
Exploration of the association between serum uric acid and testosterone in adult males: NHANES 2011–2016
Introduction
Serum uric acid (SUA) is the end product of purine nucleotide metabolism and hyperuricemia can occur as a result of overproduction or underexcretion of SUA. The prevalence of hyperuricemia has gradually increased in the past 30 years. An epidemiological investigation of the National Health and Nutrition Examination Survey (NHANES) 2007–2008 showed that the overall prevalence of hyperuricemia among US adults was 21.4%, which was significantly higher than that in the NHANES-III [1988–1994] (1). In addition to joint and kidney involvement, previous studies have suggested that elevated SUA are associated with insulin resistance, elevated blood pressure, triglyceride and the risk of metabolic syndrome (2,3). A significantly higher prevalence of hyperuricemia was observed in participants with metabolic syndrome compared with nonmetabolic syndrome (4), and the converse is also true, a higher prevalence of metabolic syndrome was also found in participants with higher SUA levels, even in those with normouricemia (5,6). The prevalence of metabolic syndrome was nearly 10-fold higher in those with SUA levels of 10 mg/dL or greater compared with less than 6 mg/dL (7). Hyperuricemia has been believed to be an independent risk factor for cardiovascular events and all-cause mortality (8).
Testosterone, the principal male sex hormone mainly secreted by Leydig cells in the testes, is responsible for the maturation of male sexual organs and development of secondary sexual characteristics. Hypogonadism is a clinical and biochemical syndrome that can occur across all ages and the diagnosis of hypogonadism is based on relevant clinical symptoms and low testosterone (9). Testosterone deficiency has become an increasingly concerning and controversial topic around the world, affecting approximately 7% of men in their 50s with an increasing prevalence with age (10). Testosterone is also an important hormone in the pathophysiology of metabolic diseases, and previous studies have shown the association between testosterone and obesity, metabolic syndrome and type 2 diabetes mellitus (T2DM). Hypogonadism is an important comorbidity of obesity (11,12). A nationwide study involves 2,615 participants have found that approximately 52.4% of obese men had low testosterone levels (13). Low testosterone levels are highly prevalent in men with metabolic syndrome and insulin resistance (14,15). Studies have found long-term testosterone replacement therapy can produce significant decrease in body mass index (BMI), improvement in insulin resistance and glycemic control (16,17).
With regard to the relationship between SUA and testosterone levels, the results of previous studies are controversial (Table S1) and lack high-level research evidence. Elevated SUA levels are associated with metabolic syndrome and insulin resistance, both of which increase the risk of low testosterone, so there might be a negative correlation between SUA and testosterone. Some studies have proved SUA is a risk factor for gonadal dysfunction (18), indicating a negative correlation between SUA and testosterone levels (19-23), which is consistent with our results. However, other studies have come to a different conclusion (24-27). Therefore, we analyzed a dataset of male Americans aged 18 years or older from NHANES to explore the relationship between SUA and testosterone and hoped it can provide some information for the treatment of hyperuricemia and low serum testosterone. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1114).
Methods
Data source and study population
The National Health and Nutrition Examination Survey (28), which was conducted by the Centers for Disease Control and Prevention of America every 2 years, is a cross-sectional survey that aims to assess the health and nutritional status of the U.S. population. The survey consists of an interview conducted in the home, followed by a standardized health examination in specially equipped mobile examination centers, which includes a physical examination administered by trained medical personnel as well as laboratory tests (29). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (30). Written informed consent was obtained from all participants prior to completing the NHANES, and all data was de-identified by the National Center for Health Statistics before being made publicly available.
The population for the present analysis consisted of individuals enrolled in three 2-year cycle surveys conducted in 2011–2012, 2013–2014 and 2015–2016. Among the 29,902 participants in the examination, we excluded those who were under the age of 18 years (n=11,933), whose testosterone data (n=1,891) and SUA data (n=17) were missing and who were female (n=8,265). Eventually, 7,796 participants were included in this cross-sectional study (Figure 1).
Variables
SUA is measured by the DxC800 synchron using a timed endpoint method (31). Serum testosterone measurement is performed via the isotope dilution liquid chromatography tandem mass spectrometry (ID-LC-MS/MS) method for routine quantitation of serum testosterone based on the National Institute for Standards and Technology’s (NIST) reference method (32). Clinically, symptomatic men whose total testosterone lower than 350 ng/dL (12 nmol/L) should be treated with testosterone therapy (33), so we categorized participants into two groups. Participants with testosterone ≤350 ng/dL belonged to the low testosterone level group, and the others were grouped into the normal group. The lower limits of detection of the assays were 0.36 ng/dL for serum testosterone, 0.5 mg/dL for SUA. Concentrations below the limit of detection were replaced with limit of detection divided by the square root of 2 (34).
