
The synergism of B and T lymphocyte attenuator (BTLA) and cytotoxic T lymphocyte associated antigen-4 (CTLA-4) attenuated acute T-cell mediated rejection and prolonged renal graft survival
Introduction
Kidney transplantation remains the optimal option for patients with end stage renal disease (ESRD) with significant promotion of life quality (1). Great progress has been made in the short-term post-transplant graft survival with the administration of potent immunosuppressants. However, the long-term survival remains suboptimal (2). There have been studies discovered that the occurrence of acute rejection (AR) is associated with the acceleration of long-term renal graft failure (3). In the past three decades, TCMR has been recognized as the major category of AR (4,5) and was attenuated by some classical therapy including induction immunosuppressive therapy (6,7). Nevertheless, the more effective therapy against TCMR is still an interesting problem to be explored (8).
The pathogenesis of acute TCMR is caused by cytotoxic T lymphocytes, activation macrophages as well as NK cell-mediated cytotoxic immune damage. The essence is the activation of T cells under isoantigenic stimulation, IL-2 production and proliferation of allergenic T cells. In addition, IL-2 plays an essential role in the transplantation immune regulation and responds T-cell immune response by activating T cells and promoting the production of cytokines. Studies have revealed that co-stimulatory and co-inhibitory receptors associated with T cell activation and proliferation, which is considered as an important mechanism during the process of TCMR (9), are recognized to influence the graft rejection and survival in organ transplantation (10,11). The BTLA is a recently identified CD28 family member and expressed on the majority of lymphocytes (12,13). A research has reported that exciting anti-BTLA mAb inhibited AR in mice heart transplant model (14). Furthermore, our previous work suggested the BTLA pathway was involved in pathogenesis of AR after kidney transplantation in biopsy-proven patients (15). On the other hand, another co-signaling molecule CTLA-4 blocks CD28 binding with its ligands and prolong long-term allograft survival in several transplantation models (16,17). Belatacept as a high-affinity variant of CTLA-4 Ig, has been applied into kidney transplantation to against AR (18). However, several studies have revealed that Belatacept is associated with a high incidence of AR compared to traditional calcineurin inhibitors therapy, suggesting that blocking CTLA-4-CD80-86 interactions might be limited (19).
Based on this, we hypothesized that the synergism between BTLA and CTLA-4 may inhibit AR by regulating T cell activation. In this paper, we examined the T cell activation and proliferation by mixed lymphocyte reaction (MLR) with BTLA-overexpressed adenovirus and Belatacept, as well as established rat kidney transplantation model, and then analyzed the postoperative pathology to investigate the role of combined BTLA and CTLA-4 in AR following transplantation. We present the following article in accordance with the ARRIVE reporting checklist (available at http://dx.doi.org/10.21037/tau-20-728).
Methods
MLR
MLR cultures were performed in triplicate in 96-well and maintained in complete medium for 48 h (20). Mature dendritic cells (DCs), extracted from the peripheral blood of Wistar rats as stimulators. Sprague Dawley (SD) rat spleen T cells were selected as the responder cells using T cell isolation kit (Miltenyi Biotec, CA, USA) in line with the manufacturer’s protocols. The T cells were previously treated negative-control (NC) vectors (Genechem, Shanghai, China) and normal saline in the MLR + Con group. BTLA overexpression adenovirus (Genechem, Shanghai, China) were pre-transfected in T cells for 48 h before MLR among the MLR + BTLA group and the MLR + BTLA + CTLA-4 group. MLR cultures were supplemented with 10ug/ml Belatacept (Nulojix; Bristol Myers-Squibb, NY, USA) in the MLR + CTLA-4 group and the MLR + BTLA + CTLA-4 group. The Naive, MLR, MLR + Con, MLR + BTLA, MLR + CTLA-4, MLR + BTLA + CTLA-4 groups of MLR cultures were established in vitro.
