The clinical utility of testicular prosthesis placement in children with genital and testicular disorders
Introduction
Testicular prosthesis placement constitutes a useful and important adjunctive component for managing children with many testicular disorders. Conditions occurring in childhood such as unilateral or bilateral testis absence (monorchia, bilateral anorchia), acquired testis loss from trauma, cancer or spermatic cord torsion, or removal of atrophic testes following herniotomy or orchidopexy are common conditions where prosthesis placement may be considered. Placement in children with various disorders of sexual development undergoing masculinizing genitoplasty constitutes another group. Though these prostheses are, of course functionless, clinical experience has shown that they are extremely beneficial in creating a more normal male body image and in preventing/relieving psychological stress in males with a missing testicle.
The history regarding development and use of testicular prostheses is of interest, from both historical as well as scientific perspectives. A variety of materials have been used over the years to create these prostheses, including metal (vitallium), rubber, plastic, polyurethane, glass and silicone, to name only some (1). Modern-day history dates to the introduction of a silicone-shell liquid silicone filled prosthesis introduced by Lattimer et al. in 1973 (2). This prosthesis was the first to have a more natural, compressible feel and was widely used over the next 20 years though it was noted at times that this prosthesis might leak liquid silicone. In 1993 concerns emerged regarding the safety of silicone implants of all types due to a suspicion of associated connective tissue disorders, and the U.S. Food and Drug Administration (FDA) mandated cessation of manufacture of all implants till further documentation of their safety and efficacy was confirmed. Their concerns are cited in detail below as they represent an insight into issues involved with the contemporary evolution of testicular implants. Additionally, an excellent review paper summarizes this interesting long history as well as elaborating on the use of testicular prostheses in general (1).
FDA testis prosthesis announcement [1993]
The FDA today proposed that manufacturers of testicular implants be required to submit scientific data to show that these products are safe and effective.
Testicular implants, which are made of silicone, are intended for cosmetic purposes. They are commonly used to correct congenital abnormalities in infants and toddlers who are born without one or both testicles. They are also used in men who have had one or both testicles removed because of cancer or other diseases or who have lost one or both testicles due to injury. An estimated 1,000 are implanted yearly.
“We need to make sure these devices are safe and effective,” said FDA Commissioner David A. Kessler, MD. “Therefore, we are proposing that companies submit data, just as we did for breast implants.”
Testicular implants are pouches that are placed in the scrotum. They are made of solid or gel silicone and have a silicone covering. Some types are coated with polyurethane foam.
These implants were on the market prior to the Medical Device Amendments of 1976, which gave FDA regulatory authority over devices. Like other pre-amendment devices, testicular implants were allowed, under the law, to remain on the market with the understanding that FDA would later require manufacturers to demonstrate their safety and effectiveness.
Although some information on the risks and benefits of testicular implants is available, there is not enough scientific evidence to determine whether the benefits outweigh the risks.
The agency’s safety concerns regarding the implants involve the lack of adequate information in these areas:
The incidence of leakage, hardening of surrounding tissue and rupture: the silicone gel in these implants may leak into adjacent tissue, causing problems similar to those seen with breast implants.
The long-term effectiveness of the implants: reported problems of unknown frequency and origin include infection, pain, discomfort, erosion of the device and its migration to other parts of the scrotum and abdomen. It is also not known how often these complications require corrective surgery.
The potential for long-term adverse effects, such as cancer, immune-related connective tissue disorders and reproductive problems: this type of information is particularly important because many of the implant users are young.
The immediate and long-term psychological benefits of the implants, such as patient satisfaction and improved self-image and psychological outlook.
If today’s proposal is made final, manufacturers planning to continue marketing testicular implants will be required to submit a Premarket Approval Application demonstrating the safety and effectiveness of these products as a condition for keeping them on the market.
FDA’s call for safety and effectiveness data on testicular implants is part of the agency’s ongoing review of pre-1976 devices. In addition to requiring safety and effectiveness data on silicone gel breast implants, FDA recently proposed calling for safety and effectiveness data on saline breast implants and will soon do the same for inflatable penile implants, heart bypass blood pumps and cranial electro-therapy stimulators.
