
Epidemiology and therapy of symptomatic lymphoceles after robot-assisted radical prostatectomy (RARP)
Introduction
Worldwide, prostate cancer is the second most frequently diagnosed cancer (after lung cancer) among men (1), with 1,276,106 new cases (7.1% of all cancer diagnoses in men) and 358,989 deaths (3.8% of all deaths caused by cancer in men) in 2018 (2). At the time of diagnosis, the majority of patients present with an organ-confined stage of the disease. In this case, surgical therapy by means of radical prostatectomy is an established therapeutic option, depending on the life expectancy and comorbidity of the patient (3). Over the course of the last few years many clinics have adopted the robot-assisted laparoscopic radical prostatectomy (RARP) as their new standard of care in surgical therapy of prostate cancer, as it is associated with lower blood loss, lower transfusion rate and less hospitalisation duration in contrast to open radical prostatectomy (4), while also showing good perioperative and oncologic outcomes (4,5).
Intraoperative pelvic lymph node dissection (PLND) is an important component of the surgical therapy of prostate cancer. Although a direct benefit on oncological outcomes has yet to be proven, PLND provides an accurate assessment of cancer spread (6), which is why the current guidelines of the European Association of Urology (EAU) on prostate cancer recommend performing PLND in high-risk patients as well as intermediate-risk patients when the estimated risk for positive lymph nodes (LNs) exceeds 5% (3).
There are, however, complications that are associated with PLND, the most common of which are lymphoceles (6). While most lymphoceles remain clinically asymptomatic, serious complications like infection or venous thromboembolism can occur (7,8). The risk of lymphocele was shown to be higher after extended PLND in comparison to limited PLND (9), no significant difference could be found between the extraperitoneal and the transperitoneal RARP approach (10). In previous studies the percentage of patients presenting with a symptomatic lymphocele (SLC) after prostatectomy and PLND was shown to be between 1.49% and 11.2% (8,10-13). Therapeutic options for the treatment of lymphoceles include cannulation and drainage (CD), instillation (e.g., of doxycycline), or laparoscopic fenestration (LF) (14-17).
The objective of this retrospective study was to explore the frequency of SLC after RARP as well as the effectiveness of different therapeutic options. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1315).
Methods
We retrospectively analysed all patients who underwent RARP (transperitoneal or extraperitoneal) at our clinic from January 1, 2014 to December 31, 2018. No exclusion criteria were applied. This resulted in a cohort of 1,029 patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee (University of Regensburg, No. 19-1393-104) and individual consent for this retrospective analysis was waived.
We recorded patients’ age, height, weight and body mass index (BMI) at the time of RARP, accompanying illnesses and regular anticoagulant or platelet inhibiting medication.
We also recorded patients’ prostate-specific antigen (PSA) levels at the time of diagnosis, their risk category according to the d’Amico risk classification for prostate cancer, whether or not preoperative diagnostic imaging had been carried out, and if so, which [abdominal computed tomography (CT), bone scintigram, Ga-68-PSMA-positron emission tomography (PET)/CT] as well as their operational risk according to the American Society of Anesthesiologists (ASA classification).
Regarding the surgery itself we documented, whether or not PLND was conducted, whether or not nerve sparing (NS) was carried out and if so, on which side(s), as well as the final histological result including the TNM stage according to the Union for International Cancer Control (UICC) as well as the Gleason score.
If PLND was conducted, bilateral PLND was performed which included at least the removal of the LNs overlying the external iliac artery and vein as well as the LNs within the obturator fossa cranially and caudally to the obturator nerve. A more extensive PLND was performed in case of pre- or intraoperatively suspect LNs. The lymphatic vessels were sealed by bipolar cauterization, clips were placed in addition according to the surgeon’s preference.
From the following days of postoperative hospitalization we recorded the length of the patients’ stay, whether or not the first postoperative cystogram (routinely done around the 5th to the 7th postoperative day, according to the surgeon who had performed the procedure) showed an insufficiency of the vesicourethral anastomosis and whether or not a secondary insufficiency of the vesicourethral anastomosis occurred.
The pelvic drainage, which was intraoperatively placed in all cases, was postoperatively removed depending on the drainage output.
All patients routinely received an ultrasound (US) examination on the day they were discharged, and it was recorded whether or not this examination already showed any lymphoceles and if so, on which side(s).