Covariates
Potential confounding factors in this study include age, race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black, and Other race), BMI (normal: below 25 kg/m2; overweight: between 25 and <30 kg/m2; obesity: ≥30 kg/m2) (35), total moderate-to-vigorous minutes of physical activity per week (MVPA, number of days do moderate-to-vigorous physical activities per week × minutes of moderate-to-vigorous physical activities on a typical day) was log-transformed for regression analysis, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), anemia (defined as hemoglobin <13 g/dL) (36), the homeostasis model assessment-insulin resistance index (HOMA-IR) [fasting blood glucose (mmol/L) × fasting insulin (mIU/L)/22.5], a widely used insulin resistance marker (37). C-reactive protein (CRP) (an inflammatory biomarker which was associated with low serum testosterone and higher prevalence of metabolic syndrome) (38,39), sex hormone binding globulin (SHBG), estradiol. A history of hypertension or diabetes was defined as a self-reported physician diagnosis of hypertension or diabetes. Based on prior studies and theoretical considerations (40-43), above covariates were selected in this study.
Statistical analysis
All statistical analyses were conducted with SPSS 24 and STATA version 14. To account for the complex sampling design and ensure nationally representative estimates, all analyses were adjusted for survey design and weighting variables. The new sample weight (the original 2-year sample weight divided by 3) was constructed according to the analytical guidelines of the NHANES (44). The normality of continuous variables was tested with the Kolmogorov-Smirnov normality test. Normally distributed variables are described as the mean ± standard deviation, and nonnormally distributed continuous variables are described as the median (interquartile range). The median values among different SUA and testosterone groups were compared with the Mann-Whitney U test and Kruskal-Wallis test. The chi-square test was adopted to compare the percentages of categorical variables among different SUA and testosterone groups. The Bonferroni test was used for the intergroup comparison. We evaluate the correlation between each independent variable and testosterone by using univariate and multivariate linear regression models. Because the distribution of serum testosterone was skewed right, testosterone was log-transformed in the regression analysis. With serum testosterone as the dependent variable, regression coefficients, standard errors and P values were determined. Due to the limitation of data, serum estradiol, SHBG and CRP were only included in the 2015–2016 cycle linear regression analysis (Table S2). Because the concentration of serum testosterone was log-transformed, the percent differences of serum testosterone was calculated by the equation: percent change = (eβ-1)×100%. We performed a sensitivity analysis by excluding participants with serum testosterone <50 or >1,000 ng/dL or outliers (defined as more than 3 standard deviations from the mean). In addition, stratified analyses by age (group 1: 18≤ age <45, group 2: 45≤ age <65, group 3: age ≥65), BMI (normal, overweight and obesity), history of hypertension and diabetes were performed to examine the association between SUA and log-transformed testosterone. We analyzed the multicollinearity of the multivariate linear regression. The variance inflation factor (VIF) was less than 10, so there was no multicollinearity. A two-sided P<0.05 was considered statistically significant.
Results
A total of 7,796 participants were included in this study and categorized into five racial groups: Mexican American (n=1,112), Other Hispanic (n=761), Non-Hispanic White (n=2,999), Non-Hispanic Black (n=1,673), and the Other race (n=1,251). The mean age was 47.63±18.51 years, the mean serum testosterone level was 417.19±189.59 ng/dL, and mean SUA level was 358.35±77.78 µmol/L. SUA levels were categorized based on SUA quartiles: Q1 (SUA ≤303.30 µmol/L), Q2 (303.30< SUA ≤350.90 µmol/L), Q3 (350.90< SUA ≤404.51 µmol/L) and Q4 (SUA >404.51 µmol/L). Characteristics of the study population among different SUA levels were summarized in Table 1. There were significant differences among the four groups with respect to age, race, BMI, serum cholesterol, triglyceride, ALT, AST, testosterone, HOMA-IR, prevalence of anemia, hypertension and diabetes. A Bonferroni test was adopted for intergroup comparison, and we found that with the increasing of SUA quartiles, the proportion of obese participants (P<0.01) and HOMA-IR (P<0.01) increased gradually, while the serum testosterone (P<0.01) decreased gradually.
