed]
- Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (PermixonR) with finasteride in the treatment of benign prostate hyperplasia: a randomised international study of 1,098 patients. Prostate 1996;29:231-40. [PubMed]
- Debruyne F, Koch G, Boyle P, et al. Comparison of a phytotherapeutic agent (Permixon) with an a-blocker (Tamsulosin) in the treatment of benign prostatic hyperplasia: a 1-year randomised international study. Eur Urol 2002;41:497-506. [PubMed]
- Bent S, Kane C, Shinohara K, et al. Saw palmetto for benign prostatic hyperplasia. N Engl J Med 2006;354:557-66. [PubMed]
- Anceschi R, Bisi M, Ghidini N, et al. Serenoa repens (Permixon) reduces intra- and postoperative complications of surgical treatments of benign prostatic hyperplasia. Minerva Urol Nefrol 2010;62:219-23. [PubMed]
- MacDonald R, Ishani A, Rutks I, et al. A systematic review of Cernilton for the treatment of benign prostatic hyperplasia. BJU Int 2000;85:836-41. [PubMed]
- Habib FK, Ross M, Buck AC, et al. In vitro evaluation of the pollen extract, cernitin T-60, in the regulation of prostate cell growth. Br J Urol 1990;66:393. [PubMed]
- Kimura M, Kimura I, Nakase K, et al. Micturition activity of pollen extract: contractile effects on bladder and inhibitory effects on urethral smooth muscle of mouse and pig. Planta Med 1986;52:148-51. [PubMed]
- Talpur N, Echard B, Bagchi D, et al. Comparison of Saw Palmetto (extract and whole berry) and Cernitin on prostate growth in rats. Mol Cell Biochem 2003;250:21-6. [PubMed]
- Aoki A, Naito K, Hashimoto O, et al. Clinical evaluation of the effect of tamsulosin hydrochloride and cernitin pollen extract on urinary disturbance associated with benign prostatic hyperplasia in a multicentered study. Hinyokika Kiyo 2002;48:259-67. [PubMed]
- Ishani A, MacDonald R, Nelson D, et al. Pygeum africanum for the treatment of patients with benign prostatic hyperplasia: a systematic review and quantitative meta-analysis. Am J Med 2000;109:654-64. [PubMed]
- Boulbès D, Soustelle L, Costa P, et al. Pygeum africanum extract inhibits proliferation of human cultured prostatic fibroblasts and myofibroblasts. BJU Int 2006;98:1106-13. [PubMed]
- Lawson RK. Role of growth factors in benign prostatic hyperplasia. Eur Urol 1997;32:22-7. [PubMed]
- Choo MS, Bellamy F, Constantinou CE. Functional evaluation of Tadenan on micturition and experimental pros tate growth induced with exogenous dihydrotestosterone. Urology 2000;55:292-8. [PubMed]
- Dedhia RC, McVary KT. Phytotherapy for lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2008;179:2119-25. [PubMed]
- Lopatkin N, Sivkov A, Walther C, et al. Long-term efficacy and safety of a combination of sabal and Urtica extract for lower urinary tract symptoms- a placebo-controlled, double-blind, multicenter trial. World J Urol 2005;23:139-46. [PubMed]
- Lopatkin N, Sivkov A, Schläfke S, et al. Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms--long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int Urol Nephrol 2007;39:1137-46. [PubMed]
- Sikora R, Sohn MH, Deutz F-J, et al. Ginkgo biloba extract in the therapy of erectile dysfunction. J Urol 1989;141:188A. (Abstract 73).
- Sikora R, Sohn MH, Engelke B, et al. Randomized placebo-controlled study on the effects of oral treatment with Ginkgo biloba extract in patients with erectile dysfunction. J Urol 1998;159:240A. (Abstract 917).