Today’s proposal, which is being published in the Jan. 13 Federal Register, provides for a 60-day comment period. Comments may be submitted to Dockets Management Branch, HFA-305, Rm 1-23, 12420 Parklawn Dr., Rockville, Md. 20857.
FDA is one of the eight public health service agencies within the U.S. Department of Health and Human Services.
For the next several years testis implants were not manufactured and therefore were unavailable for implantation for pediatric and adult patients. Extensive clinical investigations were undertaken subsequently in order to meet the FDA’s requests. The results of these studies eventually demonstrated their safety and absence of any statistical increase in diseases of concern, and both the FDA as well as the U.S. Institute of Medicine issued statements in this regard, allowing silicone prosthesis manufacture and implantation to resume.
During this time development of various additional prosthetic devices occurred. Compelled by the FDA to assess the safety and effectiveness of these devices, two sequential studies were undertaken, the first to assess a new silicone shell saline filled implant, and a subsequent to assess a silicone shell silicone gel filled (“elastomer”) implant (3). Review of the details of these studies indicate many unique and interesting aspects of the science and features of modern day testis implant devices:
Results of the safety and effectiveness of a new saline-filled testicular prosthesis [1998-1999]
A 5-year multicenter, prospective clinical trial of a new saline filled silicone shell testis prostheses (Figure 1) was undertaken in 18 centers across the U.S., including both men and boys missing one or both testis. All patients had formal autoimmune and urologic evaluation before and after prosthesis placement. Adverse events and effectiveness were carefully assessed in all patients as well as additional outcome measures assessing quality of life with three validated psychological instruments.

Among 149 patients (76 pediatric) who completed the study, there was no evidence confirming any symptoms of autoimmune disease during the study. Major complications included device extrusion in three patients (2%) and device migration in one (0.7%). All extrusions occurred in pediatric patients having prosthesis placement through a scrotal incision. The reoperation rate was 2% for these issues. Minor complications reported were discomfort or pain (9% overall, but only 2% was deemed device-related pain), allergies or sinusitis (5%), scrotal swelling (3%) and hematoma, numbness, keloid and mild prosthesis migration. No patient was noted to develop a connective tissue disorder clinically or by questionnaire during a 1 year follow-up.
The scores on 2 of 3 validated, psychological quality of life instruments were stable or improved significantly (e.g., the Body Esteem Scale, and the Body Exposure in Sexual Activities Questionnaire) after the prosthesis was placed. Overall this study demonstrated significant increases in well being in the implant patients (“improved self-esteem, physical attractiveness and behavior and feelings during sexual activity”). Amongst the pediatric patients in particular, statistically significant evidence of improvement over baseline evaluations was seen in the Rosenberg Self-Esteem Scale (3).
The study concluded that the saline filled, testis prosthesis was safe and well tolerated. In addition, the study showed that by validated self-esteem measures there is an improvement in quality of life in patients who receive such implants. Though there were previous reports alluding to these benefits, this was the first study done validating the quality of life benefits of testis implant surgery in a prospective studied manner. Because initial review of the study indicated superior results, the study was discontinued prematurely, though follow-up of these patients continued for an additional 5 years. These saline-filled prostheses remain available presently and are the only testicular prosthetic devices available in the U.S. at the present time (see below).
Results of the safety and effectiveness of a new silicone gel-filled (elastomer) testicular prosthesis [2001]
In this multi-center prospective controlled study done at 10 investigating centers over a 1 year period, a new silicone gel-filled testis prosthesis (Figure 2) was evaluated to determine the safety and effectiveness of the device. Safety was assessed by collection of all adverse device placement related events, also comparing these with the data from the previous saline-filled prosthesis study. Efficacy was measured using the Patient Assessment Questionnaire, a previously validated quality of life instrument. Of the 55 patients assessed at 1 year (20 children), the adverse event rate was 2.7%. One patient was explanted because of scrotal infection and was successfully re-implanted without subsequent problem. Patient Assessment Instrument (PAI) satisfaction results for the subject’s perception of their genitals scored a mean of 4.6±0.7 out of 5 (P<0.001). Ninety-three percent of patients agreed, or mostly agreed that “all in all I am glad I had the implant surgery”*.
