
Effects of BAP1, Ki-67 index, and Id-1 in patients with clear cell renal carcinoma and their correlation with clinical features and prognosis
Introduction
Renal cell carcinoma (RCC) is the most common renal malignant tumor, and clear cell renal carcinoma (CCRCC) is the most common histopathological type of renal cancer (1). As a multigene-related tumor, the molecular biologic mechanism of CCRCC occurrence and development has not yet been elucidated. Although treatments, such as radical nephrectomy or nephron-preservation therapies, have achieved satisfactory results among CCRCC patients, some patients still suffer from recurrence, metastasis, and even death after surgery. Therefore, it is important to find effective monitoring biomarkers and interventions to improve the prognosis of CCRCC patients (2).
Breast cancer 1 (coded by BRCA1 gene) is a tumor suppressor, with mutations or deletions in various tumor tissues. BRCA1-associated protein 1 (BAP1) is the translation product of BRCA1 and belongs to the family of deubiquitinating enzymes. The target proteins of BAP1 participate in the occurrence and development of cancer through various pathways; therefore, the expression and function of CCRCC has attracted wide attention (3). Ki-67 is a nuclear protein involved in the transcription of ribosomal RNA, and is currently often used as a sensitive indicator of cell proliferation activity (2). The Ki-67 index is widely used, and it has been noted that the expression of Ki-67 can predict the biologic behavior and prognosis of RCC (4). Inhibitor of differentiation-1 (Id-1) is involved in cell cycle regulation and plays an important role in the process of cell differentiation. Id-1 has been the focus of study for the prognosis of cancer (5). In the present study, the expression of BAP1, Ki-67, and Id-1 protein in CCRCC patients and their relationship with clinical characteristics and prognosis were studied, with the aim of developing treatment modalities for CCRCC. We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/tau-20-1258).
Methods
General information
A total of 45 CCRCC patients who were diagnosed and treated at our hospital from January 2016 to January 2018 were included in the present study. Of these patients, 23 were male and 22 were female. The patients were aged 27–70 years, with an average age of 46.75±5.16 years. A total of 36 cases involved the renal capsule, and 9 involved the renal pelvis; the tumor diameter was 1.63–13 cm, with an average diameter of 5.79±1.53 cm. Based on the TNM staging standard of RCC established by the International Anti-Cancer Association, 12 cases were pT1 stage, 13 cases were pT2 stage, 13 cases were pT3 stage, and 7 cases were pT4 stage. Tumor tissue and normal renal mucosa tissue adjacent to the tumor (>3 cm away from the CCRCC tissue) were collected, and defined as the CCRCC group (n=45) and the adjacent tissue group (n=45), respectively. There were no differences in general data, such as sex or age, between the two groups (P>0.05).
The inclusion criteria were as follows: (I) all patients had clear evidence of pathological diagnosis and were diagnosed with CCRCC; (II) patients did not undergo radiotherapy or chemotherapy before enrollment; and (III) all patients agreed with the study protocols, provided signed informed consent, and were approved by the medical ethics committee of West China Hospital. The exclusion criteria were as follows: (I) patients with hematological diseases; (II) patients who did not agree with the study protocols; (III) patients with severe mental illness or dementia who were unable to participate; (IV) patients with other primary malignant tumors other than CCRCC; and (V) patients with rheumatic immunity or connective tissue disease. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
Research methods
The clinical data of all patients were collected, and the expression of BAP1, Ki-67, and Id-1 protein were compared in CCRCC and adjacent tissues. Survival and death for all patients were recorded during the follow-up period. The correlation between BAP1, Ki-67 index, and Id-1 protein level, and the various clinical characteristics of the CCRCC patients, were then analyzed. The Kaplan-Meier survival curve was drawn to study the effect of BAP1, Ki-67 index, and Id-1 protein on the prognosis of patients, and multiple logistic regression analysis was used to analyze the risk factors affecting the prognosis of CCRCC patients.
Main reagents and instruments
Anti-BAP1 polyclonal antibody, horseradish peroxidase-conjugated secondary antibody of goat anti-rabbit immunoglobulin antibody, and ready-to-use rapid immunohistochemistry Max Vision kit were purchased from Fuzhou Maixin Biotechnology Development Company (China). Primary antibody for Id-1 was purchased from Sigma (USA); the ready-to-use EnVision kit was purchased from Dako Biological Products (Denmark) and used at a dilution of 1:200, and sections for positive control were also provided in this company. All the sections were observed under an optical microscope (Olympus, Japan). The human adult skin keratinocyte (HaCaT) cell line was purchased from the American Standard Biological Collection (USA).