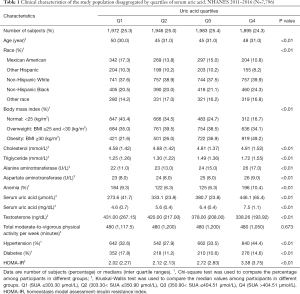
Full table
The clinical characteristics of participants with different testosterone levels were shown in Table 2. People with low testosterone levels were older (51 versus 44 years old) (P<0.01) and had higher HOMA-IR (P<0.01), higher triglyceride, ALT and SUA levels (P<0.01), and a higher prevalence of obesity (P<0.01), hypertension (P<0.01), anemia (P<0.01) and diabetes (P<0.01). Participants in the normal testosterone level group performed much more physical activity (P<0.01).
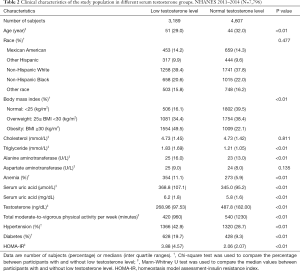
Full table
The results of the univariable and multivariate linear regression analysis of the association between each independent variable and log-transformed testosterone were shown in Table 3. In univariable linear regression analysis, we found that age, BMI, HOMA-IR, ALT and uric acid are significantly inversely associated with serum testosterone. In multivariate linear regression, we found that increasing age (estimate testosterone percent difference: −0.20% per year, P<0.01), uric acid (estimate testosterone percent difference: −4.40% per md/dL, P<0.01) and BMI (estimate testosterone percent difference: −2.86% per kg/m2, P<0.01) were associated with declining serum testosterone while increasing hemoglobin levels (estimate testosterone percent difference: 6.82% per g/dL, P<0.01) and log(MVPA) [estimate testosterone percent difference: 2.74% per log(MVPA) unit, P<0.01] were associated with elevated testosterone levels. Table 4 showed the results of sensitivity analysis by excluding participants with outlier or extreme value and the negative correlation of age, BMI and SUA with serum testosterone was still significant.

Full table

Full table
The results of stratified analyses by age, BMI, history of hypertension and diabetes were shown in Table 5. In stratified analyses by BMI and history of hypertension, the negative association between SUA and serum testosterone was still significant among different groups, but this negative correlation was not significant in man with diabetes or aged 65 and over. We then tested age and diabetes as an interaction with SUA in the model that adjusted for the same covariates. A significant interaction was noted between age (P<0.05), history of diabetes (P<0.05) and SUA.
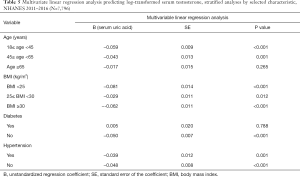
Full table
Table S2 represented the results of linear regression analysis with CRP, estradiol and SHBG were additionally adjusted. Due to the limitation of data, 2,590 participants from 2015–2016 cycle was involved. Our multiple regression model accounts for 66.2% (Adj. R2=0.662) of testosterone variation. In multivariate linear regression, we found that increasing age, SUA, BMI and HOMA-IR were associated with declining serum testosterone while increasing hemoglobin, estradiol and SHBG were associated with elevated testosterone levels.
Discussion
In this study, we combined data from the NHANES 2011–2012, 2013–2014 and 2015–2016, and a total of 7,796 men aged 18 years or older were included. In both univariable and multivariate linear regression analysis, our study indicated that increased SUA might be negatively correlated with serum testosterone. The negative association of SUA with testosterone was still significant after excluding participants with outlier or extreme value. In stratified analyses, above association was not significant in men with diabetes or aged 65 and over.
The mechanisms of the relationship between SUA and testosterone remain unclear, but several possibilities have been proposed. First, elevated SUA can lead to metabolic syndrome through mitochondrial oxidative stress and inhibition of AMP-activated protein kinase (45,46); in addition, hyperuricemia can cause decreased insulin release and insulin resistance through endothelial dysfunction, reduced nitric oxide bioavailability (6). Metabolic syndrome and insulin resistance have been shown to be associated with increased conversion from testosterone to estradiol and decreased production of testosterone by Leydig cells (47). Moreover, the deposition of urate crystals in the testicular tissue cause oxidative damage and directly affect the secretion of testosterone (18). Low testosterone can also affect the concentration of SUA, for which several mechanisms have been suggested. Low testosterone may lead to insulin resistance, which is inversely correlated with the clearance of UA (14,48,49). In addition, decreased levels of testosterone can reduce protein synthesis and increase the levels of endogenous purine, which may cause the elevation of SUA (18). In summary, metabolic syndrome, insulin resistance and obesity may play a mediating role in the relationship between low testosterone and SUA and the deposition of urate crystals in the testicular tissue may directly affects the secretion of testosterone.
