Role of application of tadalafil 5 mg once-daily (≥6 months) in men with erectile dysfunction from six randomized controlled trials
Introduction
Erectile dysfunction (ED) is defined as the inability to achieve and maintain an erection sufficient for satisfactory sexual intercourse (1). Although ED is not life-threatening, it is closely related to the patient’s quality of life, sexual relationship and family stability (2). It is also an early warning sign of many physical diseases, such as stroke, tumors, Parkinson’s disease, and spinal cord disease, etc. (3). Estimates suggest that ED affects approximately 150 million men worldwide, and the rates of ED are expected to double by the year 2025 (4,5). In view of the current trend, the research and treatment of ED is very important and meaningful. A number of therapeutic options are available to treat men with ED. Of these, oral phosphodiesterase type 5 (PDE5) inhibitors are recommended as a first line of therapy (6-8).
Tadalafil can be absorbed rapidly and possesses a longer half-life of 17.5 hours than other PDE5 inhibitor (9,10). The prolonged half-life of tadalafil and effective steady-state serum concentration makes it ideally suited for daily dosing (11,12). Although PDE5 inhibitors are easy to use, men should take these oral agents when they wish to engage in sexual activity, and therefore, they should remain temporally linked to treatment. For some patients and their partners, planning sexual activity around drug intake is troublesome. The concept of daily administration of PDE5 inhibitors was also established to provide an alternative treatment option that is closer to a natural sexual life.
Several clinical studies have evaluated the safety and efficacy of tadalafil dosed once daily in patients with ED. Compared with taking on demand, it can eliminate the embarrassment of taking medication before intercourse, increase male confidence, avoid psychological barriers between husband and wife, especially for patients with severe ED. In patients with ED, tadalafil 2.5 and 5 mg administered once daily for 12 weeks were well tolerated and significantly improved erectile function compared with placebo (13). Although tadalafil has been shown to be safe and well tolerated when taken on demand for up to 2 years (14), long-term safety and efficacy data for tadalafil dosed once daily has rarely been reported.
This analysis was to assess the efficacy and safety of taking tadalafil 5 mg once-daily for at least 6 months in the treatment of ED, which may resolve some of the current debate over use of the drug. We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/tau-19-809).
Methods
Search strategy
With Embase (2003 to Jun 2019), MEDLINE (2003 to Jun 2019), and Cochrane Central Register of Controlled Trials, we undertook a retrieval to examine published randomized controlled trials (RCTs), which investigated long-term tadalafil (5 mg once-daily) for ED at least 6 months of treatment. The searching terms were following: “ED”, “tadalafil”, “once-daily”, “long-term” and “RCT”. Additionally, we screened the retrieved references for each article.
Inclusion criteria and trial selection
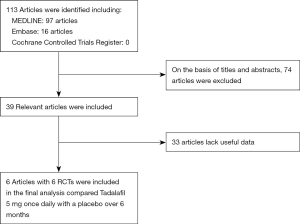
The met criteria of RCTs: (I) tadalafil (5 mg once-daily) in treating ED; (II) accurate data could be analyzed; (III) the study can be accessed. If the same study is published in a different journal, we will include the latest one. However, if the same group of researchers conducts multiple different trials on a group of subjects, we will include each study. A flow chart was used to show the selection process of the study (Figure 1).
Quality assessment
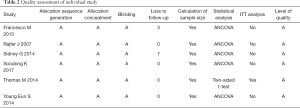
We used the Jadad scale to determine the quality of the retrieved RCTs (15). The methodological quality of each study was assessed based on how patients were assigned to the various departments of the study, the concealment of the distribution procedure, blinding, and data loss due to wear and tear. Then, according to the plan published in the Cochrane Handbook for Systematic Reviews of Interventions v.5.1.0 (16), the studies were then classified qualitatively. Each article is evaluated and assigned according to three quality classification criteria: (I) meting all quality criteria: low risk of bias; (II) partially met or ambiguous: moderate risk of bias; or (III) rarely met or not involved: high risk of bias. All authors participated in the RCTs quality assessment and resolved the differences through discussion.