In my experience this prosthetic device was the most natural feeling prosthesis, also benefitting from the utility of its five different sizes. Unfortunately, after the study was completed the device was never produced because FDA requests for further manufacturing studies were deemed to be economically not feasible.
During these years an additional device was devised consisting of a semi-solid ovoid carved from a silicone block. These devices were described by the FDA as “silicone blocks which may be fashioned for the correction and treatment of esthetic defects”, but were precluded from being labeled as a “testicular prosthesis”. Currently in the U.S., these two devices are the only ones available and approved for cosmetic testicular replacement.
Implantation technique
Choice of incision
There are several incisions utilized for prosthesis placement, each with varied benefits and disadvantages (Figure 3).
Trans-scrotal placement through a mid-line or transverse scrotal incision is frequently used in adults allowing direct easy hemi-scrotal placement and layered closure. In children the thin pre-pubertal skin does not allow for a sound closure over the device and a small but significant risk of prosthesis erosion and extrusion exists. In the saline prosthesis study cited above, all erosions/extrusions occurred in children where the prosthesis was placed trans-scrotally.
Supra-scrotal prosthesis placement (“wink” incision) precludes this problem (4). A curvilinear convex incision at the juncture of the scrotum and abdominal pubic skin allows for a reasonably direct placement but layered closure of the scrotal dartos, subcutaneous fat and Scarpa’s fascia and skin (Figure 3). In young children an inguinal incision may be utilized, finding a pathway into the scrotum, then closing the scrotal neck above the prosthesis thereby insuring that prosthesis erosion will not occur because of the distance between incision and prosthesis. In older boys at times there is a long distance between an inguinally placed incision and a dependant scrotal position making this incision more disadvantageous.
A unique “compromise” incision is sometimes of great use, especially where an existing prosthesis is to be removed and replaced with a larger adult size, or where a very under-developed scrotum exists precluding satisfactory dependent scrotal placement and symmetry. In this instance a curvilinear incision is made at the junction of the scrotal and peri-genital pubic skin on the contra-lateral opposite side from the site of the prosthesis placement. The incision is deepened and a space is developed in that hemi-scrotal sac, retracting the testicle and spermatic cord laterally. Utilizing the existing prosthesis to be removed (or an underlying finger) for elevation, the midline raphe is divided and the existing prosthesis is removed. Both hemi-scrotal sacs are then widened and joined as one single space and the new prosthesis is placed in the appropriated space with or without fixation to the underlying dependant dartos (see below). This “trans-raphe contra-lateral” approach allows for complete dependant positioning of the prosthesis, excellent scrotal symmetry as well as layered closure of the incision site well away from the prosthesis, minimizing any risk of extrusion. I have utilized this approach many times without a single instance of extrusion or ultimate scrotal asymmetry.
Device preparation and placement
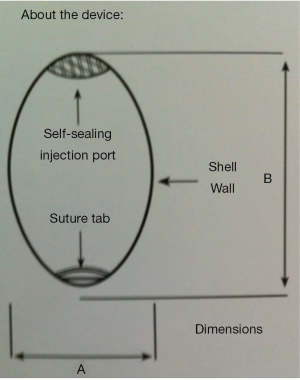
The saline-filled testis prosthesis is available in four sizes. The surgeon chooses the appropriate size based on clinical measurements with an orchidometer; measurement with a centimeter ruler offers a gross estimation of the appropriate dimensions as well (Figure 4). Peri-operative intravenous antibiotic coverage is administered and continued orally for 3 days. After completing the incision a plane is made into the scrotum, which is then progressively dilated by placement of antibiotic-soaked fully opened gauze sponges. These are left in place while the prosthesis is prepared. The device is filled with sterile saline through a self-sealing injection port on the sterile field according to manufacturer’s provided specifications. After insertion it is important to “seat” the prosthesis in the most dependant portion of the previously developed scrotal space. This site can be marked externally with a marking pen and then inverted using a gauze peanut dissector, taking care to keep the previously marked site in place. A suture tab on the inferior aspect of the device may be utilized to fasten the prosthesis to the interior dartos taking extreme care not to perforate the adjacent scrotal skin, which may cause additional risk of infection subsequently. In many cases, however, this suture tab is not needed and the device may be left to find its own placement and position. The entire wound is irrigated with an antibiotic solution and the incision is closed. Though some choose to close the neck of the scrotum over the device to prevent upward displacement, I do not utilized this maneuver routinely as at times it may causes some deformity of the scrotum with upward prosthesis movement noted later only on eventual subsequent healing.