RNA extraction and quantitative reverse transcription-PCR (qRT-PCR)
Total RNA of MLR cells was isolated by RNA extraction kits (Tiangen, China) and then reverse transcribed to cDNA using the PrimeScript RT Kit (Takara, Japan). The primers were used as follows: BTLA, forward: 5’-ATCCCAGATGCTACCAATGC-3’, reverse: 5’-TTGGGAGTTTGTCCTGGAAC-3’; CTLA-4, forward: 5’-AGTGACCCAACCTTCAGTGG-3’, reverse: 5’-AAGCCCAACGTGTTCTTCAC-3’; GAPDH, forward: 5’-GGCCTTCCGTGTTCCTACC-3’, reverse: 5’-CGCCTGCTTCACCACCTTC-3’. Quantitative RT-PCR was performed by SYBR Green PCR kit (TaKara Biotechnology, Japan). Then, 2−ΔΔCt method was adopted for the analysis of target gene expression.
Western blot analysis
The proteins of MLR cells were extracted, equal amounts of protein samples underwent SDS-PAGE by 10% gels and were blotted onto a polyvinylidene fluoride (PVDF) membrane (Billerica, MA, USA), Then, the membranes were incubated by primary antibodies included anti-rat BTLA (1:1,000, Abbiotec, CA, USA), anti-rat CTLA-4 (1:1,000, Santa Cruz CA, USA) or GAPDH (1:1,000, CST, Inc., USA) before incubation with HRP-conjugated secondary antibody (1:4,000, CST, Inc., USA). Finally, the chemiluminescent reaction was performed to reveal the bands, and the band intensity and volume were clarified by Image Lab software (Bio-Rad CA, USA).
Animals and Kidney transplantation model
The MHC mismatched SD and Wistar rats (Male, 200–250 g) (Charles River Laboratory, Beijing, China) were reared in Nanjing Medical University Animal Center with water and normal diet. All animal experiments were approved by the Animal Care and Use Committee of Nanjing Medical University (Ethical Approval Number: IACUC1601140-1), in compliance with ethical guidelines of Nanjing Medical University animal ethical and welfare committee for the care and use of animals. Rat kidney transplantation model were established by two experienced microsurgeons according to the techniques described previously (21). The left kidney of syngeneic or allogeneic donor was chipped and positioned on the left side of the SD recipient with kidneys removed. Five rats of each group were harvested to collect kidney tissues and peripheral blood on day 3 and day 7 following surgery. The day 0 samples were collected from the normal SD rats. To observe the graft function and survival, six recipients of each group were enrolled in and the end survival of grafts was defined as anuria (22).
Experimental groups
AR model was established by transplanting Wistar donor kidney to SD rat. AR recipients were assigned randomly to five groups: (I) the Allo group without intervene; (II) the Allo + Con group with pre-treated negative-control-vector and normal saline 10 mg/kg daily post-transplant; (III) the Allo + BTLA group, the SD recipient was injected with the BTLA-overexpression adenovirus 48 h before transplantation; (IV) the Allo + CTLA-4 group, Belatacept was administrated subcutaneously at dose of 60 mg/kg/per day at 0 day (day 0) and 4 days (day 4) after kidney transplantation; (V) the Allo + BTLA + CTLA-4 group with BTLA-overexpression adenovirus pretreated and Belatacept administrated. Among the syngeneic (Syn) group, surgery was performed between the SD rat donor and SD rat recipient. The graft function of rat recipients was analyzed by measuring the serum creatinine level (SCr) making use of the Creatinine Kit (Jiancheng BI, Nanjing, China) at each time point (day 0 to day 14).
Histology
The renal graft tissues were fixed in 10% formalin solution and then paraffin embedded. The tissues were sectioned at 4 µm and stained with hematoxylin and eosin (HE) according to standard protocols. T cell mediated rejection was evaluated in a two-blinded manner based on the Banff 2017 score. Based on previous work, the severity of AR was assessed semi-quantitatively with a score value of 0= normal, 1= borderline change, 2= IA, 3= IB, 4= IIA, 5= IIB, and 6= III (23,24).