With regard to deep venous thrombosis (DVT) prophylaxis, all patients received a daily dose of 5,000 IU of low molecular weight heparin (LWMH) for 4 weeks postoperatively. Patients with atrial fibrillation or a history of DVT or pulmonary embolism received a therapeutic or half-therapeutic weight-adapted LWMH dose according to their CHADS-VASc score.
If patients presented with a SLC in our emergency department and were readmitted to our clinic, further data was recorded (referring to the time of presentation at our clinic): the location of the SLC, the patients’ body temperature, the following blood test results: leucocyte count, creatinine, estimated glomerular filtration rate (eGFR), whether or not patients reported stress incontinence, as well as local pain upon pressure. All lymphoceles were primarily investigated by US; in some cases an additional CT scan was performed.
Furthermore, we recorded the length of the patients’ stay after readmission as well as the following parameters: concomitant venous thrombosis, the SLC treatment(s) applied [CD, instillation of doxycycline (including frequency and duration of instillation) or LF], if an antibiotic treatment was initiated and if the discharge examination by US showed persistent lymphoceles. Primary treatment for SLC was chosen at the surgeons’ discretion. Any therapeutic approach used to treat a case of SLC was defined as successful if the patient did not experience a recurrence and did not require further treatment.
If CD of the lymphocele was conducted, we recorded the result of the bacteriological diagnostics of the lymphocele fluid. Patients were not recorded as SLC cases when a creatinine measurement after drainage placement revealed the fluid collection to be a urinoma.
For every patient, we recorded the result of the urine culture when readmitted.
Statistical analysis
Statistical analysis was performed using SPSS (version 25). The statistical tests used to describe the relation between binary variables were chi-squared test as well as Fisher’s exact test. To describe the relation between binary and quantitative variables, Mann-Whitney U test was used after taking into account the skewness.
Results
Patient population
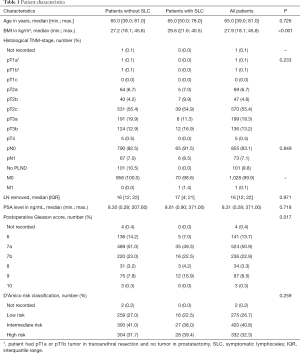
We were able to include all 1,029 patients who underwent RARP in the aforementioned period of time. Median age of all patients was 65.5 years [interquartile range (IQR), 60.0–70.0 years], median PSA level was 8.31 ng/mL (IQR, 6.00–12.83 ng/mL), with 275 patients having low-risk, 420 patients having intermediate-risk and 332 patients having high-risk disease, respectively. A detailed overview of the study population is shown in Table 1.
Full table
High BMI and high postoperative Gleason score correlate with the occurrence of SLC
There were no statistically significant correlations between the occurrence of SLC and patients’ age, their histological tumour stage including LN status, the number of LNs removed during PLND, their initial PSA levels and their risk classification according to d’Amico.
It could be shown, however, that patients with SLC had a significantly higher median BMI compared to those without SLC (29.6 vs. 27.2, P<0.001). Also, SLC was shown to be more likely among patients with a high postoperative Gleason score (P=0.017). Both higher BMI (P<0.001) and high postoperative Gleason score (P=0.016) remained statistically significant in multivariable analysis.
Frequency of SLC
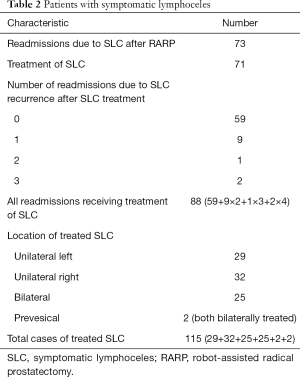
Of the 1,029 patients who underwent RARP in the aforementioned period of time, a total of 186 patients (18.1%) were diagnosed with a lymphocele, either during the standard discharge examination or when readmitted to our clinic. Seventy-three patients after RARP (7.1%) were readmitted because of a SLC, 71 of whom (6.9% of all patients who underwent RARP) had to undergo treatment. The main symptoms of these patients were local pain upon pressure and signs of infection.
In total, 115 cases of SLC were treated in the described period of time and thus included in this study. This is due to several patients being treated because of their SLC, discharged, and later readmitted because of a recurrence. Furthermore, some patients showed bilateral SLC, which were treated with varying degrees of success or different therapeutic options. A detailed overview of the patients with SLC is shown in Table 2.