Data extraction
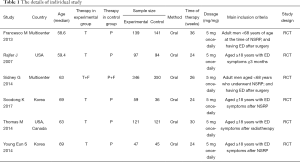
This systematic review and meta-analysis was not required ethical approval. All six articles included were obtained from open databases. The following details from individual studies: (I) regimen patients received; (II) design of study and size of sample; (III) name of the RCT; (IV) the study of country; (V) total international index of erectile function (IIEF) domain, international index of erectile function questionnaire intercourse satisfaction (IIEF-IS) domain, IIEF overall satisfaction (IIEF-OS) domain, question 3 (IIEF-5 Q3) and question 4 (IIEF-5 Q4), IIEF sexual encounter profile (SEP) question 2 (SEP2), question 3 (SEP3), question 4 (SEP4), question 5 (SEP5), treatment-emergent adverse events (AEs), headache, back pain, dyspepsia and discontinuations due to AEs. It should be noted that both IIEF-5 and IIEF-EF belong to the IIEF category, so we choose the IIEF domain as an indicator.
Statistical analysis and meta-analysis
The meta-analysis of comparable data was carried out using RevMan v.5.1.0 (Cochrane Collaboration, Oxford, UK). We used a fixed-effect and random-effects model to estimate the mean difference (MD) of continuous data and the odds ratio (OR) of the categorical results, with a corresponding 95% confidence interval (CI) (17). When the analysis results showed that the P value was >0.05, the study was considered to be homogeneous, we would use fixed-effect model. Otherwise, the random effect model was chosen to analyze the data.
Results
Characteristics of the individual studies
The study retrieved 113 articles. Based on the above inclusion criteria, after reading the titles and abstracts of these articles, 74 studies were deleted. Thirty-three articles lack valid data. Finally, six articles (18-23) were included in the study to access the long-term (≥6 months) effect of tadalafil (5 mg once-daily) in treating male with ED (Figure 1). Table 1 showed the characteristics of each trail.
Full table
Quality of the individual studies
All six trials were randomized controlled studies, both of which addressed randomized and double-blind processes. All studies included adequate patients (Table 2). All of the study has a reasonable assessment for the quality of each trail.
Full table
Efficacy
IIEF domain
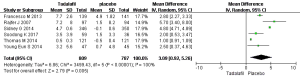
Six RCTs with 1,596 participants (Figure 2) were identified. Based on our analysis, the random-effects estimate of MD=3.09, and 95% CI: 0.92–5.26 (P=0.005). The result shows that long-term tadalafil 5mg once-daily showed greater increases in the IIEF domain compared with control group.
IIEF-IS
Figure 3 shows the results of the change in the IIEF-IS. The random-effects estimate of the MD=1.42, 95% CI: 0.04–2.80 (P=0.04). The result shows that experimental group showed greater increases in the IIEF-IS domain compared with control group.
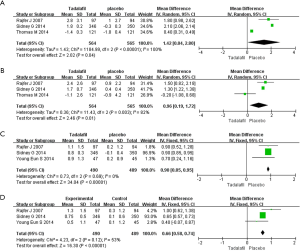
IIEF-OS
Three RCTs met the inclusion criteria (Figure 3). The fixed-effects estimate of the MD=0.96, 95% CI: 0.19–1.72 (P=0.01). The result shows that experimental group showed greater increases in the IIEF-OS domain compared with control group.
IIEF-5 Q3
Three studies failed to report the data. Three RCTs, representing 979 participants were identified (Figure 3). Heterogeneity test indicated P=0.69, the random effect model was selected for statistical analysis. The forest plots showed the SD=0.90 and 95% CI: 0.85–0.95 (P<0.00001). The result shows that experimental group showed greater improvement in the IIEF-5 Q3 compared with control group.
IIEF-5 Q4
Three RCTs including 979 participants were identified (Figure 3). No heterogeneity was found among the trials in our analysis (Figure 3), the fixed-effects model was chosen for the statistical analysis. The pooled estimate of MD was 0.66, and the 95% CI was 0.58–0.74 (P<0.00001). Our meta-analysis suggests that experimental group showed greater improvement in the IIEF-5 Q4 compared with control group.
SEP2, SEP3, SEP4 and SEP5
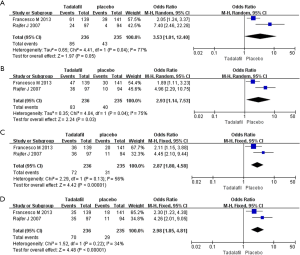
Two RCTs including 471 participants were identified (Figure 4). Based on our analysis, we concluded that (I) the pooled estimate of odds ratio (OR) of the SEP2 was 3.53, and the 95% CI was 1.01–12.40 (P=0.05); (II) the OR of the SEP3 was 2.93, and the 95% CI was 1.14–7.53 (P=0.03); (III) the OR of the SEP4 was 2.87, and the 95% CI was 1.80–4.58 (P<0.00001); (IV) the OR of the SEP5 was 2.98, and 95% CI was 1.85-4.81 (P<0.00001). Our meta-analysis suggests that the experimental group showed greater improvement in the SEP 3, SEP 4 and SEP 5 in comparison with placebo.
