Upper urinary tract disease: what we know today and unmet needs
Introduction
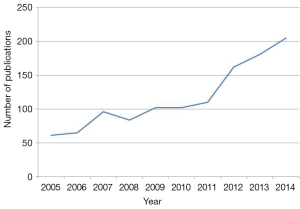
Upper tract urothelial carcinoma (UTUC) accounts for 5% of all urothelial carcinomas with an estimated annual incidence of 1 to 2 cases per 100,000 inhabitants (1). Sometimes considered the twin of urothelial carcinoma of the bladder (UCB) (2), UTUC is now considered a distinct entity by SIU and EAU guidelines (3). The annual number of UTUC-related publications has almost tripled over the last 10 years (Figure 1). First symposia and working groups such as the Upper Tract Urothelial Carcinoma Collaboration, French Collaborative National Working-Group on UTUC, and Canadian Upper Tract Collaboration reflect growing interest for UTUC.
Basic research and collaborative efforts have contributed to improve our knowledge on the natural history of UTUC. Improvements in technologies and extrapolation from UCB management, have greatly contributed to progress in UTUC management. The low incidence of the disease is, however, a limit for studies with high level of evidence. Prediction models have been developed to help physicians with evidence based personalized clinical decision making (4).
The objective of this review was to provide insights in current advances in UTUC tumorigenesis, risk stratification and treatment, and to highlight unmet needs of UTUC as we know it today.
Methods and evidence acquisition
A non-systematic Medline/PubMed literature search was performed using a combination of the terms “upper tract urothelial carcinoma” with different keywords. To select relevant articles, reviews and editorials from English literature, the following keywords were used for the needs of the different sections of the manuscript: (I) “epidemiology”, “risk factors”, and “biology”; (II) “staging” and “risk stratification”; (III) “conservative treatment”, “nephro-ureterectomy” and “lymphadenectomy”; (IV) “neo-adjuvant treatment” and “adjuvant treatment”. Time period included articles published between January 2000 and January 2015. Additional informative articles were collected by cross referencing the bibliography of previously selected articles.
Evidence synthesis
Soil and seed
Specific risk factors
UTUC has long been considered the twin tumor of UCB. Therefore, past and current practices are mainly derived from UCB management. However, several epidemiologic and basic research studies have clearly demonstrated UTUC presents specific anatomical, biological, clinical and pathological features (2). These tumors are less frequent than UCB, more invasive at diagnosis, and have a male to female ratio of 2:1 (2).
UCB and UTUC share common risk factors like smoking and exposure to aromatic amines. Specific risk factors have been identified in UTUC. It has been recently shown that UTUC is associated with Balkan endemic nephropathy, a disease linked to aristolochic acid (AA) exposure leading to DNA-adducts with specific genetic signatures such as a TP53 A to T transversion (5-7). Epidemiologic studies estimated that one third of the Taiwanese population has been exposed to AA, as it is an integral part of Chinese herbal medicine (8). This exposure also exists in China, other parts of Asia, as well as in ayurvedic medicine in India. Genetic features could also represent specific risk factors to develop UTUC even with a relatively low environmental risk exposure to known carcinogens.
Some UTUC have a hereditary predilection belonging to the hereditary nonpolyposis colorectal carcinoma (HNPCC) tumors spectrum (9). Alterations of mismatch repair genes, responsible for HNPCC, could also be involved in sporadic UTUC as a potential initiating event (10,11). It has been recommended to test all patients with UTUC who are less than 60 years old, have a personal history of an HNPCC-associated cancer, a first-degree relative <50 years of age with HNPCC-associated cancer, or two first-degree relatives with HNPCC-associated cancer, to identify hereditary cancers that have been misclassified as sporadic cancers (3). Studies on genetic variations in the population have also identified specific genetic polymorphisms associated with a higher risk of UTUC (12,13). Sasaki et al. reported DNA repair gene polymorphisms could have a prognostic value since more than two variant alleles in these genes was associated with a significant better overall survival (OS) and cancer specific survival (CSS) after radical nephroureterectomy (RNU) (14). Such genetic risk markers of UTUC could help identify patients who have an increased risk of developing UTUC but also those who are more likely to harbor biologically aggressive disease.
Molecular biomarkers
Several tissue, blood, genetic or urine based biomarkers, such as microsatellite instability (MSI) of the tumor, p53, E-cadherin, HIF1alpha, and ki-67 have been proposed to help in the prognostication of UTUC (15). Krabbe et al. reported in 475 patients treated with RNU that Ki-67 was an independent prognosticator of recurrence free survival (RFS) and CSS for high grade tumors (16). Bagrodia et al. similarly demonstrated that both PI3K and cyclin D, two mTOR biomarkers, were associated with adverse pathologic results and worse oncological outcomes in a cohort of 620 patients who underwent RNU or partial ureterectomy (17).
To date, none of these potential biomarkers have been integrated to clinical practice or predictive models. While there are many challenges to the stepwise assessment of new biomarkers before integration into clinical care (18), in UTUC, biomarkers are mainly needed to help risk stratify the disease in order to identify patients who may benefit from kidney-sparing management, neoadjuvant chemotherapy (NC), or extended lymphadenectomy. These initial studies were done on RNU specimens, so they help understand the biological potential of these biomarkers post-operatively but not in the pre-RNU setting. After RNU, adjuvant chemotherapy (AC) is not established for the reasons we discussed below. The validation of these new molecular and genetic characteristics may help physicians better appraise patient and tumor identities to guide clinical decisions and design a personalized approach to some cases. Still, biomarkers are urgently needed in the pre-RNU setting. Biomarkers that can be evaluated in small tissue samples obtained by endoscopic biopsy may help overcome the shortcomings of current staging in UTUC through refined biomarker-guided risk stratification.