BAP1, Ki-67 index, Id-1 protein detection
The collected tissues were cut into 4 µm-thick slices, and immunohistochemical staining was performed according to the instructions of the detection kits. Slices incubated with phosphate-buffered saline (PBS) instead of the primary antibody was used as negative control, and the positive control was the known positive sections of the corresponding antibody.
Ki-67 index detection
The area with high-staining cell density was magnified at 400× to count a total of 600 positive cells, and patients were divided into two groups according to a Ki-67 index of ≤10 or >10. The Ki-67 index was defined as the result of the number of positive cells/600 tumor cells.
BAP1 and Id-1 protein detection
The presence of clearly located brown or tan particles in the tumor cells was defined as positive expression. Ten randomly selected images were analyzed under high power field, and scored according to staining depth and the proportion of positive cells in the field: 0 score, no coloring; 1, <25% light; 2, 25–50% brown; and 3, >50% brown. The score of immune response was multiplied by the 2 scores; scores of 0–3 points were negative (–), and scores of >3 were positive (+). Histochemical staining results were independently read and scored by at least two senior pathologists.
Polymerase chain reaction (PCR)
The DNA of the patients was extracted from the resected specimens. After purity detection and quantitative analysis, the DNA was modified with sodium bisulfite, purified and recovered, and stored at −20 °C for later use. The primers for the promoter region of BAP1, Ki-67 and Id-1 genes were from the published literature (6), and synthesized by Dalian Bao Biological Company. And the methylation primers: (forward) 5'-TCGTGGTAACGGAAAAGCGC-3' and (reverse) 5'-AACGAACTCACGCCGCGCAA-3'; Non-methylated primers: (forward) 5'-TT-GAGAGGTTGTTGTTGTTTAGTGG-3' and (reverse) 5'-AACAAACTCACAC-CACACAA-3'. The total reaction system is 25 µL, and the reaction conditions were as follows: pre-denaturation at 95 °C for 10 min, denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s for methylated primer; annealing at 55 °C for 30 s for non-methylated primer, then extension at 72 °C for 30 s, for a total of 40 cycles, and final extension at 72 °C for 10 min. The take 5 µL reaction product to run the 1.8% agarose gel electrophoresis with EB staining and finally analyzing the gel imaging.
Follow-up methods
In the present study, follow up commenced following the operation until January 2020 or death. The average follow-up time for all patients was 20.31±2.66 months. Patients were followed up mainly by patients coming to the hospital for reexamination (telephone follow-up was used in special cases). In the present study, overall survival (OS) was defined as the length of time that CCRCC patients were alive after diagnosis.
Statistical methods
Data were analyzed using SPSS version 18.0 (IBM, USA), and the measurement data were expressed as the mean ± standard deviation (
Results
Expression of BAP1, Ki-67, and Id-1 protein in CCRCC and adjacent tissues
The negative rate of BAP1 in the CCRCC group was significantly higher than that in the adjacent tissue group (P<0.05). There were more patients in the CCRCC group with a Ki-67 index >10 than in the adjacent tissue group, and the Id-1-positive rate in the CCRCC group was higher than that in the adjacent tissue group (P<0.05) (Table 1, Figure 1).

Full table
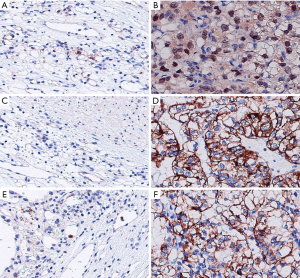
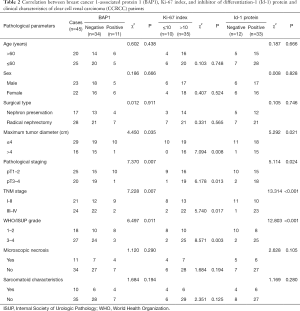
Correlation between BAP1, Ki-67 index, and Id-1 protein and the clinical characteristics of CCRCC patients
The findings indicated that the expression of BAP-1, Ki-67, and Id-1 protein were not correlated with age, sex, surgical method, microscopic features of necrosis, and sarcomatoid characteristics of CCRCC patients (P>0.05), but was correlated to tumor diameter, pathological stage, TNM stage, and WHO/ISUP classification (P<0.05) (Table 2).
