
A study of pregnancy rates in “cleared” male factor couples
Introduction
Approximately 85% of couples who attempt to conceive will succeed within one year (1). Among those who fail, more than half will have an identifiable male factor (2). This population is substantial, with 9.4% of U.S. men aged 25–44 years reporting use of infertility services (3).
Upon evaluation, semen analysis abnormalities will be revealed in almost 60% of infertile men, while the remainder will show normal semen quality (3). When a thorough male factor evaluation is performed in the setting of a normal semen analysis (4), it is also common that the history and physical exam are similarly unremarkable. Such infertility cases are designated as unexplained in nature and further evaluation with more sophisticated semen testing, including antisperm antibodies or sperm DNA fragmentation assays, are considered (5). Overall, it is estimated that a more subtle male factor underlies about 25–30% of unexplained infertility cases (6,7). Typically, whether or not an etiology is revealed, affected couples will proceed to either intrauterine insemination (IUI) or in vitro fertilization (IVF) as the next therapeutic step.
However, another interpretation of an entirely normal male factor evaluation is that there is, in fact, no male fertility impairment. Indeed, if 25–30% of unexplained infertility is male-derived, then the vast majority of men categorized as having unexplained infertility may very well be “fertile”. We hypothesize that a significant proportion of men deemed to have unexplained infertility are actually normally fertile. Since the natural history of fertility in this cohort of men is not well described, we studied the pregnancy rates in a cohort of men who were formally evaluated and “cleared” of male factor infertility. We present the following article in accordance with the TREND reporting checklist (available at: http://dx.doi.org/10.21037/tau-20-1240).
Methods
Study subjects
The study consists of consecutive infertile couples recruited from a single male infertility practice (PJT) over a 3-year period. The male factor infertility evaluation included a thorough history, a complete physical examination and at least 2 semen analyses (5). Semen analyses were performed according to World Health Organization reference ranges (8). Any and all prior general medical, hormonal metabolic laboratory studies were reviewed. Based on this formal assessment, male partners in whom the bulk semen parameters were normal on at least one of two semen samples by WHO standards and in whom no significant infertility risks factors were observed based on history and physical examination, were “cleared” from further medical evaluation. Lifestyle modifications were allowed following the male evaluation. As such, men who took antioxidant supplements and those who discontinued tobacco, finasteride, hot baths and saunas, qualified for study follow-up. However, those who received formal medical or surgical therapy for male factor issues were excluded from the study. The presence or absence of a female factor evaluation was not required for study inclusion. Subjects were followed for 12 months or until a pregnancy was achieved.
Survey technique
Patient contact
Identified subjects were contacted and consented through a telephone interview, according to HIPAA guidelines for patient contact. During the interview, the subject was provided with informed consent and HIPAA documentation and verbal consent obtained. Consent documents were then mailed to subjects for written signature with return envelopes included. During the tele-interview, the study goals were explained and a survey administered (Table 1). The total length of the phone interview was 5 minutes.

Full table
Data de-identification
After demographic and clinical information was collected from the electronic medical record and patient interviews, the data was formally de-identified for analysis. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Health Sciences Institutional Review Board at University of Southern California (HS-13-00578) and informed consent was taken from all the patients.
Statistical analysis
The primary outcome of the study was pregnancies achieved after male factor evaluation. Secondary outcomes include analyses of clinical and laboratory findings in study subjects. Simple descriptive statistics, including two tailed t-tests, were used to evaluate the significance of observations.
Results
Demographic findings
A total of 54 subjects were enrolled in the study over a 3-year period. Demographic information about the subjects and their partners is outlined in Table 2. Upon clinical presentation, the mean duration of infertility was 1.5 years (range, 0.4 to 4.0 years) and the mean male and female partner ages were 38.6 and 35.1 years respectively. The vast majority of patients (85%) presented with primary infertility and the remainder demonstrated secondary infertility.

Full table
Clinical findings
On the clinical history of study subjects, 40% were noted to have significant fertility risks that included a clinical varicocele or lifestyle exposures for infertility, including hot baths or saunas, tobacco or alcohol abuse, and the use of androgen altering medications such as finasteride (Table 3). At the time of the initial evaluation, they were advised to alter lifestyle issues to optimize fertility and encouraged to take antioxidant supplements. On physical examination, 38% presented with grade II or III left varicoceles; however, no other masses were present in any subject. No varicocele treatments were offered or performed on study subjects during the study follow-up period.