Immunohistochemistry (IHC) staining
Immunohistochemical staining was made to measure the expression of CD3 in graft sections as previously described (25,26). The CD3 antibody (CST, Inc., USA) as well as secondary antibody was used. We captured randomly 8 images under high-power field from each specimen, then used the integrated optical density (IOD) value calculated by Image Pro Plus 5.0 software (Media Cybernetics MD, USA) to express the relative quantity.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was operated by specific kits (Cusabio, Wuhan, China) to determine IL-2 concentrations in supernatants of MLR (n=3 each group) and recipient serum 100 µL at day 7 following renal transplant (n=5, each group). All procedure was performed strictly according to the manufacturer’s protocols.
Flow cytometry
Bromodeoxyuridine (BrdU) incorporation was used to measure T cell proliferation by BrdU Flow Kit (BD Pharmingen, NJ, USA). Peripheral blood (50 µL) of rat recipients (n=5, each group) at day 7 after transplantation was collected and stained with APC-labeled antiCD3 fluorochrome-conjugated antibody (eBioscience, CA, USA). Flow cytometry was determined by Gallios flow cytometer (Beckman Coulter, USA) and analyzed with FlowJo Software (Tree Star, Ashland, OR).
Statistical analysis
Comparisons among groups were subjected to student’s t-tests. Evaluation of graft survival was compared using the log-rank test. All data were expressed as the mean ± standard deviations (SDs) from at least three independent experiments. Analyses were performed using SPSS 21.0 software (SPSS, Inc., Chicago, IL, USA). P values of less than 0.05 were considered statistically significant.
Results
Combination of overexpressed BTLA and CTLA-4 inhibit activation and proliferation of T lymphocytes
Firstly, BTLA and CTLA-4 expression intervened by BTLA-overexpression adenovirus and Belatacept on T cells was measured using western blot and qRT-PCR. As shown in Figure 1A and B, the expression of BTLA and CTLA proteins were upregulated induced by BTLA-overexpression adenovirus and Belatacept (the BTLA + CTLA-4 group) compared with the naive T cell (the normal group).
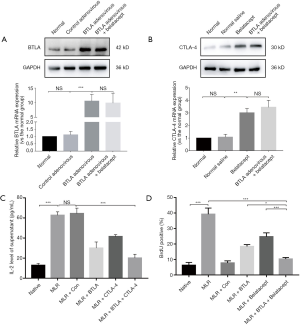
To observe the activation of T cell, we examined the IL-2 level by ELISA and found that the supernatant IL-2 level rose in the MLR and the MLR + Con group compared to the naive T cell after stimulation (Figure 1C). Additionally, the secretion of IL-2 reduced significantly in the MLR + BTLA, MLR + CTLA-4 group and especially in the MLR + BTLA + CTLA4 group. By BrdU flow analysis (Figure 1D), a prominently decreased percentage of positive stained cells was showed in the MLR + BTLA + CTLA-4 group compared with the MLR + Con group, suggesting that combined overexpressed BTLA and CTLA-4 could inhibit T cell proliferation of MLR in vitro effectively.
Establishment of the AR model after kidney transplantation
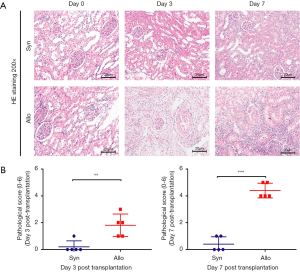
We established the rat kidney transplant model (n=5 for each group) to show the process of AR. Figure 2A shown ischemia reperfusion injury as well as renal tubule edema without graft rejection were observed at day 3 and day 7 after transplantation in the Syn group. By the contrast, serious renal tubular injury, interstitium monocyte infiltration and intimal arteritis were found at day 3, especially at day 7 in the Allo group. The pathological classification according to the Banff 2017 score revealed the occurrence of AR at day 3 and day 7 after transplantation (Figure 2B).