Our results were consistent with some previous studies (18-21,23). A cross-sectional study conducted by Chao (18) indicated a significant negative correlation between SUA and testosterone levels in T2DM males. A study conducted by Gambineri, which involved 30 obese male subjects, was designed to investigate the impact of obstructive sleep apnea syndrome on testosterone levels and the main metabolic parameters and found that testosterone values were negatively associated with SUA (20). A prospective cohort study involving 51 men aimed at investigating whether testosterone treatment had an impact on cardiovascular risk factors in patients with T2DM and late-onset hypogonadism (LOH) found that testosterone-metformin combination therapy could help reduce SUA compared with treatment by metformin alone (21). In another prospective cohort of 31 men with LOH, 16 participants received intramuscular testosterone enanthate (100 mg weekly). After 4 months of therapy, increased testosterone levels and decreased UA levels were identified in patients treated with testosterone compared with the control group (23). However, there is also some controversial evidence (24,26,27,50). A prospective study spanning two years including 47 female-to-male transsexuals documented an ensuing significant increase in SUA and a decrease in FEUA (fraction excretion of uric acid) after cross-sex hormone treatment (24). Studies conducted by Marinello and Rosen pointed out that testicular endocrine function was normal in patients with gout, and no significant difference in serum testosterone levels was found between male patients with gout or asymptomatic hyperuricemia men and controls (26,27). Borbélyová found that gonadectomy and long-term hypogonadism in male middle-aged rats had no significant effect on uric acid (50). Possible explanations of the differences in the above research results included the following: (I) previous studies on the relationship between SUA and testosterone included a relatively small sample size; (II) the inclusion criteria of the above research were different, so there were differences in the research objects; (III) there were differences in the research methods, statistical analysis and adjusted covariates.
We used a large national representative sample with participants aged 18 years and older among the general US population, which increased the statistical strength to provide a more reliable result. We also analyzed the relationship between SUA and testosterone with different statistical methods. Furthermore, we fully adjusted for potential confounding factors in the exploration of the association between SUA and testosterone levels. However, our study also has some limitations. Primarily, as a cross-sectional study, it was difficult to determine causality between SUA and testosterone. Furthermore, testosterone was measured only once, while the diagnosis of low testosterone needed at least two recorded values measured on different days and serum testosterone was not a static but a dynamic variable, which may cause some bias. Finally, due to the limited data, we only analyzed the relationship between SUA and testosterone but did not further analyze the effects of free testosterone or bioactive testosterone on SUA.
Conclusions
In conclusion, our study suggests that SUA might be negatively associated with serum testosterone levels in the general U.S. adult males. We hope that it can provide some information for the treatment of hyperuricemia and testosterone deficiency. The association we investigated in this study is biologically plausible, and further large-scale prospective studies are required to confirm the causal relationship between SUA and testosterone.
Acknowledgments
We acknowledge the support from the Capital Medical Development Research Fund. We acknowledge the staff at the National Center for Health Statistics at the CDC, who design, collect, and administer the NHANES data and release the data for public use. We are grateful to all study participants for their cooperation.
