
Imaging of childhood urologic cancers: current approaches and new advances
Introduction
Urologic tumors in children include a wide variety of neoplasms arising in the kidney, bladder, prostate, vagina, testis, or paratesticular areas. Although each tumor type is relatively rare, collectively they comprise approximately one-tenth of all childhood cancers (1). Appropriate imaging of these patients is critical for accurate staging and surveillance of these tumors, as well as for surgical and/or radiotherapy planning. In addition, techniques that can decrease the time for radiologic studies are helpful to reduce the need for sedation during imaging for very young patients. Finally, given the favorable long-term prognosis for many of these tumor types, efforts to minimize radiation exposure are important to reduce the risk of secondary malignancies.
In this review, we outline both standard and innovative approaches to the imaging of pediatric patients with suspected urologic malignancy. For the three most common anatomic locations, we review the epidemiology, differential diagnosis, initial imaging and staging considerations, surveillance strategies, and controversies and new developments for imaging of tumors. We emphasize the important contribution of imaging to the management of these patients, and how that contribution can be optimized.
Renal tumors
Kidney neoplasms comprise up to 7% of all childhood cancer, with Wilms tumor (nephroblastoma) being by far the most common histology. The age at presentation provides an important first clue in guiding the differential diagnosis for kidney tumors. For example, the incidence of Wilms tumors peaks between ages 2–3 years, with 80% occurring before the age of 5 years (2). In contrast, congenital mesonephric tumor is usually seen in infants, and is the most common kidney neoplasm in the first 3 months of life (3). In the second decade of life, renal cell carcinoma is the most common primary tumor of the kidney (4). Other clues that guide the differential diagnosis include the presence of a known genetic predisposition such as Beckwith-Wiedemann syndrome, or evidence of genitourinary anomalies or other features that may suggest other syndromes seen with Wilms tumor (5). For older children, there is a known association between tuberous sclerosis and angiomyolipoma, as well as von-Hippel-Lindau disease and renal cell carcinoma. Finally, renal involvement does not always mean a primary renal tumor, as some teenagers with lymphoma may have marked kidney involvement.
There are no imaging features that consistently distinguish Wilms tumors from the less common renal malignancies. Ultrasound (US) is commonly used for the initial evaluation of renal tumors, and imaging features associated with a renal origin include a mass that moves with respiration and displaces the renal parenchyma to create the characteristic “claw sign” (Figure 1). The entirety of both kidneys should be thoroughly investigated with imaging, as 10% of Wilms tumors will have multiple lesions within a single kidney, and 5% of patients have bilateral tumors (6,7). Other important imaging findings include the presence of free fluid beyond the cul-de-sac to suggest tumor rupture, metastatic liver lesions, pleural effusions, or intravascular extension into the renal vein, inferior vena cava, and occasionally even right atrium. The use of color Doppler US may be useful to facilitate identification of vascular involvement by tumor.
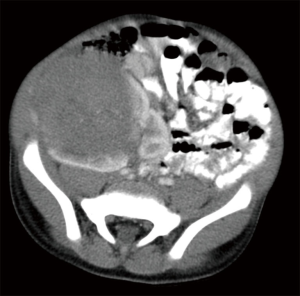
These important features are critical for accurate staging, as preoperative identification of any of the above findings can affect staging and treatment assignment (6). While US is a useful starting point for imaging, all pediatric patients with renal masses should undergo cross-sectional imaging with computed tomography (CT) or magnetic resonance imaging (MRI), as in over half of patients these studies provide additional important information beyond what can be obtained with US (8). For example, contrasted CT or MRI can provide even more definitive information about resectability of tumors and the presence of intravascular tumor, which occurs in 6% of Wilms tumor patients (9). Because vascular extension of tumor greatly increases the surgical complication rate, upfront nephrectomy is usually deferred while patients are treated with neoadjuvant chemotherapy in an attempt to retract the clot and facilitate a safer surgery done later (10).
Synchronous contralateral renal involvement of Wilms tumor is termed stage V disease. This finding is seen in 5% of all Wilms tumor patients, and creates special diagnostic and management issues. These patients are more likely to have genetic predispositions leading to the development of their tumors, as well as histologic evidence of anaplasia associated with aggressive clinical behavior (7). Smaller lesions, as well as benign nephrogenic rests, are often underestimated with renal US, as some may be isoechoic with renal parenchyma. Instead, these lesions may be best appreciated on contrast-enhanced MRI scans done in the nephrogenic phase, and MRI is recommended by the Children’s Oncology Group for evaluation of patients with known or suspected bilateral Wilms tumor (11). In younger patients presenting with bilateral renal tumors, chemotherapy is often initiated even without surgical biopsy. Assessment of response by imaging is done after several weeks of treatment, and guides decisions about biopsy or nephron-sparing surgery at later time points. The focus of treatment is not just on disease control but also preservation of renal function, as both issues can affect long-term survival (7). For older patients with bilateral renal tumors, the diagnosis of lymphoma should be strongly considered.