- Solomon PR, Adams F, Silver A, et al. Ginkgo for memory enhancement: a randomized controlled trial. JAMA 2002;288:835-40. [PubMed]
- Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res 1998;10:37-43. [PubMed]
- Hong B, Ji YH, Hong JH, et al. A doubleblind crossover study evaluating the efficacy of Korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol 2002;168:2070-3. [PubMed]
- Lue TF, Lee KL. Pharmacotherapy for erectile dysfunction. Chin Med J (Engl) 2000;113:291-8. [PubMed]
- Toda N, Ayajiki K, Okamura T. Nitric oxide and penile erectile function. Pharmacol Ther 2005;106:233-66. [PubMed]
- Zorgniotti AW, Lizza AF. Effect of large doses of nitric oxide precursor L-arginine, on erectile dysfunction. Int J Impot Res 1994;6:33-5. [PubMed]
- Klotz T, Mathers MJ, Braun M, et al. Effectiveness of oral L-arginine in the first-line treatment of erectile dysfunction in a controlled crossover study. Urol Int 1999;63:220-3. [PubMed]
- Chen J, Wollman Y, Chernichovsky T, et al. Effect of oral administration of high-dose nitric oxide donor L-arginine in men with organic erectile dysfunction: results of a double-blind, randomized, placebo-controlled study. BJU Int 1999;83:269-73. [PubMed]
- Xin ZC, Kim EK, Lin CS, et al. Effects of icariin on cGMPspecific PDE5 and cAMP-specific PDE4 activities. Asian J Androl 2003;5:15-8. [PubMed]
- Ning H, Xin ZC, Lin G, et al. Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells. Urology 2006;68:1350-4. [PubMed]
- Chye PH. Traditional Asian folklore medicines in sexual health. Indian J Urol 2006;22:241-5.
- Zamble A, Carpentier M, Kandoussi A, et al. Paullinia pinnata extracts rich in polyphenols promote vascular relaxation via endothelium-dependent mechanisms. J Cardiovasc Pharmacol 2006;47:599-608. [PubMed]
- Bogani P, Simonini F, Iriti M, et al. Lepidium meyenii (Maca) does not exert direct androgenic activities. J Ethnopharmacol 2006;104:415-7. [PubMed]
- Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-344. [PubMed]
- Choudhary I, Ur-Rahman A. Elixirs of love. Chem Br 1997.25-27.
- Samuelsson G. Drugs of natural origin: a textbook of pharmacognosy. 4th ed. Stockholm: Swedish Pharmaceutical 1999;171.
- Tode T, Kikuchi Y, Hirata J, et al. Effect of Korean red ginseng on psychological functions in patients with severe climacteric syndromes. Int J Gynaecol Obstet 1999;67:169-74. [PubMed]
- Wiklund IK, Mattson LA, Lindgren R, et al. Effects of a standardized ginseng extract on quality of life and physiological parameters in symptomatic postmenopausal women women: a double-blind, placebo-controlled trial. Swedish Alternative Medicine Group. Int J Clin Pharmacol Res 1999;19:89-99. [PubMed]
- Sung J, Han KH, Zo JH, et al. Effects of red ginseng upon vascular endothelial function in patients with essential hypertension. Am J Chin Med 2000;28:205-16. [PubMed]
- Zheng HZ, Dong ZH, She Q. Modern research and application of traditional Chinese medicine. Beijing: Xueyuan, 1998: 4225-54.
- Chiu JH, Chen KK, Chien TM, et al. Epimedium brevicornum Maxim extract relaxes rabbit corpus cavernosum through multitargets on nitric oxide/cyclic guanosine monophosphate signaling pathway. Int J Impot Res 2006;18:335-42. [PubMed]
- Panossian A, Wikman G. Pharmacology of Schisandra chinensis Bail: an overview of Russian research and uses in medicine. J Ethnopharmacol 2008;118:183-212. [PubMed]
- Sohn DW, Kim HY, Kim SD, et al. Elevation of intracavernous pressure and NO-cGMP activity by a new herbal formula in penile tissues of spontaneous hypertensive male rats. J Ethnopharmacol 2008;120:176-80. [PubMed]
- Park JY, Shin HK, Lee YJ, et al. The mechanism of vasorelaxation induced by Schisandra chinensis extract in rat thoracic aorta. J Ethnopharmacol 2009;121:69-73. [PubMed]
- Han DH, Lee JH, Kim H, et al. Effects of Schisandra chinensis extract on the contractility of corpus cavernosal smooth muscle (CSM) and Ca2+ homeostasis in CSM cells. BJU Int 2012;109:1404-13. [PubMed]