“Plant anatomy and morphology”
Imaging and biopsy
Imaging and ureteroscopic biopsy now play a critical role to define stage and grade of UTUC, which are the most accurate independent factors of outcome (15). However, despite technological advances, current modalities yield limited samples that preclude optimal staging and grading. Multi-detector computed tomography urography (MDCTU) with images during excretory phase (10-15 min) is the standard technique used for staging today (3). Its accuracy to stage the tumor ranges from 59% to 88% (19,20). Assessment of nodal involvement by MDCTU is even less accurate since only 60% of the patients with positive lymph nodes (LNs) at LN dissection (LND) are considered N+ on preoperative imaging (21). Nevertheless, if invasion is seen on MDCTU, it indicates at least muscle invasive disease (22). In addition, hydronephrosis has also been associated with invasive disease which may not benefit from kidney-sparing management (23).
Flexible ureteroscopy has revolutionized preoperative evaluation of UTUC allowing to visualize all upper urinary tract and to perform tumor biopsy. There are anatomical and instrumental limitations to sample the tumor adequately (24). Even when the biopsy can be properly analyzed, the accuracy of biopsy to define T stage is limited. Smith et al. reported a stage discrepancy between final RNU pathology and endoscopic biopsy in 38% of the cases (25). Biopsy is more efficient regarding grading assessment with an accuracy ranging from 69% to 91% when compared with RNU pathology (26). Biopsy grading can enhance T staging evaluation: 68-100% of grade 1 biopsies are associated with ≤ pT1 tumors while 62-100% of biopsies with grade 3 correctly predict muscle invasive stage (≥ pT2) (26).
To improve T staging by imaging and compensate the paucity of current pathological data from biopsy, new modalities of acquisition and evaluation have been developed. Matin et al. tested a promising endoluminal ultrasound (US) (27). In this pilot study, seven patients with UTUC underwent RNU after endoluminal US evaluation to stage the tumor. PPV and NPV for invasive disease status were 66.7% and 100%, respectively. Other technologies such as optical coherence tomography and confocal laser endomicroscopy are under evaluation (28,29). Preliminary reports suggest multiparametric MRI, especially ADC, could also be useful tools for staging and grading the tumor (30,31). Sassa et al. evaluated 11C-choline positron emission tomography-computed tomography (PET/CT) for primary diagnosis and staging of UTUC and demonstrated encouraging results, especially regarding nodal evaluation. In this study, among 12 patients with UTUC on final pathology and pre-operative PET/CT, 11 had choline tumor uptake on pre-operative PET/CT. LN or distant metastases were diagnosed in five patients on pre-operative PET/CT and all metastatic sites displayed choline uptake (32).
To improve the quality of biopsies, new instrumental methods have been tested and showed that tumor removal using baskets could better determinate tumor grade in some cases (33,34). New technologies such as narrow band imaging (NBI) and high definition digital ureteroscopy can also help better characterize tumor characteristics (35).
Predictive models
To overcome current limited accuracy of imaging and biopsy sampling and to combine all available data to improve outcome prediction, multi-institutional clinical research groups have developed preoperative predictive models to guide clinical decision-making (36). Favaretto et al. proposed a model based on the combination of data from imaging (local invasion and hydronephrosis) and ureteroscopy (tumor location and high grade at biopsy) (22). Margulis et al. combined grade, architecture and tumor location (37). These models were able to predict non organ confined disease with an accuracy of 70% and 77%, respectively. Brien et al. proposed a simple model based on the presence of hydronephrosis, high grade at biopsy and positive cytology. The positivity or negativity of all three features was able to predict the muscle invasion with 89% PPV and 100% NPV (38).
To date, guidelines propose a risk stratification on low risk and high risk tumors based on pre-operative parameters to guide therapeutic management of patients with UTUC (3,39). This decision making definition relies on relatively small studies and experts’ opinion. These predictive models represent evidence based data that may be integrated in treatment decision algorithms. However, no external validation of these models has been published yet. Therefore, large and multicenter external validation of these models are the first step before considering their use in the management of UTUC.
“The best harvest”
Kidney sparing approach using flexible ureteroscopy
Kidney sparing management of UTUC was historically limited to imperative indications (renal insufficiency or solitary functional kidney). The previously described concept of “low risk” tumors, the high percentage of pTa tumors at time of RNU, and the development of flexible ureterorenoscopy and novel instrumentations lead to a shift of the indications to elective cases (when the contralateral kidney is functional) (40). The tumor has to be resectable and with a low risk of recurrence and progression, and the patient has to understand that a close follow up is necessary (3). This can be achieved with flexible/semi-rigid ureteroscopy today. Open and percutaneous resection of tumors of the renal pelvis or calices have almost disappeared (3). Distal ureteral segmentectomy remains, however, an option for tumors of the distal ureter or in case of ureteroscopic failure (41).
Recently, using the Surveillance, Epidemiology, and End Results (SEER) database, Simhan et al. reported similar CSS with RNU and kidney sparing procedure (KSP), including ureteral segmentectomy and endoscopic KSP (42). Patients treated with KSP were older with a greater proportion of grade 1 tumors and underwent segmental ureterectomy in 62.5% of cases. To date, oncological outcomes of endoscopic KSP with percutaneous resection and/or flexible ureteroscopy tumor ablation have been compared to RNU in nine non-randomized studies (43-51). A recent meta-analysis included eight of these studies and revealed no difference in terms of OS and CSS between both strategies (52). These studies were all retrospective with small cohorts and limited follow-up. Selection bias was clearly a major limitation since most tumors in the KSP group were unifocal, <2 cm and low grade, in contrast with a higher incidence of invasive tumors in the RNU group. Local recurrence rate, a major issue in endoscopic conservative management, ranged from 6% to 71% in these heterogeneous cohorts. Results were so variable that no reliable RFS meta-analysis could be performed. Yakoubi et al. partly related the high heterogeneity among studies to differences in expertise of endoscopy between centers (52). Progression rate, another major concern regarding conservative management, remains unclear because of the inability to accurately grade and stage UTUC. Grade and stage migration during follow up has been estimated to reach 19% and 14%, respectively, and varied widely according grade at first biopsy (26). A delayed RNU is finally performed in 28-43% patients initially treated endoscopically (26). A major issue to address is the oncologic impact of such delayed radical treatment. Two studies compared delayed RNU after endoscopic KSP to immediate RNU and reported similar oncologic outcomes (53,54). However, these results should be considered with caution due to small populations and short follow-up.