Funding: This work was supported by a grant from the Capital Medical Development Research Fund (2018-1-4012).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1114
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1114). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all participants prior to completing the NHANES, and all data was de-identified by the NCHS before being made publicly available.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007-2008. Arthritis Rheum 2011;63:3136-41. [Crossref] [PubMed]
- Biradar MI, Chiang KM, Yang HC, et al. The causal role of elevated uric acid and waist circumference on the risk of metabolic syndrome components. Int J Obes (Lond) 2020;44:865-74. [Crossref] [PubMed]
- Wan X, Xu C, Lin Y, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism. J Hepatol 2016;64:925-32. [Crossref] [PubMed]
- Uaratanawong S, Suraamornkul S, Angkeaw S, et al. Prevalence of hyperuricemia in Bangkok population. Clin Rheumatol 2011;30:887-93. [Crossref] [PubMed]
- Li Q, Yang Z, Lu B, et al. Serum uric acid level and its association with metabolic syndrome and carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol 2011;10:72. [Crossref] [PubMed]
- Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia. Curr Opin Rheumatol 2013;25:210-6. [Crossref] [PubMed]
- Choi HK, Ford ES. Prevalence of the metabolic syndrome in individuals with hyperuricemia. Am J Med 2007;120:442-7. [Crossref] [PubMed]
- Chen JH, Chuang SY, Chen HJ, et al. Serum uric acid level as an independent risk factor for all-cause, cardiovascular, and ischemic stroke mortality: a Chinese cohort study. Arthritis Rheum 2009;61:225-32. [Crossref] [PubMed]
- Bhasin S, Cunningham GR, Hayes FJ, et al. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2010;95:2536-59. [Crossref] [PubMed]
- Halpern JA, Brannigan RE. Testosterone Deficiency. JAMA 2019;322:1116. [Crossref] [PubMed]
- Lamm S, Chidakel A, Bansal R. Obesity and Hypogonadism. Urol Clin North Am 2016;43:239-45. [Crossref] [PubMed]
- Rotter I, Ryl A, Grzesiak K, et al. Cross-Sectional Inverse Associations of Obesity and Fat Accumulation Indicators with Testosterone in Non-Diabetic Aging Men. Int J Environ Res Public Health 2018;15:1207. [Crossref] [PubMed]
- Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 2006;60:762-9. [Crossref] [PubMed]
- Dimopoulou C, Goulis DG, Corona G, et al. The complex association between metabolic syndrome and male hypogonadism. Metabolism 2018;86:61-8. [Crossref] [PubMed]
- Li C, Ford ES, Li B, et al. Association of testosterone and sex hormone-binding globulin with metabolic syndrome and insulin resistance in men. Diabetes Care 2010;33:1618-24. [Crossref] [PubMed]
- Rao PM, Kelly DM, Jones TH. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat Rev Endocrinol 2013;9:479-93. [Crossref] [PubMed]
- Saad F, Yassin A, Doros G, et al. Effects of long-term treatment with testosterone on weight and waist size in 411 hypogonadal men with obesity classes I-III: observational data from two registry studies. Int J Obes (Lond) 2016;40:162-70. [Crossref] [PubMed]
- Cao W, Zheng RD, Xu SH, et al. Association between Sex Hormone and Blood Uric Acid in Male Patients with Type 2 Diabetes. Int J Endocrinol 2017;2017:4375253. [Crossref] [PubMed]
- Fukai S, Akishita M, Miyao M, et al. Age-related changes in plasma androgen levels and their association with cardiovascular risk factors in male Japanese office workers. Geriatr Gerontol Int 2010;10:32-9. [Crossref] [PubMed]
- Gambineri A, Pelusi C, Pasquali R. Testosterone levels in obese male patients with obstructive sleep apnea syndrome: relation to oxygen desaturation, body weight, fat distribution and the metabolic parameters. J Endocrinol Invest 2003;26:493-8. [Crossref] [PubMed]
- Krysiak R, Gilowski W, Okopien B. The effect of testosterone on cardiovascular risk factors in men with type 2 diabetes and late-onset hypogonadism treated with metformin or glimepiride. Pharmacol Rep 2016;68:75-9. [Crossref] [PubMed]
- Wan H, Zhang K, Wang Y, et al. The Associations Between Gonadal Hormones and Serum Uric Acid Levels in Men and Postmenopausal Women With Diabetes. Front Endocrinol (Lausanne) 2020;11:55. [Crossref] [PubMed]
- Krysiak R, Gilowski W, Okopien B. The effect of testosterone on cardiometabolic risk factors in atorvastatin-treated men with late-onset hypogonadism. Pharmacol Rep 2016;68:196-200. [Crossref] [PubMed]
- Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab 2008;93:2230-3. [Crossref] [PubMed]
- Kurahashi H, Watanabe M, Sugimoto M, et al. Testosterone replacement elevates the serum uric acid levels in patients with female to male gender identity disorder. Endocr J 2013;60:1321-7. [Crossref] [PubMed]
- Marinello E, Riario-Sforza G, Marcolongo R. Plasma follicle-stimulating hormone, luteinizing hormone, and sex hormones in patients with gout. Arthritis Rheum 1985;28:127-31. [Crossref] [PubMed]
- Rosen R, Tomer Y, Carel R, et al. Serum 17-beta-estradiol and testosterone levels in asymptomatic hyperuricaemic men. Clin Rheumatol 1994;13:219-23. [PubMed]
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Available online: https://wwwn.cdc.gov/nchs/nhanes/AnalyticGuidelines.aspx. Accessed 25 July 2020.
- Centers for Disease, Control, and Prevention. National Health and Nutrition Examination Survey. Survey Methods and Analytic Guidelines. Available online: https://www.cdc.gov/nchs/nhanes/index.htm. Accessed 20 June 2020.