Full table
Prognosis of the 45 CCRCC patients
By the last follow up, 17 of 45 CCRCC patients had died, with a mortality rate of 37.78% and a survival rate of 62.22%.
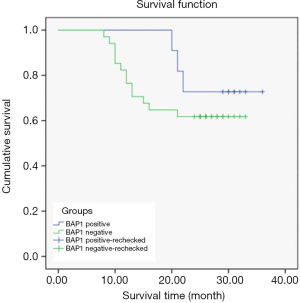
Relationship between BAP1 and prognostic survival of CCRCC patients
A total of 20 patients survived in the BAP1-negative group and 14 died. In the BAP1-positive group, 8 patients survived and 3 died.
Kaplan-Meier survival curves differed according to BAP1 expression level. The survival time of the BAP1-negative group was, on average, 25.09±1.76 months, and was 31.91±2.02 months in the BAP1-positive group, indicating that the average OS of patients in the BAP1-negative group was shorter than that in the positive group (P<0.05) (Figure 2).
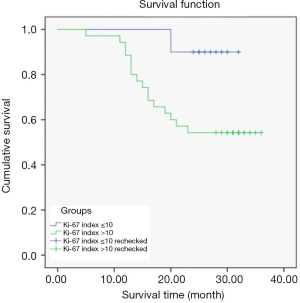
Relationship between Ki-67 index and prognostic survival of CCRCC patients
In the Ki-67 index ≤10 group, 9 patients survived and 1 died; in the Ki-67 index >10 group, 19 patients survived and 16 died.
The Kaplan-Meier survival curves differed according to Ki-67 index expression level. The average survival time of patients in the Ki-67 index ≤10 group was 30.80±1.14 months, and 26.40±1.84 months in the Ki-67 index >10 group, indicating that the average survival time of CCRCC patients was significantly shorter in the Ki-67 index >10 group than the Ki-67 index ≤10 group (P<0.05) (Figure 3).
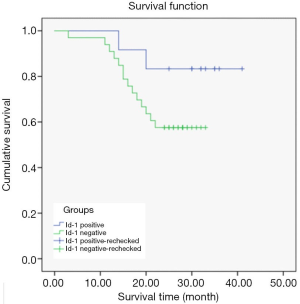
Relationship between Id-1 protein and prognostic survival of CCRCC patients
In the Id-1-negative group, 10 of 12 patients survived; in the Id-1-positive group 18 of 33 patients survived.
The Kaplan-Meier survival curves significantly differed depending on Id-1 protein expression level. The average survival time of patients in the Id-1-negative group was 37.00±2.61 months, and 25.55±1.60 months in the Id-1 protein-positive group, indicating that the average survival time of patients in the Id-1-negative group was significantly longer than that in the Id-1-positive group (P<0.05) (Figure 4).
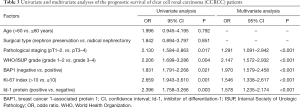
Univariate and multivariate analyses of the prognostic survival of CCRCC patients
The results of the univariate and multivariate analyses showed that factors, such as pathological staging, WHO/ISUP classification, BAP1 negativity, Ki-67 index >10, and Id-1 protein positivity, were independent risk factors affecting the prognosis of CCRCC patients (P<0.05) (Table 3).
Full table
Discussion
CCRCC is the most common histopathological type of renal cancer. Related studies have found that the survival rate of CCRCC patients is mainly affected by pathological staging and tumor cell grading. In addition, molecular indicators and their prognostic significance are still the focus of current research.