Many improvements with digital ureteroscopes such as NBI and photodynamic diagnosis are currently under evaluation (35). These new technologies could help better diagnose UTUC but also perform a complete tumoral ablation during endoscopic KSP. Despite the lack of prospective randomized studies, the differential indications for KSP versus RNU seem reasonable based on the available evidence in order to provide optimal risk-based therapy for the individual patient.
Radical nephro-ureterectomy
Because of the limits of KSP and since more than 60% of tumors are invasive at presentation, RNU still remains the standard treatment for the majority of UTUC (3). To ensure negative margin, complete removal of the ureter including a bladder cuff is mandatory during RNU. In high risk UTUC (pT3N0, pT4N0 and/or N+ and/or M+), positive margins have been identified as an independent prognostic factor for CSS and OS (55). Lughezzani et al. showed that avoiding bladder cuff excision increased cancer specific mortality (CSM), especially in high risk UTUC (56). Several approaches have been proposed to perform bladder cuff excision with no difference in RFS, CSS, and OS between transvesical, extravesical, or endoscopic approaches in a large multicenter study of 2,681 patients treated with RNU (57). However, endoscopic approach was associated with a higher risk of intravesical recurrence. Recently, Kapoor et al. reported an improved overall and intravesical RFS with open intravesical excision of the distal ureter compared with endoscopic but also extravesical approaches (58).
Similarly to other fields of urology, laparoscopy and robotic assistance have been adopted to perform RNU. Robotic assisted RNU is still in its infancy and comparative studies are scarce (59,60). Conversely, many studies have compared laparoscopic RNU (LRNU) to open RNU (ORNU), and a recent meta-analysis reported similar oncologic outcome (61). Caution should be advocated especially in locally advanced disease since LRNU is generally performed in favorable-risk patients (62). Indeed, Fairey et al. reported that LRNU may be associated with poorer RFS compared to ORNU in a study of 849 patients (403 ORNU vs. 446 LRNU) (P=0.06) (63). In the only randomized controlled trial, Simone et al. found CSS and metastasis free survival were significantly different between the two procedures for pT3 tumors, in favor of ORNU (P=0.039 and P=0.004, respectively) (64). However, this and other studies were limited by their small size and other potential biases of selection or expertise, but one main limitation may be the use or extent of LND during LRNU.
The importance of LND remains a question of debate, yet all the evidence shows improved outcomes with higher number of LN removed, specially in LN negative patients (65). Capitanio et al. reported that LND was not commonly performed during ORNU and LRNU [42% and 24% of cases, respectively (62)]. Guidelines advocate LND in RNU for two reasons: (I) improve prognostication; (II) a potential therapeutic effect (3). Indeed, LN status is one of the most powerful predictor of CSS in patients treated with RNU, possibly guiding treatment decision for follow-up scheduling and AC (66). Roscigno et al. estimated that removal of eight LNs was the critical cut off to reach a prognostic significance and a 75% probability to correctly stage the patients (67). Therapeutic effect remains, however, unclear. A potential survival benefit in patients who underwent a LND during RNU has been reported in several monocentric studies with small cohorts (68-70). Two retrospective studies in large cohorts of patients reported this benefit could only be valuable in muscle invasive or locally advanced UTUC (71,72). Indeed, the risk of LN involvement is limited in Ta T1 UTUC, probably less than 5% (65,73). Recently, Yang et al. included 6,000 patients in a meta-analysis and confirmed a benefit of LND only in the group of patients with muscle invasive tumors (74). One question that remains unclear in these studies is the template for LND. Kondo et al. proposed a template for LND according to tumor location in the upper two-thirds of ureter, or in the lower third of the ureter (68). The former implies a dissection of iliac vessels, the latter a dissection of the aorta or the vena cava that could limit its performance minimally-invasively.
Therefore, prospective comparative studies are mandatory to assess the oncologic outcomes according to surgical approach and the extent of LND. Futures studies with RNU should match patients for grade and stage, but also for surgical approach. Strict definitions of the extent of LND using predefined templates will be necessary to make evidence-based recommendations.
“Prevent growth and regrowth”
Local instillations
One major concern with each management is the prediction, prevention and treatment of disease recurrence. Urothelial carcinoma can either recur in the bladder, contralateral ureter and/or in the ipsilateral ureter if KSP has been attempted.
After KSP, recurrence rate in the upper tract can be reported in up to 70% of the cases (52). Instillations of topical agents in the upper tract have been proposed to decrease this risk. Different approaches have been reported (percutaneous nephrostomy, retrograde catheterisation and vesico-ureteral reflux) with bacille calmette guerin (BCG) and mitomycin C (MMC) (75,76). BCG instillations for carcinoma in situ (CIS) may be the only one with sufficient evidence today. Only one study compared BCG instillation and RNU for CIS in 11 and 6 patients, respectively, and reported no significant difference in 5-year RFS and CSS (77). Topical instillations with BCG and MMC have been also reported as therapy after endoscopic management of Ta/T1 UTUC. Rastinehad et al. performed the largest comparative study with adjuvant antegrade BCG therapy after percutaneous resection and demonstrated no benefit in terms of recurrence and progression rates (78). These studies were retrospective, mostly non comparative, and included small cohorts treated mainly by percutaneous resection. These limitations preclude any conclusion regarding the use of instillations in the upper tract for UTUC after conservative treatment. Therefore, new studies should investigate its efficacy but also the best way to administrate it in the era of flexible ureteroscopic management.