Full table
Treatment of SLC: fenestration as the only effective option
The therapeutic options used were CD, instillation of doxycycline and LF. A detailed description of how these therapeutic measures were applied to the 115 cases of SLC is shown in Figure 1. Any therapeutic approach used to treat a case of SLC was defined as successful if the patient did not experience a recurrence and did not require further treatment. This refers to the specific case of SLC treated; a contralateral recurrence, for example, was defined as a separate case.
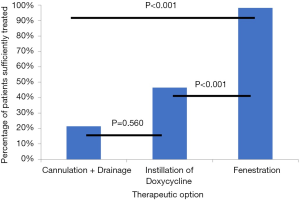
The success rates of the different approaches are shown in Figure 2. In total, 102 cases of SLC received CD. Of those, 21 cases (20.6%) were successfully treated this way. The rest required further treatment or experienced a recurrence of the SLC. In 56 cases of SLC, the next treatment option chosen was instillation of doxycycline, which proved successful in 26 cases (46.4%). LF of the SLC was applied in 54 cases (in 21 cases following CD as well as instillation of doxycycline, in 20 cases following CD only, and in 13 cases as primary therapeutic option). Fifty-three cases of SLC (98.1%) were successfully treated this way, corresponding to 20 cases of LF after CD as well as instillation of doxycycline (95.2%), 20 cases of LF after CD only (100.0%) and 13 cases of primary LF (100.0%). The success rate of LF was significantly higher than the success rates of CD and drainage and instillation (DI) (P<0.001, respectively).
Complications of SLC
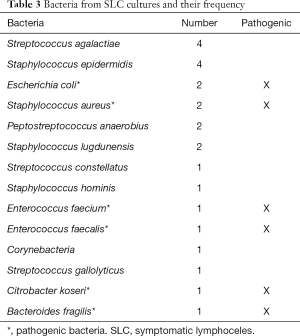
As mentioned before, SLC can present with several complications such as thromboembolism or infection. Of those readmitted because of SLC, 10 patients (13.7%) presented with an accompanying deep vein thrombosis. With regard to infection of the SLC, in 23 of the 88 (26.1%) readmissions due to SLC the bacteriological diagnostic of the SLC fluid after cannulation showed bacteria. The bacteria which could be found and their frequency distribution are shown in Table 3 (in one case, the SLC culture showed a colonisation with two bacteria). Most of these bacteria are commensal with low pathogenic potential. This results in 8 of 88 (9.1%) readmissions with pathogenic bacteria in SLC culture (Table 3).
Full table
Of the 88 hospital readmissions due to SLC, 10 patients (11.4%) presented with fever (defined as a body temperature above 38.4 °C), while another 6 patients (6.8%) presented with an elevated body temperature between 37.5 and 38.4 °C. An accompanying urinary tract infection as shown by a positive urine culture could be found in 31 patients (35.2%). The overlap of patients presenting with elevated body temperature above 37.5 °C, a positive SLC culture and a sterile urine culture showed a total of 6 patients equalling 6 cases of SLC (6.8% of all readmissions due to SLC, 5.2% of all treated cases of SLC).
Discussion
RARP has emerged as the new standard of care for surgical treatment of prostate cancer. However, SLC remain a common complication even with this minimally invasive method, regardless of the approach (extraperitoneal or transperitoneal) (10).
As mentioned above, in previous studies the percentage of patients presenting with SLC after prostatectomy and PLND was shown to be between 1.49% and 11.2% (8,10-13). With 7.2% of all RARP patients experiencing SLC, our study showed a frequency of SLC similar to those reported previously. The variance of the reported frequencies may in part be due to the use of different imaging techniques (US vs. CT scan) to detect SLC.
PLND had previously been shown to be the main risk factor for SLC formation after RARP (18). Correspondingly, our data showed that patients who did not receive PLND were significantly less likely to develop SLC (P<0.001). As additional risk factors for SLC formation previous studies listed patients’ age, BMI and the number of LNs removed (8,13,19). Our study, however, showed patients’ BMI and their postoperative Gleason score to be the only statistically significant risk factors for SLC, with patients with high BMI (P<0.001) and patients with a high postoperative Gleason score (P=0.017) being more likely to experience SLC.