Combined overexpressed BTLA and CTLA-4 attenuated the acute TCMR after kidney transplantation and improved the graft function and prolonged the graft survival
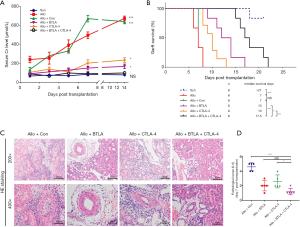
We overexpressed BTLA and CTLA-4 expression in the model as described in Methods. Results of SCr detection from the serum samples among each day showed that recipients of the Allo and the Allo + Con group had taller SCr level than those in the Syn group, but no difference found between the Allo and the Allo + Con group. On the contrary, a significantly lower SCr level existed in the Allo + BTLA + CTLA-4 group in contrast to the Allo + Con group (Figure 3A, P<0.05). Recipients in the Allo + BTLA + CTLA-4 group had a prolonged graft survival time compared with the Allo + Con group (17.5 vs. 7 days, median survival time) (Figure 3B). Our data exhibited the graft tissues of the Allo + BTLA group showed remarkly reduced renal injury with monocyte infiltration compared with the Allo + Con group, which displayed acute TCMR at day 7 similar to the Allo group in Figure 2A. Furthermore, kidney tubulitis, interstitium monocyte infiltration and intimal arteritis reduced in the Allo + BTLA + CTLA-4 group than that in the Allo + Con group (Figure 3C). The pathological score shed light to a reduction of TCMR following renal transplant in the Allo + BTLA + CTLA-4 group in contrast to the Allo + Con group (Figure 3D).
Combined overexpressed BTLA and CTLA-4 reduced the numbers of T cell in peripheral blood and graft and down-regulated serum IL-2 level
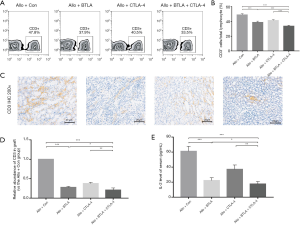
To explore the influence of BTLA and CTLA-4 on the post-transplant T cells number in peripheral blood and graft, we use the flow cytometry and immunohistochemical staining to analyze the expression of CD3+ T cells. As shown in Figure 4A and B, the proportion of CD3+ cells in peripheral total lymphocyte were less on 7 days after transplantation from the Allo + BTLA + CTLA4 group compared to the Allo + Con group, which were rose compared to the Syn group. Based on IHC analysis, infiltration of CD3+ cells in graft were decreased in the Allo + BTLA and the Allo + CTLA-4 group compared to the Allo + Con group, and in the Allo + BTLA + CTLA-4 group were further reduced (Figure 4C,D). Serum levels of inflammatory cytokines IL-2 were tested at day 7 in recipients with ELISA (Figure 4E). The Allo and the Allo + Con groups showed further release of IL-2. However, significantly decreased release of IL-2 was found in the Allo + BTLA, the Allo + CTLA-4 and the Allo + BTLA + CTLA-4 group compared with the Allo + Con group.
Discussion
The benefits of transplantation for ESRD patients have attracted much attention and excellent progress has been taken in the research area of kidney transplantation (27,28). However, AR remains a major challenge, there is an urgent need for a more effective immunosuppressive theory. Recent reports have engaged the co-signaling pathways play an important role in the activation of T cells (29,30). The co-signaling molecules BTLA and CTLA-4 have similar structure and function (31). But little is known with the effects of the combined co-signaling factors after kidney transplantation. Based on this, we examined the T cell activation and proliferation in MLR with BTLA-overexpressed adenovirus and Belatacept, established rat kidney transplantation model, and then analyzed the postoperative pathology to investigate the role of combined BTLA and CTLA-4 in AR following transplantation.
The data obtained from MLR showed overexpressed BTLA and CTLA-4 inhibited production of IL-2 and proliferation of T cells in vitro and more effectively than single overexpressed BTLA or CTLA-4. The secretion of IL-2 presents a marker of the T cells activation (32) and is considered essential for T cells proliferation (33). These results shed light to inhibitory effect of combined BTLA and CTLA-4 in activation and proliferation of T cells. Then we successfully established the rat graft AR model, in which typical interstitium monocyte infiltration and intimal arteritis were observed in the kidney graft on 3 and 7 days after transplantation. Based on the Banff score, the representations of acute TCMR were more serve critical at day 7 compared to day 3 post-transplant, suggesting a successful TCMR model was established.