Instillation of post-operative topical agents in the bladder have also been proposed to decrease the risk of intravesical recurrence after RNU. Indeed, 30% to 50% of patients will develop UCB during the first 5 years after RNU for UTUC (79). O’Brien et al. demonstrated, in a prospective multicentre randomized study, that a single post-operative intravesical dose of MMC after RNU decreased the relative risk of bladder tumor by 40% within the first year (80). In a phase II trial using intravesical pirarubicin (THP) within 48 h after RNU, Ito et al. reported similar results (81). Xylinas et al. developed a tool to identify the patients most likely to benefit from immediate post RNU intravesical chemotherapy (82). No study on the role of early post operative bladder instillation has been yet published after endoscopic management. Therefore, high level of evidence regarding the usefulness of post operative instillation of MMC after RNU now exits but further evaluation is needed to conclude on its efficacy after KSP management, another area of high likelihood of benefit.
Chemotherapy
Systemic NC before radical cystectomy has demonstrated survival benefit in patients with T2-4 N0 M0 UCB with high level of evidence (83). To date, no level 1 evidence exists to state on the role of peri-operative chemotherapy in UTUC. A recent review and meta-analysis identified ten studies that investigated the role of chemotherapy in an adjuvant setting (84). All but one were retrospective studies. These studies harbored many potential biases with most patients who received AC having worse prognostic factors and more likely to have LN metastasis. Conversely, patients receiving AC may have better renal function and performance status. Meta-analysis demonstrated only a statistically significant benefit for OS and disease free survival (DFS) among the three studies using cisplatin-based AC (HR, 0.43; 95% CI, 0.21-0.89; P=0.023). Furthermore, recent studies suggested that AC may only benefit high risk patients with pT3-4 UTUC and LN involvement (85,86). With potential benefit restricted to cisplatin based chemotherapy in locally advanced disease, the impact of AC appears limited since most patients with UTUC will experience renal function loss after RNU, becoming ineligible (87). Even before RNU, only 49% of patients have a glomerular filtration rate that would allow cisplatin based chemotherapy. This rate decreases to 19% after RNU.
Potential use and efficacy of chemotherapy in a pre-operative setting is, therefore, a critical issue. To date, two prospective studies assessed the role of NC in patients with urothelial carcinoma but only recruited 21 patients with UTUC. These studies suggested NC could be associated with a significant downstaging. The small cohorts and the inaccuracy of current methods to pre-operatively stage the tumor limit any conclusion (84). Results from four larger retrospective and comparative studies that specifically evaluated NC in UTUC have been published so far (88-91). Matin et al. reported outcomes of 43 patients with high grade UTUC who received NC compared to a historical cohort. A significant higher pathologic downstaging and a complete response in 14% of patients were observed in the NC group (89). In a recent study, use of NC in 31 patients was associated with a significant improvement of OS and CSS compared to a cohort of 81 patients who underwent RNU alone (91). Upper Tract Urothelial Carcinoma Collaboration group reported as well outcome in a large cohort of 313 patients including 18 patients with biopsy proven LN involvement who received NC and demonstrated favorable oncologic outcomes in this group: 5-yr DFS and CSS rates of 49% and 44%, respectively (88). Considering these two last studies, a recent meta-analysis reported a CSS benefit of 59% with NC (HR, 0.41; 95% CI: 0.22-0.76; P=0.005) (84). These retrospective data suggest that all eligible patients should be proposed cisplatin combination chemotherapy in UTUC. Which patients are most likely to benefit from NC remains to be defined. Patients with clinically suspect LN should receive definitive chemotherapy and a RNU in case of response. However, the level of evidence of the studies does not allow any firm conclusion. Further prospective trials are needed to assess the role of peri-operative chemotherapy in UTUC. One randomized controlled phase 3 trial, the peri-operative chemotherapy versus surveillance in upper tract urothelial cancer (POUT) trial, is ongoing (92). This trial will randomize 345 patients undergoing RNU for UTUC between adjuvant platin-based chemotherapy and surveillance. Results from phase 2 trials that investigate impact of neoadjuvant gemcitabine in patients with high grade or T2-T4 N0/X M0 UTUC before RNU will probably help us further to define the place of perioperative chemotherapy in UTUC.
Predictive models
The lack of randomized trials and reliable preoperative staging and grading evaluation leads to complex decision making in UTUC management. Intense collaborative and multi-institutional efforts resulted in propositions of predictive tools. To date, three pre-operative models have been proposed to predict muscle invasive and non-organ-confined UTUC (22,23,37,38). We previously discussed the usefulness of these models to decide between KSP and RNU. Other predictive models using pre-operative data represent promising tools. Two models based on imaging, urine cytology, or neutrophil count demonstrated significant ability to predict RFS and CSS (93,94). To manage the potential risk of renal function loss after RNU, several prognostic factors have been identified and corresponding predictive models constructed to identify patients that would not be suitable for post-operative chemotherapy (95-97). These prediction tools could help physicians identify patients who may benefit from neoadjuvant medical treatments in UTUC or LND during surgery.
Several postoperative prognostic risk factors after UTUC have been identified and were combined to propose post-operative prediction tools (36). Jeldres et al. were the first to propose a post-operative nomogram for UTUC. Within a cohort of almost 6,000 patients from the SEER database, a model based on age, tumor stage, tumor grade, and LN status predicted 5-year CSS with a discrimination of 75.4%. Since this first nomogram, four new models have been published. The French collaborative group and international UTUC collaboration proposed their own models to predict CSS (98,99). Both cohorts (3,387 patients) were combined and the model predicted 5-year CSS with 80% discrimination (100). Recently, Seisen et al. proposed a model including only patients without NC from both collaborative groups (101). However, to date, only one external validation focusing on the French collaborative group model has been published (102). Xylinas et al. recently published a predictive model of intravesical recurrence after RNU (82). Based on age, gender, previous history of bladder cancer, tumor location, tumor stage, presence of concomitant CIS, and LN status, the model discrimination was 68%. With this model, considering a risk of intravesical recurrence of 15% at 2 years to perform post operative instillation would spare 23% of the patients while not preventing only 0.3% of intravesical recurrences. These models could be particularly relevant to help physicians identifying patients whose disease is more likely to recur and therefore benefit from adjuvant therapy.