Full table
Semen parameters on routine analysis were within normal range for at least one of two semen analyses obtained from each study subject (Table 4). The first semen analysis was performed before the initial infertility evaluation, and the second sample was performed subsequent to this evaluation. The mean interval between two semen analyses performed on individuals was 8.8 weeks (range, 1–28 weeks). Interestingly, despite the fact that all semen analyses were considered technically “normal”, there was a trend toward improved semen quality between the first and second semen samples (Table 4), driven mainly by an increase in sperm motility. This led to a significant improvement in total motile sperm counts between samples (65.8 million sperm to 150.4 million sperm, P<0.05).

Full table
Pregnancy outcomes
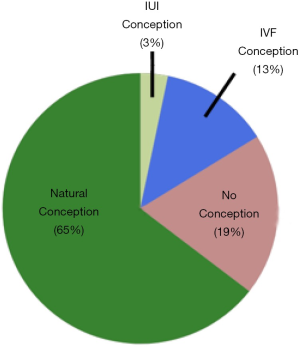
As illustrated in Figure 1, among n=31 couples with known pregnancy outcomes, 20/31 (65%) conceived naturally at a mean of 9 months after evaluation (range, 3–30 mos). Another 1/31 (3%) couples conceived with IUI and 4/31 (13%) conceived with IVF-ICSI. If reproductive assistance was used, time to conception was longer at 22.3 months (range, 8–53). The remaining 6 couples had not conceived by one year.
Conclusions
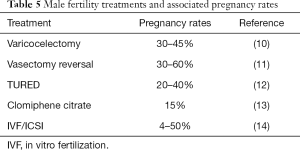
We investigated the natural and assisted fertility rates among a small cohort of infertile couples who demonstrated no evidence of male factor infertility on a standard urologic evaluation. Indeed, this subset of men conceived naturally at a robust rate (65%) after the male factor evaluation and did so without specific medical or surgical intervention. Another 16% of couples conceived with either IUI or IVF within a year of follow-up. Notably, this conception rate is markedly higher than the 10–15% pregnancy rate that is expected for untreated couples who are followed for a second year after an infertility diagnosis (9). These pregnancy rates compare favorably to other classic medical or surgical treatments offered to infertile men (Table 5). It also suggests that the male factor evaluation is reasonably robust in identifying relevant male factor issues that may impact fertility and that this straightforward evaluation may be a better predictor of true male fertility than previously believed. As such, the word “unexplained” may be inappropriate for many men with unremarkable male factor evaluations, as they may actually be fertile. Indeed, this pilot study suggests that two-thirds of these men may be perfectly fertile.
Full table
There is another possible interpretation of these findings. Although men were “cleared” of male factor issues after a standard evaluation and were not offered formal medical or surgical treatment, they may have pursued lifestyle modifications that augmented natural fertility. Discontinuation of wet heat exposures (15), stopping medications such as finasteride, and reducing habitual alcohol or other drug use may have factored into improving the natural fertility of study subjects. In addition, dietary changes that increased antioxidant intake and the regular use of antioxidant supplements by study subjects could influence fertility potential (16). Although not quantified in this study, the effects of such lifestyle modifications are hard to quantify but likely contributed to improved fertility potential of men in the study. Given this alternative interpretation of the study findings, it is provocative to suggest that simple lifestyle modifications could substantially improve male fertility potential, but very reasonable to suggest that lifestyle issues merit further study for their potential to improve the fertility of men.
It is reassuring to see that the prevalence of varicoceles detected in this study cohort approximates what one would expect for an unselected population of men with infertility (17). More novel is the finding that 40% of infertile men had what could be considered significant lifestyle risk factors, independent of varicocele, revealed on urologic evaluation. Although no malignancies were detected, the ability of the urologic assessment to detect clinical risk for male infertility is clearly apparent from the study. Indeed, the burden of lifestyle risk identified among infertile men supports the common medical idiom for other significant human diseases, like cancer or metabolic syndrome, that “lifestyle matters”.
One statistically significant finding in the study was that the semen quality following an initial male factor evaluation improved compared to the intake semen analysis. Given that the mean time from the initial to subsequent semen analyses was almost 9 weeks, this could represent real clinical improvement following lifestyle modifications recommended at the initial visit. However, it may also reflect the great variability that exists within intraindividual semen analyses over time, and between different laboratories (18,19). Therefore, caution is advised when evaluating the true underlying significance of the semen analysis findings.
Of course, significant study limitations exist, the foremost among them being limited patient numbers. Indeed, this may limit the ability to generalize the study findings. An accurate characterization of potential female factor issues was also not undertaken, although excluding couples based on defined female factors would likely have increased the proportion of those achieving pregnancies by making the denominator smaller. In addition, we were not able to confirm exactly which lifestyle changes were pursued by patients subsequent to their male factor evaluation. Lastly, this study did not address the issue of sperm “quality” as may be garnered from assays such as sperm DNA fragmentation (20). However, it is our belief that including these assays would likely have refined the relatively “blunt” instrument that is the standard semen analysis in predicting whom among study subjects would be fertile vs. infertile at study completion (21).