We overexpressed BTLA and CTLA-4 by pre-injecting BTLA-overexpression adenovirus before transplantation and continuous intragastric administration of Belatacept after surgery. We cannot see statistical difference between the Allo group and the Allo + Con group in pathology. Similar to the previous work (34), our research found combined overexpressed BTLA and CTLA-4 downregulated the postoperative serum Cr level. In addition, the median survival time was prolonged statistically in the Allo + BTLA + CTLA-4 group compared to the Allo + Con group, the Allo + BTLA and the Allo + CTLA-4 group, suggesting a protection of graft function and survival. In the same way, we also found a remarkable decrease of interstitium monocyte infiltration and especially intimal arteritis in the graft of the Allo + BTLA + CTLA-4 group on 7 days following transplantation, although the less reduce was observed in the Allo + BTLA and the Allo + CTLA-4 group, suggesting a more effective therapy against TCMR via combined overexpressed BTLA and CTLA-4 instead of single-agent therapy.
It is well recognized that the increase infiltration of CD3+ T cells are largely responsible for acute T-cell mediated rejection (TCMR) (35). In our models, combination therapy reduced the number of CD3+ T cell in peripheral blood, leading to a decrease infiltration of T cells in grafts confirmed by IHC. Our data also showed that the level of serum IL-2 level was significantly reduced upon combination therapy. Several studies have suggested that the presence of T cells involve in TCMR and T cells were activated after upregulating IL-2 level (36,37). Given the IL-2 level displayed a decline trend in serum among the Allo + BTLA + CTLA-4 group, it was supposed that the combination therapy may also suppress the activation of T cells, which contributed to the attenuation of TCMR after kidney transplantation. It should be noted that this study has examined the combination therapy on TCMR and found the more effective attenuate effects than separately over-expressed BTLA and CTLA-4. Considering that the side-effects induced by high-lose of single-agent therapy, the combination therapy may be safe and effective. However, the changes of T cells differentiation after combination therapy as well as the specific mechanisms of the attenuation and potential interaction between BTLA and CTLA-4 remains to be determined in further studies.
Conclusions
We demonstrate that combined BTLA and CTLA-4 were capable of suppressing T cell activation in vitro. Subsequently, we providence that the synergism of BTLA and CTLA-4 attenuated acute TCMR after kidney transplantation in rat model, which contributes to the improvement of function and survival in kidney graft. Our research suggests new combined therapeutic opportunities, more effective than single-agent therapy, for co-signaling factors in the prevention of TCMR in kidney transplantation.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China [grant numbers 81900684, 81870512, 81770751, 81570676, 81470981, 81100532], the Standardized Diagnosis and Treatment Research Program of Key Diseases in Jiangsu Province [grant number BE2016791], Project of Jiangsu Province for Important Medical Talent [grant number ZDRCA2016025], the “333 High Level Talents Project” in Jiangsu Province [grant numbers BRA2017532, BRA2016514, BRA2015469], the Open Project Program of Health Department of Jiangsu Province [grant number JSY-2-2016-099] and Jiangsu Province Natural Science Foundation Program [grant number BK20191063].