Conclusions
Ten years of intense collaborative efforts in basic and clinical research have made the natural history of UTUC more comprehensible and predictable. Current management is based, however, on low level evidence and there are many challenges to face in the future. There is a need to clarify the role of KSP management, topical agents, LND, and perioperative chemotherapy. New further collaborative efforts are mandatory to propose ambitious multi-institutional studies with preferentially prospective design.
Acknowledgements
Romain Mathieu is supported by the Scholarship Foundation of the Republic of Austria—OeAD and by the EUSP Scholarship—European Association of Urology.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics 2012. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Green DA, Rink M, Xylinas E, et al. Urothelial carcinoma of the bladder and the upper tract: disparate twins. J Urol 2013;189:1214-21. [PubMed]
- Rouprêt M, Babjuk M, Comperat E, et al. European guidelines on upper tract urothelial carcinomas: 2013 update. Eur Urol 2013;63:1059-71. [PubMed]
- Rai BP, Shelley M, Coles B, et al. Surgical management for upper urinary tract transitional cell carcinoma. Cochrane Database Syst Rev 2011.CD007349. [PubMed]
- Matin SF, Shariat SF, Milowsky MI, et al. Highlights from the first symposium on upper tract urothelial carcinoma. Urol Oncol 2014;32:309-16. [PubMed]
- Moriya M, Slade N, Brdar B, et al. TP53 Mutational signature for aristolochic acid: an environmental carcinogen. Int J Cancer 2011;129:1532-6. [PubMed]
- Chen CH, Dickman KG, Moriya M, et al. Aristolochic acid-associated urothelial cancer in Taiwan. Proc Natl Acad Sci U S A 2012;109:8241-6. [PubMed]
- Lai MN, Wang SM, Chen PC, et al. Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J Natl Cancer Inst 2010;102:179-86. [PubMed]
- Rouprêt M, Yates DR, Comperat E, et al. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol 2008;54:1226-36. [PubMed]
- Rouprêt M, Fromont G, Azzouzi AR, et al. Microsatellite instability as predictor of survival in patients with invasive upper urinary tract transitional cell carcinoma. Urology 2005;65:1233-7. [PubMed]
- Roupret M, Catto J, Coulet F, et al. Microsatellite instability as indicator of MSH2 gene mutation in patients with upper urinary tract transitional cell carcinoma. J Med Genet 2004;41:e91. [PubMed]
- Rouprêt M, Drouin SJ, Cancel-Tassin G, et al. Genetic variability in 8q24 confers susceptibility to urothelial carcinoma of the upper urinary tract and is linked with patterns of disease aggressiveness at diagnosis. J Urol 2012;187:424-8. [PubMed]
- Lin HH, Ke HL, Hsiao KH, et al. Potential role of CCND1 G870A genotype as a predictor for urothelial carcinoma susceptibility and muscle-invasiveness in Taiwan. Chin J Physiol 2011;54:196-202. [PubMed]
- Sasaki M, Sakano S, Okayama N, et al. DNA repair gene polymorphisms may be associated with prognosis of upper urinary tract transitional cell carcinoma. Neoplasia 2008;10:255-65. [PubMed]
- Lughezzani G, Burger M, Margulis V, et al. Prognostic factors in upper urinary tract urothelial carcinomas: a comprehensive review of the current literature. Eur Urol 2012;62:100-14. [PubMed]
- Krabbe LM, Bagrodia A, Haddad AQ, et al. Multi-institutional Validation of the Predictive Value of Ki-67 in Patients with High Grade Urothelial Carcinoma of the Upper Urinary Tract. J Urol 2015;193:1486-93. [PubMed]
- Bagrodia A, Krabbe LM, Gayed BA, et al. Evaluation of the prognostic significance of altered Mammalian target of rapamycin pathway biomarkers in upper tract urothelial carcinoma. Urology 2014;84:1134-40. [PubMed]
- Bensalah K, Montorsi F, Shariat SF. Challenges of cancer biomarker profiling. Eur Urol 2007;52:1601-9. [PubMed]
- Scolieri MJ, Paik ML, Brown SL, et al. Limitations of computed tomography in the preoperative staging of upper tract urothelial carcinoma. Urology 2000;56:930-4. [PubMed]
- Fritz GA, Schoellnast H, Deutschmann HA, et al. Multiphasic multidetector-row CT (MDCT) in detection and staging of transitional cell carcinomas of the upper urinary tract. Eur Radiol 2006;16:1244-52. [PubMed]
- Secin FP, Koppie TM, Salamanca JI, et al. Evaluation of regional lymph node dissection in patients with upper urinary tract urothelial cancer. Int J Urol 2007;14:26-32. [PubMed]
- Favaretto RL, Shariat SF, Savage C, et al. Combining imaging and ureteroscopy variables in a preoperative multivariable model for prediction of muscle-invasive and non-organ confined disease in patients with upper tract urothelial carcinoma. BJU Int 2012;109:77-82. [PubMed]
- Messer JC, Terrell JD, Herman MP, et al. Multi-institutional validation of the ability of preoperative hydronephrosis to predict advanced pathologic tumor stage in upper-tract urothelial carcinoma. Urol Oncol 2013;31:904-8. [PubMed]
- Tavora F, Fajardo DA, Lee TK, et al. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol 2009;33:1540-6. [PubMed]
- Smith AK, Stephenson AJ, Lane BR, et al. Inadequacy of biopsy for diagnosis of upper tract urothelial carcinoma: implications for conservative management. Urology 2011;78:82-6. [PubMed]