BAP1 is a deubiquitinating enzyme, which contains an 80.4-kDa nuclear localization protein and ubiquitin carboxy-terminal hydrolase domain (7). BAP1 appears to be a tumor suppressor in cultured cells in vitro, and its deubiquitinating enzyme domain and nuclear localization sequence are the main pathways of BAP1 in inhibiting cell growth. Shankar et al. found that 47% of uveal melanomas are inactivated by BAP1 protein mutations (8). They reported that BAP1 regulates a variety of cellular pathways related to tumorigenesis and closely related to tumor metastasis. The results of previous sequencing studies have shown that there are base substitutions at the BAP1 mRNA site, and the mutation of BAP1 gene occurs in 15% of the CCRCC cases, and the mutation of BAP1 gene was considered as a pathogenic factor of CCRCC (9). The findings of the present study indicate that the BAP1 protein in cancer tissues of CCRCC patients was mainly negatively expressed, and the expression of BAP1 and Ki-67 was related to the tumor diameter, pathological stage, TNM stage, and WHO/ISUP grade of the CCRCC patients (P<0.05), indicating that the BAP1 protein can inhibit cell proliferation and the metastasis of cancer cells. The Kaplan-Meier survival curve was further used to analyze its impact on the prognosis of patients. The average OS of patients in the BAP1-negative group was shorter than that in the BAP1-positive group (P<0.05), indicating that BAP1-negative expression is also an independent risk factor affecting CCRCC patients (P<0.05). This indicates that the overexpression of BAP1 may be an important way to promote the progression and deterioration of CCRCC patients, and it is expected to be a good biologic target for controlling CCRCC progression.
Immortal proliferation is one of the hallmarks of malignant tumors. Ki-67 is a proliferation-related nuclear antigen involved in the transcription of ribosomal RNA that can reflect cell proliferation activity (10). In normal cells, Ki-67 is a sign of the late-stage cell cycle. The mRNA level of Ki-67 is highest around the G2 phase, while its protein level increases throughout the cell cycle and reaches a peak during mitosis (11). At the same time, ki-67 was found to play a key role in mitosis by regulating recombinant chromatin, promoting epithelial mesenchymal transformation and thus inducing the occurrence and development of CCRCC. The relationship between its expression and prognostic factors and pathological stage has also been confirmed by several studies. The Id-1 protein belongs to the helix-loop-helix protein family and has the same characteristics as oncogenes, which inhibit cell differentiation and promote cell proliferation, thereby playing a role in tumor occurrence and invasion (12). At present, the well-defined mechanisms of the Id-1 protein in promoting tumor progression include downregulating the downstream MAPK signals of thrombotic constrictor gene (tsp-1), upregulating phosphorylation levels of Raf and mitogen-activated protein kinase (MEK1/2), thereby promoting the expression of genes on early growth response. In recent years, Ki-67 and Id-1 have been used as sensitive indicators to assess prognosis in a variety of malignant tumors (13,14). However, there are few studies on the expression of Ki-67 index and Id-1 protein in patients with different clinical characteristics and prognoses (15). Therefore, in the present study, we studied the expression of the Ki-67 index and Id-1 protein in CCRCC patients and their correlation with clinical features and prognosis. The results showed that the majority of CCRCC patients were those with Ki-67 index >10 and a high expression of Id-1 positivity in cancer tissues (P<0.05). The average OS of patients in the Ki-67 index >10 group and the Id-1-positive group was shorter than those in the Ki-67 index ≤10 group and the Id-1-negative group (P<0.05), respectively, indicating that Ki-67 index and Id-1 protein are closely related to the development and prognosis of CCRCC. These results suggest that Ki-67 and Id-1 may be potential biomarkers for clinical prognoses.
Risk factors affecting the prognosis of CCRCC patients are very complex, and differ among regions (16). The results of the present study showed that pathological staging, WHO/ISUP grade, negative expression of BAP1, Ki-67 index >10, and positive expression of Id-1 protein were all independent risk factors affecting the prognosis of CCRCC patients (P<0.05). These findings indicate that patients with adverse prognostic factors should be treated differently to those without. Effective measures and comprehensive treatment should be adopted for patients with unfavorable prognoses to further improve the survival rate of patients.
In CCRCC patients in the present study, the BAP1 expression level decreased, and the Ki-67 index and Id-1 protein expression levels increased, indicating that the abnormal expression levels of BAP1, Ki-67, and Id-1 protein are involved in the occurrence and development of CCRCC, and are closely related to the prognosis of CCRCC. These indicators can be used as molecular biomarkers for predicting the prognosis of CCRCC patients, as well as potential targets for tumor treatment.