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/wp-content/uploads/2016/11/DoH-Oct2013-JAMA.pdf. Accessed 21 October 2020.
- Centers for Disease Control and Prevention (CDC). National center for health Statistics (NCHS). National health and nutrition examination survey laboratory protocol. Standard Biochemistry Profile (BIOPRO_H). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/BIOPRO_H_MET_URIC_ACID.pdf. Accessed 20 June 2020.
- Centers for Disease Control and Prevention (CDC). National center for health Statistics (NCHS). National health and nutrition examination survey laboratory protocol. Sex Steroid Hormone - Serum (TST_H). Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/labmethods/TST_H_MET_Total_Estradiol_and_Total_Testosterone.pdf. Accessed 20 June 2020.
- Khera M, Adaikan G, Buvat J, et al. Diagnosis and Treatment of Testosterone Deficiency: Recommendations From the Fourth International Consultation for Sexual Medicine (ICSM 2015). J Sex Med 2016;13:1787-804. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). National center for health Statistics (NCHS). NHANES 2015-2016 Laboratory Data Overview. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/overviewlab.aspx?BeginYear=2015. Accessed 2 October 2020.
- Defining Adult Overweight and Obesity. Available online: https://www.cdc.gov/obesity/adult/defining.html. Accessed 20 June 2020.
- World Health Organization. Haemoglobin Concentration for the Diagnosis of Anaemia and Assessment of Severity. Vitamin and Mineral Nutrition Information System. 2011 (online). Available online: http://www.who.int/vmnis/indicators/haemoglobin.pdf. Accessed 20 June 2020.
- Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487-95. [Crossref] [PubMed]
- Tsilidis KK, Rohrmann S, McGlynn KA, et al. Association between endogenous sex steroid hormones and inflammatory biomarkers in US men. Andrology 2013;1:919-28. [Crossref] [PubMed]
- Ren Z, Zhao A, Wang Y, et al. Association between Dietary Inflammatory Index, C-Reactive Protein and Metabolic Syndrome: A Cross-Sectional Study. Nutrients 2018;10:831. [Crossref] [PubMed]
- Leong JY, Blachman-Braun R, Patel AS, et al. Association between polychlorinated biphenyl 153 exposure and serum testosterone levels: analysis of the National Health and Nutrition Examination Survey. Transl Androl Urol 2019;8:666-72. [Crossref] [PubMed]
- Yang H, Li D, Song X, et al. Joint associations of serum uric acid and ALT with NAFLD in elderly men and women: a Chinese cross-sectional study. J Transl Med 2018;16:285. [Crossref] [PubMed]
- Phan H, Richard A, Lazo M, et al. The association of sex steroid hormone concentrations with non-alcoholic fatty liver disease and liver enzymes in US men. Liver Int 2020. Epub ahead of print. [Crossref] [PubMed]
- Duca Y, Calogero AE, Cannarella R, et al. Erectile dysfunction, physical activity and physical exercise: Recommendations for clinical practice. Andrologia 2019;51:e13264. [Crossref] [PubMed]
- National Health and Nutrition Examination Survey. Tutorials. Module 3: Weighting. Available online: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx. Accessed 20 June 2020.
- King C, Lanaspa MA, Jensen T, et al. Uric Acid as a Cause of the Metabolic Syndrome. Contrib Nephrol 2018;192:88-102. [Crossref] [PubMed]
- Sharaf El Din UAA, Salem MM, Abdulazim DO. Uric acid in the pathogenesis of metabolic, renal, and cardiovascular diseases: A review. J Adv Res 2017;8:537-48. [Crossref] [PubMed]
- Corona G, Monami M, Rastrelli G, et al. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med 2011;8:272-83. [Crossref] [PubMed]
- Perez-Ruiz F, Aniel-Quiroga MA, Herrero-Beites AM, et al. Renal clearance of uric acid is linked to insulin resistance and lower excretion of sodium in gout patients. Rheumatol Int 2015;35:1519-24. [Crossref] [PubMed]
- Facchini F, Chen YD, Hollenbeck CB, et al. Relationship between resistance to insulin-mediated glucose uptake, urinary uric acid clearance, and plasma uric acid concentration. Jama 1991;266:3008-11. [Crossref] [PubMed]
- Borbelyova V, Domonkos E, Babickova J, et al. Does long-term androgen deficiency lead to metabolic syndrome in middle-aged rats? Exp Gerontol 2017;98:38-46. [Crossref] [PubMed]