- Cutress ML, Stewart GD, Zakikhani P, et al. Ureteroscopic and percutaneous management of upper tract urothelial carcinoma (UTUC): systematic review. BJU Int 2012;110:614-28. [PubMed]
- Matin SF, Kamat AM, Grossman HB. High-frequency endoluminal ultrasonography as an aid to the staging of upper tract urothelial carcinoma: imaging findings and pathologic correlation. J Ultrasound Med 2010;29:1277-84. [PubMed]
- Chen SP, Liao JC. Confocal laser endomicroscopy of bladder and upper tract urothelial carcinoma: a new era of optical diagnosis? Curr Urol Rep 2014;15:437. [PubMed]
- Bus MT, Muller BG, de Bruin DM, et al. Volumetric in vivo visualization of upper urinary tract tumors using optical coherence tomography: a pilot study. J Urol 2013;190:2236-42. [PubMed]
- Yoshida S, Kobayashi S, Koga F, et al. Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol 2013;23:2206-14. [PubMed]
- Akita H, Jinzaki M, Kikuchi E, et al. Preoperative T categorization and prediction of histopathologic grading of urothelial carcinoma in renal pelvis using diffusion-weighted MRI. AJR Am J Roentgenol 2011;197:1130-6. [PubMed]
- Sassa N, Kato K, Abe S, et al. Evaluation of (11)C-choline PET/CT for primary diagnosis and staging of urothelial carcinoma of the upper urinary tract: a pilot study. Eur J Nucl Med Mol Imaging 2014;41:2232-41. [PubMed]
- Kleinmann N, Healy KA, Hubosky SG, et al. Ureteroscopic biopsy of upper tract urothelial carcinoma: comparison of basket and forceps. J Endourol 2013;27:1450-4. [PubMed]
- Al-Qahtani SM, Legraverend D, Gil-Diez de Medina S, et al. Can we improve the biopsy quality of upper urinary tract urothelial tumors? Single-center preliminary results of a new biopsy forceps. Urol Int 2014;93:34-7. [PubMed]
- Bus MT, de Bruin DM, Faber DJ, et al. Optical diagnostics for upper urinary tract urothelial cancer: technology, thresholds, and clinical applications. J Endourol 2015;29:113-23. [PubMed]
- Xylinas E, Kluth L, Mangal S, et al. Predictive tools for clinical decision-making and counseling of patients with upper tract urothelial carcinoma. World J Urol 2013;31:31-6. [PubMed]
- Margulis V, Youssef RF, Karakiewicz PI, et al. Preoperative multivariable prognostic model for prediction of nonorgan confined urothelial carcinoma of the upper urinary tract. J Urol 2010;184:453-8. [PubMed]
- Brien JC, Shariat SF, Herman MP, et al. Preoperative hydronephrosis, ureteroscopic biopsy grade and urinary cytology can improve prediction of advanced upper tract urothelial carcinoma. J Urol 2010;184:69-73. [PubMed]
- Rouprêt M, Colin P, Yates DR. A new proposal to risk stratify urothelial carcinomas of the upper urinary tract (UTUCs) in a predefinitive treatment setting: low-risk versus high-risk UTUCs. Eur Urol 2014;66:181-3. [PubMed]
- Lucca I, Klatte T, Rouprêt M, et al. Kidney-sparing surgery for upper tract urothelial cancer. Curr Opin Urol 2015;25:100-4. [PubMed]
- Xylinas E, Roupret M, Shariat SF. Segmental ureterectomy for upper tract urothelial carcinoma: two procedures with different indications. Urol Oncol 2013;31:1841-3. [PubMed]
- Simhan J, Smaldone MC, Egleston BL, et al. Nephron-sparing management vs radical nephroureterectomy for low- or moderate-grade, low-stage upper tract urothelial carcinoma. BJU Int 2014;114:216-20. [PubMed]
- Lee BR, Jabbour ME, Marshall FF, et al. 13-year survival comparison of percutaneous and open nephroureterectomy approaches for management of transitional cell carcinoma of renal collecting system: equivalent outcomes. J Endourol 1999;13:289-94. [PubMed]
- Rouprêt M, Hupertan V, Traxer O, et al. Comparison of open nephroureterectomy and ureteroscopic and percutaneous management of upper urinary tract transitional cell carcinoma. Urology 2006;67:1181-7. [PubMed]
- Lucas SM, Svatek RS, Olgin G, et al. Conservative management in selected patients with upper tract urothelial carcinoma compares favourably with early radical surgery. BJU Int 2008;102:172-6. [PubMed]
- Gadzinski AJ, Roberts WW, Faerber GJ, et al. Long-term outcomes of nephroureterectomy versus endoscopic management for upper tract urothelial carcinoma. J Urol 2010;183:2148-53. [PubMed]
- Raymundo EM, Lipkin ME, Banez LB, et al. Third prize: the role of endoscopic nephron-sparing surgery in the management of upper tract urothelial carcinoma. J Endourol 2011;25:377-84. [PubMed]
- Grasso M, Fishman AI, Cohen J, et al. Ureteroscopic and extirpative treatment of upper urinary tract urothelial carcinoma: a 15-year comprehensive review of 160 consecutive patients. BJU Int 2012;110:1618-26. [PubMed]
- Fajkovic H, Klatte T, Nagele U, et al. Results and outcomes after endoscopic treatment of upper urinary tract carcinoma: the Austrian experience. World J Urol 2013;31:37-44. [PubMed]
- Cutress ML, Stewart GD, Tudor EC, et al. Endoscopic versus laparoscopic management of noninvasive upper tract urothelial carcinoma: 20-year single center experience. J Urol 2013;189:2054-60. [PubMed]
- Hoffman A, Yossepowitch O, Erlich Y, et al. Oncologic results of Nephron sparing endoscopic approach for upper tract low grade transitional cell carcinoma in comparison to nephroureterectomy - a case control study. BMC Urol 2014;14:97. [PubMed]