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at http://dx.doi.org/10.21037/tau-20-728
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tau-20-728
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-20-728). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All animal experiments were approved by the Animal Care and Use Committee of Nanjing Medical University (ethical approval Number: IACUC1601140-1), in compliance with ethical guidelines of Nanjing Medical University animal ethical and welfare committee for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wang Z, Han Z, Tao J, et al. Clinical efficacy and safety of pamidronate therapy on bone mass density in early post-renal transplant period: a meta-analysis of randomized controlled trials. PLoS One 2014;9:e108106. [Crossref] [PubMed]
- Nickel P, Bestard O, Volk HD, et al. Diagnostic value of T-cell monitoring assays in kidney transplantation. Curr Opin Organ Transplant 2009;14:426-31. [Crossref] [PubMed]
- El Ters M, Grande JP, Keddis MT, et al. Kidney allograft survival after acute rejection, the value of follow-up biopsies. Am J Transplant 2013;13:2334-41. [Crossref] [PubMed]
- Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 2018;18:293-307. [Crossref] [PubMed]
- Cherukuri A, Mehta R, Sharma A, et al. Post-transplant donor specific antibody is associated with poor kidney transplant outcomes only when combined with both T-cell-mediated rejection and non-adherence. Kidney Int 2019;96:202-13. [Crossref] [PubMed]
- Takeuchi A, Kato K, Akashi K, et al. Cyclophosphamide-induced tolerance in kidney transplantation avoids long-term immunosuppressive therapy. Int J Urol 2018;25:112-20. [Crossref] [PubMed]
- Martinez-Mier G, Soto-Miranda E, Avila-Pardo SF, et al. Induction Immunosuppressive Therapy Use in Deceased Donor Kidney Transplantation: 11-Year Experience in Veracruz, Mexico. Transplant Proc 2016;48:600-4. [Crossref] [PubMed]
- Ko EJ, Yu JH, Yang CW, et al. Usefulness of valacyclovir prophylaxis for cytomegalovirus infection after anti-thymocyte globulin as rejection therapy. Korean J Intern Med 2019;34:375-82. [Crossref] [PubMed]
- Bobka S, Ebert N, Koertvely E, et al. Is Early Complement Activation in Renal Transplantation Associated with Later Graft Outcome? Kidney Blood Press Res 2018;43:1488-504. [Crossref] [PubMed]
- Long M, Beckwith K, Do P, et al. Ibrutinib treatment improves T cell number and function in CLL patients. J Clin Invest 2017;127:3052-64. [Crossref] [PubMed]
- Ezzelarab MB, Lu L, Shufesky WF, et al. Donor-Derived Regulatory Dendritic Cell Infusion Maintains Donor-Reactive CD4(+)CTLA4(hi) T Cells in Non-Human Primate Renal Allograft Recipients Treated with CD28 Co-Stimulation Blockade. Front Immunol 2018;9:250. [Crossref] [PubMed]
- Gonzalez LC, Loyet KM, Calemine-Fenaux J, et al. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A 2005;102:1116-21. [Crossref] [PubMed]
- Hobo W, Norde WJ, Schaap N, et al. B and T lymphocyte attenuator mediates inhibition of tumor-reactive CD8+ T cells in patients after allogeneic stem cell transplantation. J Immunol 2012;189:39-49. [Crossref] [PubMed]
- Uchiyama M, Jin X, Matsuda H, et al. An agonistic anti-BTLA mAb (3C10) induced generation of IL-10-dependent regulatory CD4+ T cells and prolongation of murine cardiac allograft. Transplantation 2014;97:301-9. [Crossref] [PubMed]
- Wang Z, Yang H, Liu X, et al. Role of B and T Lymphocyte Attenuator in Renal Transplant Recipients with Biopsy-Proven Acute Rejection. Med Sci Monit 2018;24:387-96. [Crossref] [PubMed]
- Hancock WW, Sayegh MH, Zheng XG, et al. Costimulatory function and expression of CD40 ligand, CD80, and CD86 in vascularized murine cardiac allograft rejection. Proc Natl Acad Sci U S A 1996;93:13967-72. [Crossref] [PubMed]