Safety
Treatment-emergent AEs (TEAEs)
Six RCTs with 1,596 participants reported the TEAEs data (Figure 5). We adopted the fixed-effects model (The pooled OR 1.25, 95% CI: 0.99–1.58, P=0.06). The results showed no difference in the incidence of TEARs between the two groups.
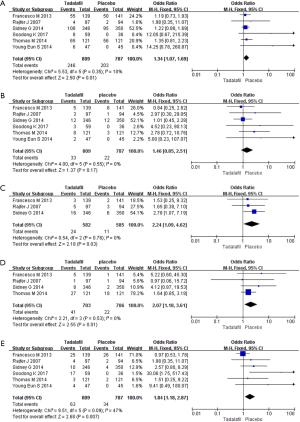
Discontinuation due to AEs
Six RCTs with 1,596 participants were included. The random-effects estimate of the MD=1.29, 95% CI: 0.79–2.09 (P=0.31). In terms of the incidence of discontinuation due to AEs, the result showed that tadalafil and placebo are similar.
Headache, back pain and dyspepsia
Six RCTs including 1,596 participants were involved in the analysis for headache. The pooled estimate of the OR was 1.46, and the 95% CI was 0.85 to 2.51 (P=0.17). Three RCTs included data on back pain (OR 2.24; 95% CI: 1.09–4.62; P=0.03), and 4 RCTs included data on dyspepsia (OR 1.82; 95% CI: 1.01–3.26; P=0.05). These results indicate that there is no significant difference between the two groups in terms of headache, back pain and indigestion (Figure 5).
Discussion
ED is a common disease that affects men worldwide and is associated with increased age, underlying cardiovascular, neurological, psychological, and even some urology surgery (24). Male sexual arousal is a complex process that involves the brain, hormones, emotions, nerves, muscles and blood vessels. ED can result from a problem with any of these (25,26). In addition, reconstructive radical prostatectomy (NSRP) has become the main treatment for localized prostate cancer, and ED is a common sequela of NSRP (27,28). PDE5 is a phosphodiesterase that is cGMP specific and highly expressed in corpus cavernosum smooth muscle cells. PDE5 inhibitors enhance erectile function by maintaining adequate cGMP levels in the corpus cavernosum and supplying vascular smooth muscle cells during sexual stimulation, increasing the expansion of the cavernous sinus (29,30). PDE5 inhibitor therapies, which include tadalafil, sildenafil and vardenafil, have provided nonaggressive, productive and well-tolerated treatments for ED (31).
The fact that tadalafil is effective in treating ED once a day has been confirmed or a consensus has been established. So, the effectiveness of long-term use of tadalafil is also certain, as evidenced by our research. Our study suggests that long-term tadalafil 5mg once- daily is efficacious for ED patients over 6 months. Patients take orally tadalafil 5 mg once-daily showed greater improvement in the IIEF domain. Long-term low-dose tadalafil is one of the first-line treatments for repairing damaged vascular endothelium and promoting long-term improvement of erectile function (32). Our present study also confirmed this point. What is worth mentioning is that in the early stages of treatment, low-dose tadalafil cannot achieve sufficient erectile stiffness as fast and clearly as sildenafil (33). For most ED patients seeking treatment, rapid and satisfactory sexual intercourse erection hardness is the key to success. Sildenafil has a quick onset of action of 30 min after the initial dose, a duration of action of 4–6 h and a maximum duration of 12 h (34). Therefore, sildenafil in early treatment may be necessary. However, it may lead to higher costs, the uncertainty of the agreement and possible side effects.
Recent global trends indicate that drug expenditures have become a major financial burden for ED patients due to the increasing use of PDE5 inhibitors. Cost analysis showed that the direct cost of tadalafil was higher than that of sildenafil (35). ED was not life-threatening and there was no physical pain, so patients were willing to pay for treatment, which also proved the value of tadalafil. Therefore, the economics of the long-term once-daily tadalafil is a potential problem that deserves further discussion.
The meta-analysis involved a total of 1,596 patients in six publications. The long-term tadalafil once-daily group perform a better in terms of the international index of erectile function-erectile function (IIEF) domain (P=0.005) for the treatment of ED compared with the placebo group. Safety assessments including discontinuations due to adverse events (AEs) (P=0.31) and treatment-emergent AEs (P=0.06) indicated that the long-term tadalafil group had a better tolerate. The analysis elucidates that tadalafil 5mg once-daily showed a good effect after the treatment of at least 6 months relative to the control group with fewer side effects.