- Yakoubi R, Colin P, Seisen T, et al. Radical nephroureterectomy versus endoscopic procedures for the treatment of localised upper tract urothelial carcinoma: A meta-analysis and a systematic review of current evidence from comparative studies. Eur J Surg Oncol 2014;40:1629-34. [PubMed]
- Boorjian S, Ng C, Munver R, et al. Impact of delay to nephroureterectomy for patients undergoing ureteroscopic biopsy and laser tumor ablation of upper tract transitional cell carcinoma. Urology 2005;66:283-7. [PubMed]
- Gadzinski AJ, Roberts WW, Faerber GJ, et al. Long-term outcomes of immediate versus delayed nephroureterectomy for upper tract urothelial carcinoma. J Endourol 2012;26:566-73. [PubMed]
- Vassilakopoulou M, de la Motte Rouge T, Colin P, et al. Outcomes after adjuvant chemotherapy in the treatment of high-risk urothelial carcinoma of the upper urinary tract (UUT-UC): results from a large multicenter collaborative study. Cancer 2011;117:5500-8. [PubMed]
- Lughezzani G, Sun M, Perrotte P, et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol 2010;57:956-62. [PubMed]
- Xylinas E, Rink M, Cha EK, et al. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 2014;65:210-7. [PubMed]
- Kapoor A, Dason S, Allard CB, et al. The impact of method of distal ureter management during radical nephroureterectomy on tumour recurrence. Can Urol Assoc J 2014;8:E845-52. [PubMed]
- Ambani SN, Weizer AZ, Wolf JS, et al. Matched comparison of robotic vs laparoscopic nephroureterectomy: an initial experience. Urology 2014;83:345-9. [PubMed]
- Trudeau V, Gandaglia G, Shiffmann J, et al. Robot-assisted versus laparoscopic nephroureterectomy for upper-tract urothelial cancer: A population-based assessment of costs and perioperative outcomes. Can Urol Assoc J 2014;8:E695-701. [PubMed]
- Ni S, Tao W, Chen Q, et al. Laparoscopic versus open nephroureterectomy for the treatment of upper urinary tract urothelial carcinoma: a systematic review and cumulative analysis of comparative studies. Eur Urol 2012;61:1142-53. [PubMed]
- Capitanio U, Shariat SF, Isbarn H, et al. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol 2009;56:1-9. [PubMed]
- Fairey AS, Kassouf W, Estey E, et al. Comparison of oncological outcomes for open and laparoscopic radical nephroureterectomy: results from the Canadian Upper Tract Collaboration. BJU Int 2013;112:791-7. [PubMed]
- Simone G, Papalia R, Guaglianone S, et al. Laparoscopic versus open nephroureterectomy: perioperative and oncologic outcomes from a randomised prospective study. Eur Urol 2009;56:520-6. [PubMed]
- Lughezzani G, Jeldres C, Isbarn H, et al. A critical appraisal of the value of lymph node dissection at nephroureterectomy for upper tract urothelial carcinoma. Urology 2010;75:118-24. [PubMed]
- Novara G, De Marco V, Gottardo F, et al. Independent predictors of cancer-specific survival in transitional cell carcinoma of the upper urinary tract: multi-institutional dataset from 3 European centers. Cancer 2007;110:1715-22. [PubMed]
- Roscigno M, Shariat SF, Freschi M, et al. Assessment of the minimum number of lymph nodes needed to detect lymph node invasion at radical nephroureterectomy in patients with upper tract urothelial cancer. Urology 2009;74:1070-4. [PubMed]
- Kondo T, Nakazawa H, Ito F, et al. Impact of the extent of regional lymphadenectomy on the survival of patients with urothelial carcinoma of the upper urinary tract. J Urol 2007;178:1212-7; discussion 1217. [PubMed]
- Brausi MA, Gavioli M, De Luca G, et al. Retroperitoneal lymph node dissection (RPLD) in conjunction with nephroureterectomy in the treatment of infiltrative transitional cell carcinoma (TCC) of the upper urinary tract: impact on survival. Eur Urol 2007;52:1414-8. [PubMed]
- Roscigno M, Cozzarini C, Bertini R, et al. Prognostic value of lymph node dissection in patients with muscle-invasive transitional cell carcinoma of the upper urinary tract. Eur Urol 2008;53:794-802. [PubMed]
- Roscigno M, Shariat SF, Margulis V, et al. Impact of lymph node dissection on cancer specific survival in patients with upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol 2009;181:2482-9. [PubMed]
- Burger M, Shariat SF, Fritsche HM, et al. No overt influence of lymphadenectomy on cancer-specific survival in organ-confined versus locally advanced upper urinary tract urothelial carcinoma undergoing radical nephroureterectomy: a retrospective international, multi-institutional study. World J Urol 2011;29:465-72. [PubMed]
- Xylinas E, Rink M, Margulis V, et al. Prediction of true nodal status in patients with pathological lymph node negative upper tract urothelial carcinoma at radical nephroureterectomy. J Urol 2013;189:468-73. [PubMed]
- Yang D, Chen Q, Song X, et al. Effect of lymph node dissection on the outcomes of upper tract urothelial carcinomas: a meta-analysis. Expert Rev Anticancer Ther 2014;14:667-75. [PubMed]
- Audenet F, Traxer O, Bensalah K, et al. Upper urinary tract instillations in the treatment of urothelial carcinomas: a review of technical constraints and outcomes. World J Urol 2013;31:45-52. [PubMed]
- Carmignani L, Bianchi R, Cozzi G, et al. Intracavitary immunotherapy and chemotherapy for upper urinary tract cancer: current evidence. Rev Urol 2013;15:145-53. [PubMed]