Acknowledgments
Funding: This research was supported by Science and Technology Department of Sichuan (2019YFS0223).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-1258
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-1258
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-1258). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). All patients agreed with the study protocols, provided signed informed consent, and the study were approved by the medical ethics committee of West China Hospital.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Swami U, Nussenzveig RH, Haaland B, et al. Revisiting AJCC TNM staging for renal cell carcinoma: quest for improvement. Ann Transl Med 2019;7:S18. [Crossref] [PubMed]
- Wang L, Cai W, Kong W, et al. Plasma fibrinogen as prognostic predictor in patients with metastatic renal cell carcinoma receiving target therapy. Transl Cancer Res 2018;7:1384-92. [Crossref]
- Zhao H, Zhao H, Xia X, et al. MicroRNA-599 targets high-mobility group AT-hook 2 to inhibit cell proliferation and invasion in clear cell renal carcinoma. Mol Med Rep 2018;17:7451-9. [Crossref] [PubMed]
- Wentink MQ, Verheul HMW, Pal SK, et al. Phase I Study of dalteparin in combination with sunitinib in patients with metastatic clear cell renal carcinoma. Clin Genitourin Cancer 2017;S1558-7673(17)30201-X.
- Chen J, Zhang F, Wang D, et al. Prognostic ability of DNA-binding protein inhibitor ID-1 expression in patients with oral squamous cell carcinoma. Oncol Lett 2020;19:3917-22. [PubMed]
- Watchorn RE, Calonje E, Taibjee SM. Germline BRCA1-associated protein 1 mutation presenting as BAP1 inactivated melanocytic nevi in a child of a father with fatal paraganglioma. Pediatr Dermatol 2018;35:e316-e318. [Crossref] [PubMed]
- Gao S, Sun H, Cheng C, et al. BRCA1-Associated Protein-1 Suppresses Osteosarcoma Cell Proliferation and Migration Through Regulation PI3K/Akt Pathway. DNA Cell Biol 2017;36:386-93. [Crossref] [PubMed]
- Shankar GM, Santagata S. BAP1 mutations in high-grade meningioma: implications for patient care. Neuro Oncol 2017;19:1447-56. [Crossref] [PubMed]
- Owen D, Sheffield BS, Ionescu D, et al. Loss of BRCA1-associated protein 1 (BAP1) expression is rare in non-small cell lung cancer. Hum Pathol 2017;60:82-5. [Crossref] [PubMed]
- Cozzi I, Oprescu FA, Rullo E, et al. Loss of BRCA1-associated protein 1 (BAP1) expression is useful in diagnostic cytopathology of malignant mesothelioma in effusions. Diagn Cytopathol 2018;46:9-14. [Crossref] [PubMed]
- Bersanelli M, Brunelli M, Gnetti L, et al. Pazopanib as a possible option for the treatment of metastatic non-clear cell renal carcinoma patients: a systematic review. Ther Adv Med Oncol 2020;12:1758835920915303. [Crossref] [PubMed]
- Weitzel JN, Neuhausen SL, Adamson A, et al. Pathogenic and likely pathogenic variants in PALB2, CHEK2, and other known breast cancer susceptibility genes among 1054 BRCA-negative Hispanics with breast cancer. Cancer 2019;125:2829-36. [Crossref] [PubMed]
- Del Rosario Taco Sanchez M, Soler-Monsó T, Petit A, et al. Digital quantification of KI-67 in breast cancer. Virchows Arch 2019;474:169-76. [Crossref] [PubMed]
- Hatem L, McIntire PJ, He B, et al. The role of BRCA1-associated protein 1 in the diagnosis of malignant mesothelioma in effusion and fine-needle aspiration cytology. Diagn Cytopathol 2019;47:160-5. [Crossref] [PubMed]
- Parrotta R, Okonska A, Ronner M, et al. A Novel BRCA1-Associated Protein-1 Isoform Affects Response of Mesothelioma Cells to Drugs Impairing BRCA1-Mediated DNA Repair. J Thorac Oncol 2017;12:1309-19. [Crossref] [PubMed]
- Sharma GP, Geethadevi A, Mishra J, et al. Phosphorylation of BRCA1-associated protein 1 as an important mechanism in the evasion of tumorigenesis: A perspective. Cancer Translational Medicine 2019;5:25-9. [Crossref]
(English Language Editor: R. Scott)