Among these TEAEs, generally in the diagnosis of investigators adverse events related to drugs studied are back pain, dyspepsia, myalgia and headache. In laboratory parameters, vital signs or electrocardiogram readings no clinically significant changes were observed for patients in all of the six RCTs. Safety assessments including discontinuations due to AEs (P=0.31) and treatment-emergent AEs (P=0.06) indicated that the long-term tadalafil group had a better tolerate. There were no deaths or serious adverse events in the six studies, and most of the adverse events were well-tolerated. The effectiveness of tadalafil in the treatment of ED has been proven, and the focus of our analysis is primarily on the safety of long-term use of tadalafil, which has not been well studied and confirmed before.
PDE5 has been found to have relatively high expression in only a few limited tissues, such as the corpus cavernosum, the reproductive system of the pulmonary blood vessels and smooth muscle, platelets and visceral smooth muscle (36,37). Therefore, the most common side effects of tadalafil are headache, flushing, dyspepsia, nasal congestion, conjunctivitis and hypotension, which are also common to PDE5 inhibitors, but these side effects are usually transient and reversible (38-40).
From a scientific and daily clinical practice perspective, the results of this analysis are very important and may provide some treatment recommendations for ED patients and may resolve some of the current debate over use of the drug. In addition, unpublished research data is not included in the analysis and these factors may lead to bias. Individual nature of study design, patient inclusion and exclusion criteria and eventual patient demographics including severity may also led to bias. In addition, it is worth mentioning that the cost of drugs is a factor that cannot be ignored, especially for those patients who need to take drugs for a long time but have to bear the cost, which is a considerable financial burden. Therefore, we hope that more effective studies and long-term data will be included in our analysis in the future, so as to better prove the effectiveness and safety of long-term tadalafil 5 mg once-daily in treating ED.
Conclusions
The analysis elucidates that tadalafil 5 mg once-daily showed a good effect after the treatment of at least 6 months relative to the control group with fewer side effects.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA checklist. Available at http://dx.doi.org/10.21037/tau-19-809
Conflict of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-809). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Irwin GM. Erectile Dysfunction. Prim Care 2019;46:249-55. [Crossref] [PubMed]
- Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J 2017;93:679-85. [Crossref] [PubMed]
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83-90. [Crossref] [PubMed]
- Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int 1999;84:50-6. [Crossref] [PubMed]
- McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res 2000;12 Suppl 4:S6-11. [Crossref] [PubMed]
- Porst H, Rosen R, Padma-Nathan H, et al. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int J Impot Res 2001;13:192-9. [Crossref] [PubMed]
- Eardley I, Mirone V, Montorsi F, et al. An open-label, multicentre, randomized, crossover study comparing sildenafil citrate and tadalafil for treating erectile dysfunction in men naive to phosphodiesterase 5 inhibitor therapy. BJU Int 2005;96:1323-32. [Crossref] [PubMed]
- Vardenafil. In: LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD), National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012.
- Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag 2008;4:1315-30. [Crossref] [PubMed]
- Francis SH, Corbin JD. Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep 2003;4:457-65. [Crossref] [PubMed]
- McMahon C. Efficacy and safety of daily tadalafil in men with erectile dysfunction previously unresponsive to on-demand tadalafil. J Sex Med 2004;1:292-300. [Crossref] [PubMed]
- McMahon C. Comparison of efficacy, safety, and tolerability of on-demand tadalafil and daily dosed tadalafil for the treatment of erectile dysfunction. J Sex Med 2005;2:415-25; discussion 425-7. [Crossref] [PubMed]
- Hatzichristou D, Gambla M, Rubio-Aurioles E, et al. Efficacy of tadalafil once daily in men with diabetes mellitus and erectile dysfunction. Diabet Med 2008;25:138-46. [Crossref] [PubMed]