In summary, a significant proportion of men diagnosed with unexplained infertility have lifestyle risk factors ascertainable on a standard urologic evaluation. Medical care in the form of counseling at risk patients regarding lifestyle issues, in the absence of formal medical or surgical treatment, may have value in improving the fertility potential in this population. Indeed, the natural conception rates among men identified with unexplained infertility can be substantial and suggest that many of these men have no male factor infertility.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1240
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1240
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-1240
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1240). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Health Sciences Institutional Review Board at University of Southern California (HS-13-00578) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Infertility: A Tabulation of Available Data on Prevalence of Primary and Secondary Infertility. Geneva, Switzerland: WHO Programme on Maternal and Child Health and Family Planning, Division of Family Health. World Health Organization 1991.
- Thonneau P, Marchand S, Tallec A, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989). Hum Reprod 1991;6:811-16. [Crossref] [PubMed]
- Chandra A, Copen CE, Stephen EH. Infertility service use in the United States: data from the National Survey of Family Growth, 1982-2010. Natl Health Stat Report 2014.1-21. [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103: e18-25. [Crossref] [PubMed]
- Turek PJ. Practical approach to the diagnosis and management of male infertility. Nat Clin Pract Urol 2005;2:226-38. [Crossref] [PubMed]
- Mackenna A, Barratt CLR, Kessepoulu E, et al. The contribution of a hidden male factor to unexplained infertility. Fertil Steril 1993;59:405-11. [Crossref] [PubMed]
- Hamada A, Esteves SC, Nizza M, et al. Unexplained male infertility: Diagnosis and management. Int Braz J Urol 2012;38:576-94. [Crossref] [PubMed]
- Cooper TG, Noonan E, Eckardstein S, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update 2010;16:231-45. [Crossref] [PubMed]
- Snick HK, Snick TS, Ever JL, et al. The spontaneous pregnancy prognosis in untreated subfertile couples: The Walcheren primary care study. Hum Reprod 1997;12:1582-8. [Crossref] [PubMed]
- Çayan S, Shavakhabov S, Kadioğlu A. Treatment of palpable varicocele in infertile men: A meta‐analysis to define the best technique. J Androl 2009;30:33-40. [Crossref] [PubMed]
- Belker AM, Thomas AJ, Fuchs EF, et al. Results of 1,469 microsurgical vasectomy reversals by the vasovasostomy study group. J Urol 1991;145:505-11. [Crossref] [PubMed]
- Kadioglu A, Cayan S, Tefekli A, et al. Does response to treatment of ejaculatory duct obstruction in infertile men vary with pathology? Fertil Steril 2001;76:138-42. [Crossref] [PubMed]
- Vandekerckhove P, Lilford R, Vail A, et al. WITHDRAWN: Clomiphene or tamoxifen for idiopathic oligo/asthenospermia. Cochrane Database Syst Rev 2007.CD000151. [PubMed]
- Sart.org. Survey of 2017 U.S. IVF pregnancy rates. Available online: https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2017
- Shefi S, Tarapore PE, Walsh TJ, et al. Wet heat exposure: a potentially reversible cause of low semen quality in infertile men. International Braz J Urol 2007;33:50-6; discussion 6-7. [Crossref] [PubMed]
- Smits RM, Mackenzie-Proctor R, Yazdani A, et al. Antioxidants for male subfertility. The Cochrane database of systematic reviews. 2019.13. [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [Crossref] [PubMed]
- Auger J, Eustache F, Ducot B, et al. Intra-and inter-individual variability in human sperm concentration, motility and vitality assessment during a workshop involving ten laboratories. Hum Reprod 2000;15:2360-8. [Crossref] [PubMed]
- Leushuis E, van der Steeg JW, Steures P, et al. Reproducibility and reliability of repeated semen analyses in male partners of subfertile couples. Fertil Steril 2010;94:2631-5. [Crossref] [PubMed]
- McReynolds S, Dzieciatkowska M, Stevens J, et al. Toward the identification of a subset of unexplained infertility: a sperm proteomic approach. Fertil Steril 2014;102:692-9. [Crossref] [PubMed]
- Turek PJ. Does the male infertility clinical evaluation adequately assess toxicologic exposures? Fertil Steril 2008;89 suppl:E69. [Crossref] [PubMed]