- Kojima Y, Tozawa K, Kawai N, et al. Long-term outcome of upper urinary tract carcinoma in situ: effectiveness of nephroureterectomy versus bacillus Calmette-Guerin therapy. Int J Urol 2006;13:340-4. [PubMed]
- Rastinehad AR, Ost MC, Vanderbrink BA, et al. A 20-year experience with percutaneous resection of upper tract transitional carcinoma: is there an oncologic benefit with adjuvant bacillus Calmette Guerin therapy? Urology 2009;73:27-31. [PubMed]
- Xylinas E, Kluth LA, Rieken M, et al. Impact of smoking status and cumulative exposure on intravesical recurrence of upper tract urothelial carcinoma after radical nephroureterectomy. BJU Int 2014;114:56-61. [PubMed]
- O’Brien T, Ray E, Singh R, et al. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial). Eur Urol 2011;60:703-10. [PubMed]
- Ito A, Shintaku I, Satoh M, et al. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol 2013;31:1422-7. [PubMed]
- Xylinas E, Kluth L, Passoni N, et al. Prediction of intravesical recurrence after radical nephroureterectomy: development of a clinical decision-making tool. Eur Urol 2014;65:650-8. [PubMed]
- Griffiths G, Hall R, Sylvester R, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 2011;29:2171-7. [PubMed]
- Leow JJ, Martin-Doyle W, Fay AP, et al. A systematic review and meta-analysis of adjuvant and neoadjuvant chemotherapy for upper tract urothelial carcinoma. Eur Urol 2014;66:529-41. [PubMed]
- Lucca I, Kassouf W, Kapoor A, et al. The role of adjuvant chemotherapy for lymph node-positive upper tract urothelial carcinoma following radical nephroureterectomy: a retrospective study. BJU Int 2014. [Epub ahead of print]. [PubMed]
- Shirotake S, Kikuchi E, Tanaka N, et al. Impact of an adjuvant chemotherapeutic regimen on the clinical outcome in high risk patients with upper tract urothelial carcinoma: a Japanese multi-institution experience. J Urol 2015;193:1122-8. [PubMed]
- Kaag MG, O’Malley RL, O’Malley P, et al. Changes in renal function following nephroureterectomy may affect the use of perioperative chemotherapy. Eur Urol 2010;58:581-7. [PubMed]
- Youssef RF, Shariat SF, Lotan Y, et al. Upper urinary tract urothelial carcinoma with loco-regional nodal metastases: insights from the Upper Tract Urothelial Carcinoma Collaboration. BJU Int 2011;108:1286-91. [PubMed]
- Matin SF, Margulis V, Kamat A, et al. Incidence of downstaging and complete remission after neoadjuvant chemotherapy for high-risk upper tract transitional cell carcinoma. Cancer 2010;116:3127-34. [PubMed]
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33. [PubMed]
- Porten S, Siefker-Radtke AO, Xiao L, et al. Neoadjuvant chemotherapy improves survival of patients with upper tract urothelial carcinoma. Cancer 2014;120:1794-9. [PubMed]
- Birtle AJ, Lewis R, Johnson M, et al. Time to define an international standard of postoperative care for resected upper urinary tract transitional cell carcinoma (TCC) - opening of the peri-operative chemotherapy versus surveillance in upper tract urothelial cancer (POUT) Trial. BJU Int 2012;110:919-21. [PubMed]
- Sakano S, Matsuyama H, Kamiryo Y, et al. Risk group stratification based on preoperative factors to predict survival after nephroureterectomy in patients with upper urinary tract urothelial carcinoma. Ann Surg Oncol 2013;20:4389-96. [PubMed]
- Hashimoto T, Ohno Y, Nakashima J, et al. Clinical significance of preoperative peripheral blood neutrophil count in patients with non-metastatic upper urinary tract carcinoma. World J Urol 2013;31:953-8. [PubMed]
- Xylinas E, Rink M, Margulis V, et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int 2013;112:453-61. [PubMed]
- Kaag M, Trost L, Thompson RH, et al. Preoperative predictors of renal function decline after radical nephroureterectomy for upper tract urothelial carcinoma. BJU Int 2014;114:674-9. [PubMed]
- Fang D, Zhang Q, Li X, et al. Nomogram predicting renal insufficiency after nephroureterectomy for upper tract urothelial carcinoma in the Chinese population: exclusion of ineligible candidates for adjuvant chemotherapy. Biomed Res Int 2014;2014:529186.
- Yates DR, Hupertan V, Colin P, et al. Cancer-specific survival after radical nephroureterectomy for upper urinary tract urothelial carcinoma: proposal and multi-institutional validation of a post-operative nomogram. Br J Cancer 2012;106:1083-8. [PubMed]
- Cha EK, Shariat SF, Kormaksson M, et al. Predicting clinical outcomes after radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol 2012;61:818-25. [PubMed]
- Rouprêt M, Hupertan V, Seisen T, et al. Prediction of cancer specific survival after radical nephroureterectomy for upper tract urothelial carcinoma: development of an optimized postoperative nomogram using decision curve analysis. J Urol 2013;189:1662-9. [PubMed]
- Seisen T, Colin P, Hupertan V, et al. Postoperative nomogram to predict cancer-specific survival after radical nephroureterectomy in patients with localised and/or locally advanced upper tract urothelial carcinoma without metastasis. BJU Int 2014;114:733-40. [PubMed]
- Ku JH, Moon KC, Jung JH, et al. External validation of an online nomogram in patients undergoing radical nephroureterectomy for upper urinary tract urothelial carcinoma. Br J Cancer 2013;109:1130-6. [PubMed]