- Lunenfeld B, Saad F, Hoesl CE. ISA, ISSAM and EAU recommendations for the investigation, treatment and monitoring of late-onset hypogonadism in males: scientific background and rationale. Aging Male 2005;8:59-74. [PubMed]
- Comhaire FH. Andropause: hormone replacement therapy in the aging male. Eur Urol 2000;38:655-62. [PubMed]
- Yan W, Ohtani K, Kasai R, et al. Steroidal saponins from fruits of Tribulus terrestris. Phytochemistry 1996;42:1417-22. [PubMed]
- Bedir E, Khan IA. New steroidal glycosides from the fruits of Tribulus terrestris. J Nat Prod 2000;63:1699-701. [PubMed]
- Cai L, Wu Y, Zhang J, et al. Steroidal saponins from Tribulus terrestris. Planta Med 2001;67:196-8. [PubMed]
- Adimoelja A. Phytochemicals and the breakthrough of traditional herbs in the management of sexual dysfunctions. Int J Androl 2000;23:82-4. [PubMed]
- Arcasoy HB, Erenmemisoglu A, Tekol Y, et al. Effect of Tribulus terrestris L. saponin mixture on some smooth muscle preparations: a preliminary study. Boll Chim Farm 1998;137:473-5. [PubMed]
- Adaikan PG, Gauthaman K, Prasad RN, et al. Proerectile pharmacological effects of Tribulus terrestris extract on the rabbit corpus cavernosum. Ann Acad Med Singapore 2000;29:22-6. [PubMed]
- Antonio J, Uelmen J, Rodriguez R, et al. The effects of Tribulus terrestris on body composition and exercise performance in resistance-trained males. Int J Sport Nutr Exerc Metab 2000;10:208-15. [PubMed]
- Gauthaman K, Adaikan PG, Prasad RNV. Aphrodisiac properties of Tribulus Terrestris extract (Protodioscin) in normal and castrated rats. Life Sci 2002;71:1385-96. [PubMed]
- Koyama T. Role of Kampo (herbal) medicine for management in postmenopausal women in Japan. J Jpn Menopause Soc 1993;1:75-9.
- Amano T, Imao T, Takemae K. Clinical efficacy of Japanese traditional herbal medicine (Kampo) in patients with late-onset hypogonadism. Aging Male 2010;13:166-73. [PubMed]
- Devi PR, Laxmi V, Charulata C, et al. ‘‘Alternative medicine’’- a right choice for male infertility management. Int Cong Series 2004;1271:67-70.
- Storey BT. Biochemistry of the induction and prevention of lipoperoxidative damage in human spermatozoa. Mol Hum Reprod 1997;3:203-13. [PubMed]
- Dhuley JN. Effect of ashwagandha on lipid peroxidation in stressinduced animals. J Ethnopharmacol 1998;60:173-8. [PubMed]
- Abdel-Magied EM, Abdel-Rehman HA, Harraz FM. The effect of aqueous extracts of Cynomorium coccineum and Withania somnifera on testicular development in immature Wistar rats. J Ethnopharmacol 2001;75:1-4. [PubMed]
- Shukla KK, Mahdi AA, Mishra V, et al. Withania somnifera improves semen quality by combating oxidative stress and cell death and improving essential metal concentrations. Reprod Biomed Online 2011;22:421-7. [PubMed]
- Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res 2001;44:183-93. [PubMed]
- Carrasco OF, Vidrio H. Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta. Vascul Pharmacol 2007;47:68-73. [PubMed]
- Kamischke A, Nieschlag E. Varicocele treatment in the light of evidence-based andrology. Hum Reprod Update 2001;7:65-9. [PubMed]
- Fang Y, Zhao L, Yan F, et al. Escin improves sperm quality in male patients with varicocele-associated infertility. Phytomedicine 2010;17:192-6. [PubMed]
- Francis FJ. Food colorants: anthocyanins. Crit Rev Food Sci Nutr 1989;28:273-314. [PubMed]
- Harborne JB, Wiliam CA. Advances in flavonoid research since 1992. Phytochemistry 2000;55:481-504. [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [PubMed]
- Aitken RJ, Buckingham D, West K, et al. Differential contribution of leucocytes and spermatozoa to the generation of reactive oxygen species in the ejaculates of oligozoospermic patients and fertile donors. J Reprod Fertil 1992;94:451-62. [PubMed]
- Jang H, Kim SJ, Yuk SM, et al. Effects of anthocyanin extracted from black soybean seed coat on spermatogenesis in a rat varicocele-induced model. Reprod Fertil Dev 2012;24:649-55. [PubMed]