The procedure for placement of the silicone carving block device is similar, though there is no fixation site present on this device.
Complications
Complications related to prosthesis placement are infrequent and may be divided into those related immediately to surgery and those that are later and delayed. The immediate surgically related complications to a great extent may be minimized by careful planning and attention to detail. Choice of incision, especially avoiding a scrotal incision in the thin pediatric scrotum or in previously operated scrotum will minimize the risk of post-operative extrusion. Careful hemostasis and antibiotic wound irrigation will minimize post-operative infection. Less than ideal dependant scrotal placement is the cause of more common complaint and certainly can be minimized with careful attention to placement location. Upward migration of the prosthesis has been described as well. Post-operative scrotal pain (“phantom orchalgia”) occurs in some and it may be difficult to diagnose the cause and to treat.
While immune complications related to silicone shell shedding or silicone leakage were highly suspected and worrisome in the 1990’s subsequent investigations have not validated these concerns and are rarely commented on nowadays. Traumatic rupture remains an infrequent occurrence, however cases of spontaneous non-traumatic rupture have been reported as well, in some instances many years after prosthesis implant (5).
Controversies
Patient choice
It is clear that not all males with an absent testis need to have a testicular prosthesis (6,7). The absence of a testis per se is not an indication since many males are not disturbed by its absence and are perfectly happy with their body image. The patient or parent should make the decision about prosthesis placement with full information provided regarding the benefits and risks. It is noted that there is a wide divergence of feelings among boys and men regarding the desire and need for prosthetic testicle placement.
Age at implantation
Testis prostheses are often placed in adolescence, usually as a result of significant expressed concern about the appearance of his genitals and concerns about body image. During the time when prostheses were unavailable as cited previously, in my practice I cared for three adolescent boys who expressed suicidal ideations because of these above concerns. In more usual circumstances, adolescent boys express a curiosity or less drastic desire, which after consultations leads to a frequent choice to proceed with prosthesis placement.
A more difficult decision is encountered by parents in deciding whether a prosthesis should be placed at an early age. Consider the 1-year old who undergoes orchiectomy for acute testicular torsion. Should a prosthesis be placed at that time? Is there any merit? Is there benefit in placing a prosthesis subsequent to the acute episode, i.e., torsion, yet early-on? Controversy exists regarding early prosthesis placement for an absent testis, and clearly there are advantages and disadvantages, benefits and potential risks. Besides the need for subsequent surgery and anesthesia and for prosthesis change subsequently, as well as the potential risks of infection and extrusion, the effects on psychological well-being remain.
In following these boys with an empty scrotum, as time passes the scrotum usually becomes significantly shrunken and asymmetrical. Prosthesis placement early-on can minimize this in some though this preventative maneuver is not universally successful in accomplishing this goal (Figure 5), which clearly can be helpful in placing a proper size, dependant symmetrical prosthesis in adolescence subsequently. Choice of technique influences this desired outcome: placement of an oversized prosthesis early on leads to initial asymmetry, which at that time is far less noticeable and of less concern than the cosmetic appearance of the empty scrotum, i.e., in boys of high school age having a shrunken empty scrotum at that time. In these circumstances later satisfactory prosthesis placement is also more difficult because of the extreme asymmetry. As a result, in most cases, when feasible I favor prosthesis placement early on, which serves the patient well through puberty at which time an adult prosthesis is substituted.

So clearly, there are advantages and disadvantages: for some parents, early initial synchronous or asynchronous prosthesis placement offers a psychological benefit, for others, this approach is unimportant. Proper counseling and information is essential in helping parents arrive at a personalized meaningful decision.
The small underdeveloped scrotum
As mentioned, even though prosthesis placement may be achieved in an underdeveloped scrotum, the ultimate cosmetic appearance may be poor due to inadequate prosthesis dependency leading to frequent patient dissatisfaction. Little is gained when a prosthesis is successfully placed in the most dependant portion of the scrotum but visually is very high riding compared with the contra-lateral testis because of inadequate ipsilateral scrotal size. This event is a common cause for patient dissatisfaction after surgery.