- Judge TA, Wu Z, Zheng XG, et al. The role of CD80, CD86, and CTLA4 in alloimmune responses and the induction of long-term allograft survival. J Immunol 1999;162:1947-51. [PubMed]
- Vincenti F, Larsen C, Durrbach A, et al. Costimulation blockade with belatacept in renal transplantation. N Engl J Med 2005;353:770-81. [Crossref] [PubMed]
- Ville S, Poirier N, Branchereau J, et al. Anti-CD28 Antibody and Belatacept Exert Differential Effects on Mechanisms of Renal Allograft Rejection. J Am Soc Nephrol 2016;27:3577-88. [Crossref] [PubMed]
- Ye Q, Wang L, Wells AD, et al. BAFF binding to T cell-expressed BAFF-R costimulates T cell proliferation and alloresponses. Eur J Immunol 2004;34:2750-9. [Crossref] [PubMed]
- Martins PN. Kidney transplantation in the rat: a modified technique using hydrodissection. Microsurgery 2006;26:543-6. [Crossref] [PubMed]
- Triantos CK, Samonakis D, Thalheimer U, et al. Terlipressin therapy for renal failure in cirrhosis. Eur J Gastroenterol Hepatol 2010;22:481-6. [Crossref] [PubMed]
- Li S, Liang P, Zhao Y, et al. Detection and mechanism of action of ESM-1 in rat kidney transplantation under various immune states. Cell Immunol 2013;283:31-7. [Crossref] [PubMed]
- Zhang J, Zhang H, Wang Z, et al. BTLA suppress acute rejection via regulating TCR downstream signals and cytokines production in kidney transplantation and prolonged allografts survival. Sci Rep 2019;9:12154. [Crossref] [PubMed]
- Wang Z, Han Z, Tao J, et al. Transforming Growth Factor-beta1 Induces Endothelial-to-Mesenchymal Transition via Akt Signaling Pathway in Renal Transplant Recipients with Chronic Allograft Dysfunction. Ann Transplant 2016;21:775-83. [Crossref] [PubMed]
- Zhang J, Zhang H, Qin Y, et al. MicroRNA-200c-3p/ZEB2 loop plays a crucial role in the tumor progression of prostate carcinoma. Ann Transl Med 2019;7:141. [Crossref] [PubMed]
- Zhou J, Cheng H, Wang Z, et al. Bortezomib attenuates renal interstitial fibrosis in kidney transplantation via regulating the EMT induced by TNF-alpha-Smurf1-Akt-mTOR-P70S6K pathway. J Cell Mol Med 2019;23:5390-402. [Crossref] [PubMed]
- Zhang H, Shi G, Hu Q, et al. Transcriptional dissection of differentially expressed long non-coding RNAs and messenger RNAs reveals the potential molecular mechanism after kidney transplantation. Ann Transl Med 2019;7:458. [Crossref] [PubMed]
- Greisen S, Kunder R, Deleuran B. T cell co-stimulatory factors. Rheumatology (Oxford) 2017;56:861-2. [PubMed]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 2013;13:227-42. [Crossref] [PubMed]
- Paulos CM, June CH. Putting the brakes on BTLA in T cell-mediated cancer immunotherapy. J Clin Invest 2010;120:76-80. [Crossref] [PubMed]
- Liu Y, Gao X, Deeb D, et al. Anticancer agent pristimerin inhibits IL-2 induced activation of T lymphocytes. J Exp Ther Oncol 2016;11:181-8. [PubMed]
- Bitar M, Boldt A, Freitag MT, et al. Evaluating STAT5 Phosphorylation as a Mean to Assess T Cell Proliferation. Front Immunol 2019;10:722. [Crossref] [PubMed]
- Truong W, Plester JC, Hancock WW, et al. Combined coinhibitory and costimulatory modulation with anti-BTLA and CTLA4Ig facilitates tolerance in murine islet allografts. Am J Transplant 2007;7:2663-74. [Crossref] [PubMed]
- Parkes MD, Halloran PF, Hidalgo LG. Mechanistic Sharing Between NK Cells in ABMR and Effector T Cells in TCMR. Am J Transplant 2018;18:63-73. [Crossref] [PubMed]
- Chen G, Mi J, Xiao MZ, et al. PDIA3 mRNA expression and IL-2, IL-4, IL-6, and CRP levels of acute kidney allograft rejection in rat. Mol Biol Rep 2012;39:5233-8. [Crossref] [PubMed]
- Tefik T, Ciftci HS, Karadeniz MS, et al. Predictive Value of Interleukin 2 and Interleukin 8 on Early Rejection in Living Related Kidney Transplant Recipients. Transplant Proc 2019;51:1078-81. [Crossref] [PubMed]