- Montorsi F, Verheyden B, Meuleman E, et al. Long-term safety and tolerability of tadalafil in the treatment of erectile dysfunction. Eur Urol 2004;45:339-44; discussion 344-5. [Crossref] [PubMed]
- Moher D, Jadad AR, Tugwell P. Assessing the quality of randomized controlled trials. Current issues and future directions. Int J Technol Assess Health Care 1996;12:195-208. [Crossref] [PubMed]
- Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:Ed000142. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Rajfer J, Aliotta PJ, Steidle CP, et al. Tadalafil dosed once a day in men with erectile dysfunction: a randomized, double-blind, placebo-controlled study in the US. Int J Impot Res 2007;19:95-103. [Crossref] [PubMed]
- Kim S, Sung GT. Efficacy and Safety of Tadalafil 5 mg Once Daily for the Treatment of Erectile Dysfunction After Robot-Assisted Laparoscopic Radical Prostatectomy: A 2-Year Follow-Up. Sex Med 2018;6:108-14. [Crossref] [PubMed]
- Pisansky TM, Pugh SL, Greenberg RE, et al. Tadalafil for prevention of erectile dysfunction after radiotherapy for prostate cancer: the Radiation Therapy Oncology Group 0831 randomized clinical trial. JAMA 2014;311:1300-7. [Crossref] [PubMed]
- Seo YE, Kim SD, Kim TH, et al. The Efficacy and Safety of Tadalafil 5 mg Once Daily in the Treatment of Erectile Dysfunction After Robot-Assisted Laparoscopic Radical Prostatectomy: 1-Year Follow-up. Korean J Urol 2014;55:112-9. [Crossref] [PubMed]
- Glina S, Roehrborn CG, Esen A, et al. Sexual function in men with lower urinary tract symptoms and prostatic enlargement secondary to benign prostatic hyperplasia: results of a 6-month, randomized, double-blind, placebo-controlled study of tadalafil coadministered with finasteride. J Sex Med 2015;12:129-38. [Crossref] [PubMed]
- Montorsi F, Brock G, Stolzenburg JU, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT). Eur Urol 2014;65:587-96. [Crossref] [PubMed]
- Schout B, Meuleman EJ. Erectile dysfunction and incontinence after prostatectomy. Treating the complications of surgery for prostate cancer. Ned Tijdschr Geneeskd 2012;156:A4667. [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Sadovsky R, Miner M. Erectile dysfunction is a signal of risk for cardiovascular disease: a primary care view. Prim Care 2005;32:977-93. vii. [Crossref] [PubMed]
- Salonia A, Burnett AL, Graefen M, et al. Prevention and management of postprostatectomy sexual dysfunctions. Part 1: choosing the right patient at the right time for the right surgery. Eur Urol 2012;62:261-72. [Crossref] [PubMed]
- Ficarra V, Novara G, Ahlering TE, et al. Systematic review and meta-analysis of studies reporting potency rates after robot-assisted radical prostatectomy. Eur Urol 2012;62:418-30. [Crossref] [PubMed]
- Wespes E, Amar E, Hatzichristou D, et al. EAU Guidelines on erectile dysfunction: an update. Eur Urol 2006;49:806-15. [Crossref] [PubMed]
- Corbin JD, Francis SH. Pharmacology of phosphodiesterase-5 inhibitors. Int J Clin Pract 2002;56:453-9. [PubMed]
- Brant WO, Bella AJ, Lue TF. Treatment options for erectile dysfunction. Endocrinol Metab Clin North Am 2007;36:465-79. [Crossref] [PubMed]
- Wrishko R, Sorsaburu S, Wong D, et al. Safety, efficacy, and pharmacokinetic overview of low-dose daily administration of tadalafil. J Sex Med 2009;6:2039-48. [Crossref] [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404. [Crossref] [PubMed]
- Gong B, Ma M, Xie W, et al. Direct comparison of tadalafil with sildenafil for the treatment of erectile dysfunction: a systematic review and meta-analysis. Int Urol Nephrol 2017;49:1731-40. [Crossref] [PubMed]
- Martin AL, Huelin R, Wilson D, et al. A systematic review assessing the economic impact of sildenafil citrate (Viagra) in the treatment of erectile dysfunction. J Sex Med 2013;10:1389-400. [Crossref] [PubMed]
- Zucchi A, Costantini E, Scroppo FI, et al. The first-generation phosphodiesterase 5 inhibitors and their pharmacokinetic issue. 2019;7:804-17.
- Dolci S, Belmonte A, Santone R, et al. Subcellular localization and regulation of type-1C and type-5 phosphodiesterases. Biochem Biophys Res Commun 2006;341:837-46. [Crossref] [PubMed]
- Ventimiglia E, Capogrosso P, Montorsi F, et al. The safety of phosphodiesterase type 5 inhibitors for erectile dysfunction. Expert Opin Drug Saf 2016;15:141-52. [Crossref] [PubMed]
- Burnett AL. Phosphodiesterase 5 mechanisms and therapeutic applications. Am J Cardiol 2005;96:29m-31m. [Crossref] [PubMed]
- Hellstrom WJ. Current safety and tolerability issues in men with erectile dysfunction receiving PDE5 inhibitors. Int J Clin Pract 2007;61:1547-54. [Crossref] [PubMed]