This difficulty may be averted at times by utilizing the entire scrotum as the reservoir, as mentioned above. Striking symmetry is achieved by this maneuver. In some, staged tissue expansion may be attempted (8), however this is difficult prepubertally due to the thin scrotal skin, risking erosion and extrusion of the expansion device. I have also utilized a single prosthesis to fill the scrotum fully and symmetrically in boys with bilateral testis absence, where two individual smaller prostheses appeared to give a less beneficial cosmetic appearance.
Simultaneous versus delayed placement
The underlying condition as well as prosthesis availability affect the decision whether to place a prosthesis at the time of surgery. When conditions allow and an appropriate pre-operative discussion can take place, i.e., for known absent testes, low-grade neoplasms, orchiectomy for benign disease, etc., a prosthesis may be on hand for simultaneous placement; there is little risk described in using this approach as long as good hemostasis occurs and the scrotal skin is not violated (9,10). In other instances, i.e., for situations of acute testis loss from torsion, trauma, etc., a prosthesis may not be available and pre-operative discussions are usually not undertaken for prosthesis placement, though one can certainly be placed in a staged procedure subsequently.
Placement in previously violated or compromised scrotum
If a previous prosthesis was extruded, previous scrotal surgery occurred, or if radiotherapy to the region severely compromised the scrotal skin, an increased risk of prosthesis extrusion exists. Prosthesis placement is not contra-indicated, however; rather, careful surgical planning and implementation should be undertaken. A supra-scrotal incision should be used for prosthesis placement in these instances. Extreme care needs to be done in dilating the scrotum so as to not perforate or thin the impaired scrotal skin. A prosthesis of suitable size should be utilized. This is not a situation where “the largest size possible” should be utilized.
Conclusions
Testis prostheses provide an important psychological and cosmetic benefit for children with testicular disorders. The high patient/parent satisfaction rate and established psychological benefits and very predictable and excellent safety profile suggest that prosthesis placement should be strongly considered in most children who have appropriate indications of testis loss or absence.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
*Data kindly provided by the Coloplast Corporation, Minneapolis, MN, USA.
References
- Bodiwala D, Summerton DJ, Terry TR. Testicular prostheses: development and modern usage. Ann R Coll Surg Engl 2007;89:349-53. [PubMed]
- Lattimer JK, Vakili BF, Smith AM, et al. A natural-feeling testicular prosthesis. J Urol 1973;110:81-3. [PubMed]
- Turek PJ, Master VA. Testicular Prosthesis Study Group. Safety and effectiveness of a new saline filled testicular prosthesis. J Urol 2004;172:1427-30. [PubMed]
- Libman JL, Pippi-Salle JL, Chan PT. The use of a suprascrotal or 'wink' incision for placing a testicular prosthesis. BJU Int 2006;98:1051-3. [PubMed]
- Floyd MS Jr, Williams H, Agarwal SK, et al. Unilateral spontaneous rupture of a testicular implant thirteen years after bilateral insertion: a case report. J Med Case Rep 2010;4:341. [PubMed]
- Incrocci L, Bosch JL, Slob AK. Testicular prostheses: body image and sexual functioning. BJU Int 1999;84:1043-5. [PubMed]
- Adshead J, Khoubehi B, Wood J, et al. Testicular implants and patient satisfaction: a questionnaire-based study of men after orchidectomy for testicular cancer. BJU Int 2001;88:559-62. [PubMed]
- Lattimer JK, Stalnecker MC. Tissue expansion of underdeveloped scrotum to accommodate large testicular prosthesis. A technique. Urology 1989;33:6-9. [PubMed]
- Bush NC, Bagrodia A. Initial results for combined orchiectomy and prosthesis exchange for unsalvageable testicular torsion in adolescents: description of intravaginal prosthesis placement at orchiectomy. J Urol 2012;188:1424-8. [PubMed]
- Robinson R, Tait C, Clarke N, et al. Is it Safe to Insert a Testicular Prosthesis at the Time of Radical Orchidectomy for Testis Cancer - an Audit of 904 Men Undergoing Radical Orchidectomy. BJU Int 2014. [Epub ahead of print].