Regarding therapy of SLC, there is no standard of care, with different therapeutic approaches having been reported with varying rates of success. While some authors recommend that LF be used as first-line treatment for SLC (14,15), others report success rates of 70% to 100% for conservative approaches (8,17,20). The success rates for LF given by the aforementioned studies are 97% to 100%, with a mean hospitalisation time of 2.3 days postoperatively (14,15). Our success rate of 98.1% for LF in general (100% for primary LF) is well comparable to these results. Comparison of success rates for instillation therapy, however, is made difficult by the fact that in the aforementioned studies povidone-iodine and ethanol were used as sclerosing agents, whereas we used doxycycline. Apart from that, no differences can be found in conducting the instillation therapy. In our study, LF of the SLC proved significantly more effective than CD (P<0.001) and instillation of doxycycline (P<0.001). There was no significant difference between the two conservative approaches (P=0.560).
Furthermore, our patients’ average time of hospitalisation (from admission until discharge) depended on their therapy sequence and was found to be 7 days for CD only, 12 days for CD followed by instillation of doxycycline, 14 days for CD followed by instillation of doxycycline followed by LF, and 9 days for primary LF. The mean hospitalisation time of 9 days for our primary LF patients is longer in comparison to the one given by the aforementioned studies. This is explained by the delay until reaching the decision to conduct primary LF and getting a slot in the operating room. By reducing the time from admission to primary LF (for example, by establishing primary LF as the standardised treatment option for SLC), the total time of hospitalisation for SLC patients can probably be reduced even further (3–4 days).
Thromboembolism is described as a common complication of SLC, with reported rates of deep vein thrombosis of up to 8% for patients with lymphoceles (20). PLND is a known risk factor for thromboembolic events (21). In our study, 13.5% of patients presenting with SLC also experienced a deep vein thrombosis. As most other studies only show the percentage of deep vein thrombosis in relation to all patients receiving RARP and not in relation to patients experiencing SLC, a precise evaluation of this percentage is difficult.
Infection is also often mentioned as a complication of SLC. Infection of SLC as indicated by a positive SLC culture and a sterile urine culture was reported by Hamada et al. to affect up to 42% of SLC after RARP, with Staphylococcus aureus being the most frequently isolated organism (11). In our study, in 26.1% of readmissions due to SLC the bacteriological diagnostic of the SLC fluid after cannulation showed bacteria. Most of these bacteria, however, are commensal with low pathogenic potential or possibly due to contamination (Table 3), which results in 9.1% of readmissions presenting with pathogenic bacteria in SLC culture.
Hamada et al. furthermore reported that fever, CT findings of abnormally thickened walls, leucocytosis and younger age were significant predictors for SLC fluid culture positivity (11).
In our study, however, no significant correlation between a positive SLC culture and elevated body temperature, leucocytosis, elevated C-reactive protein (CRP) or lower abdominal pain could be found. There often was an accompanying urinary tract infection, which is another possible reason for fever and elevated inflammation markers.
As previously described, the overlap of patients presenting with elevated body temperature above 37.5 °C, a positive SLC culture and a sterile urine culture showed a total of 6 cases of SLC (6.7% of all readmissions due to SLC, 5.2% of all treated cases of SLC). Thus, a clinically significant infection of a SLC is a rare complication.
Conclusions
SLC are a common postoperative complication of RARP. However, serious complications like deep vein thrombosis or clinically relevant infections of SLCs with pathogenic bacteria are rare. Our retrospective analysis shows that the most efficient treatment method for SLC is LF, which also results in a shortened hospital stay and should be considered as the initial treatment of SLC. However, an additional cost-benefit-analysis as well as larger prospective studies are necessary to further confirm this recommendation.
Limitations
The main limitation of this study is its retrospective design over a time period of five years. On the one hand, this makes analysis of the parameters of interest more difficult, as surgical techniques and clinical approaches are in constant change. On the other hand, we were able to acquire a comparatively large number of patients, which certainly contributes to the relevance of the reported results. Certainly, though, further prospective data is necessary to confirm the reported results.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1315
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1315
Peer Review File: Available at http://dx.doi.org/10.21037/tau-20-1315
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1315). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics committee (University of Regensburg, No. 19-1393-104) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Global Cancer Facts & Figures 4th Edition. Atlanta: American Cancer Society, 2018.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Briers E, et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. Last edited 2019. Available online: https://uroweb.org/guideline/prostate-cancer/. Access date February 14th, 2020.
- Cao L, Yang Z, Qi L, et al. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: Perioperative, functional, and oncological outcomes: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e15770. [Crossref] [PubMed]
- Liss MA, Lusch A, Morales B, et al. Robot-assisted radical prostatectomy: 5-year oncological and biochemical outcomes. J Urol 2012;188:2205-10. [Crossref] [PubMed]
- Fossati N, Willemse PM, Van den Broeck T, et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur Urol 2017;72:84-109. [Crossref] [PubMed]
- Liss MA, Skarecky D, Morales B, et al. Preventing perioperative complications of robotic-assisted radical prostatectomy. Urology 2013;81:319-23. [Crossref] [PubMed]
- Keskin MS, Argun ÖB, Öbek C, et al. The incidence and sequela of lymphocele formation after robot-assisted extended pelvic lymph node dissection. BJU Int 2016;118:127-31. [Crossref] [PubMed]
- Briganti A, Chun FK, Salonia A, et al. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer. Eur Urol 2006;50:1006-13. [Crossref] [PubMed]
- Horovitz D, Lu X, Feng C, et al. Rate of Symptomatic Lymphocele Formation After Extraperitoneal vs Transperitoneal Robot-Assisted Radical Prostatectomy and Bilateral Pelvic Lymphadenectomy. J Endourol 2017;31:1037-43. [Crossref] [PubMed]
- Hamada A, Hwang C, Fleisher J, et al. Microbiological evaluation of infected pelvic lymphocele after robotic prostatectomy: potential predictors for culture positivity and selection of the best empirical antimicrobial therapy. Int Urol Nephrol 2017;49:1183-91. [Crossref] [PubMed]
- Orvieto MA, Coelho RF, Chauhan S, et al. Incidence of lymphoceles after robot-assisted pelvic lymph node dissection. BJU Int 2011;108:1185-90. [Crossref] [PubMed]
- Capitanio U, Pellucchi F, Gallina A, et al. How can we predict lymphorrhoea and clinically significant lymphocoeles after radical prostatectomy and pelvic lymphadenectomy? Clinical implications. BJU Int 2011;107:1095-101. [Crossref] [PubMed]
- Khoder WY, Becker AJ, Seitz M, et al. Modified laparoscopic lymphocele marsupialization for the treatment of lymphoceles after radical prostatectomy: first results. J Laparoendosc Adv Surg Tech A 2011;21:145-8. [Crossref] [PubMed]
- Khoder WY, Gratzke C, Haseke N, et al. Laparoscopic marsupialisation of pelvic lymphoceles in different anatomic locations following radical prostatectomy. Eur Urol 2012;62:640-8. [Crossref] [PubMed]
- Akhan O, Karcaaltincaba M, Ozmen MN, et al. Percutaneous transcatheter ethanol sclerotherapy and catheter drainage of postoperative pelvic lymphoceles. Cardiovasc Intervent Radiol 2007;30:237-40. [Crossref] [PubMed]
- Alago W, Deodhar A, Michell H, et al. Management of postoperative lymphoceles after lymphadenectomy: percutaneous catheter drainage with and without povidone-iodine sclerotherapy. Cardiovasc Intervent Radiol 2013;36:466-71. [Crossref] [PubMed]
- Khoder WY, Trottmann M, Buchner A, et al. Risk factors for pelvic lymphoceles post-radical prostatectomy. Int J Urol 2011;18:638-43. [Crossref] [PubMed]
- Seetharam Bhat KR, Onol F, Rogers T, et al. Can we predict who will need lymphocele drainage following robot assisted laparoscopic prostatectomy (RALP)? J Robot Surg 2020;14:439-45. [Crossref] [PubMed]
- Musch M, Klevecka V, Roggenbuck U, et al. Complications of pelvic lymphadenectomy in 1,380 patients undergoing radical retropubic prostatectomy between 1993 and 2006. J Urol 2008;179:923-8; discussion 928-9. [Crossref] [PubMed]
- Tyritzis SI, Wallerstedt A, Steineck G, et al. Thromboembolic complications in 3,544 patients undergoing radical prostatectomy with or without lymph node dissection. J Urol 2015;193:117-25. [Crossref] [PubMed]