The preoperative identification of intra-adnominal lymphadenopathy can also help guide nodal sampling, which is standard for all children with Wilms tumor. For patients with renal cell carcinoma, there is a relatively high rate of nodal involvement even with smaller primary tumors, and this may be underestimated by imaging. In a large series of adolescent renal cell cancer, tumor was identified in nearly half of patients who underwent nodal sampling, and 42% of nodes with histologically documented tumor did not meet imaging criteria for malignancy (12).
The extent of evaluation for distant metastatic disease depends on the tumor type. For example, clear cell sarcoma and rhabdoid tumor are rare but aggressive renal malignancies seen in young children, and have a propensity to develop metastases in bone and the central nervous system. Therefore, imaging of these sites is warranted in these diseases, as well as in symptomatic patients with renal cell carcinoma.
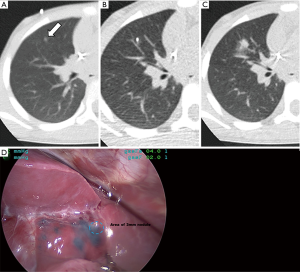
The lungs are the most common site of metastasis in Wilms tumor, and historically patients were considered to have lung involvement if nodules were identified on chest X-ray (CXR). However, CT of the chest is a more sensitive modality for identifying metastatic lung nodules, especially when done preoperatively in awake patient in order to reduce the atelectasis associated with postoperative or sedated imaging studies in young children. Five percent of Wilms tumor patients will have nodules identified only on CT but not conventional CXR (13). Importantly, the improved sensitivity of chest CT comes with decreased specificity, as Ehrlich and colleagues demonstrated that up to one-fourth of patients undergoing biopsy of “CT only” nodules actually had histologically benign lesions (13). A recent report from the Children’s Oncology Group is instructive in how the response assessment of lung nodules following neoadjuvant chemotherapy can improve treatment assignment and outcomes for favorable histology Wilms tumor patients (14). In that study, lung nodules identified on chest CT were considered metastatic disease if they were round, non-calcified, and not located in a pulmonary fissure. Patients with such lung nodules were treated with 6 weeks of three-drug therapy, with 42% showing complete resolution on follow-up CT. These patients had excellent overall survival while avoiding whole lung radiotherapy, which has been associated with long-term cardiopulmonary toxicities. Of the patients whose nodules remained stable or only slightly diminished with neoadjuvant chemotherapy, no histologic evidence of tumor was seen in 17 of the 24 patients undergoing biopsy. These findings again underscore the observation that determining the nature of small lung nodules can be difficult, given that indeterminate lung nodules can be seen on CT scan in as many as one-third of healthy children (15). In certain situations, histologic confirmation of lung nodules can help direct patient care by reducing the need for lung radiotherapy, and resection of even small nodules can often be done with video-assisted thoracoscopic surgery (VATS). This procedure can be greatly facilitated by localizing techniques such as administration of image-guided blue dye (Figure 2) and/or placement of a hook wire to help the surgeon identify these small nodules (16).
Imaging with positron emission tomography (PET) is very helpful for staging renal lymphoma, but has been less beneficial for primary renal tumors. In Europe, patients commonly undergo neoadjuvant chemotherapy prior to nephrectomy. In that setting, PET imaging before and after neoadjuvant chemotherapy did not improve disease staging compared to conventional imaging, and did not predict tumor responsiveness or outcome (17). PET is not routinely used in the management of renal tumors, although perhaps could be of some utility in the evaluation of residual findings at the end of therapy, or at relapse (18). The use of diffusion weighted MRI has been reported to show correlations between the apparent diffusion coefficient measurements and the blastemal component of residual tumors after neoadjuvant chemotherapy (19). However, the applicability of this strategy may be less in North America, where most patients undergo upfront nephrectomy prior to starting chemotherapy.
The surveillance imaging strategy for children following completion of Wilms tumor therapy has been drawn into question based on the concerns of radiation exposure from repeated CT scans. Most relapses occur within 2 years of initial diagnosis, with the lungs being the most common sites (20). Given that the vast majority of children the Wilms tumor are long-term survivors, efforts to reduce radiation exposure are especially important. Previous strategies for surveillance imaging after completed therapy have included either CXR combined with abdominal US, or CT of the chest and abdomen, or alternating modalities. In one large series, 75% of all relapses of favorable histology Wilms tumor were detected by some type of surveillance imaging as opposed to signs and symptoms (21), and patients whose disease was detected by symptoms had a trend toward a worse 5-year overall survival compared to those whose relapse was identified by surveillance imaging (59% vs. 70%, P=0.23). However, although relapse was identified at an earlier time with CT compared to CXR/US, there was no difference in outcome. These results suggest that if routine surveillance imaging of asymptomatic patients with favorable histology Wilms tumor is used, use of CXR/US does not compromise outcome and can reduce radiation exposure. For asymptomatic patients who do undergo surveillance CT, omitting the pelvis does not compromise disease identification, but can reduce radiation exposure by as much as 45% (22).
Finally, surveillance imaging is also utilized in pre-cancerous patients who remain at high risk for the development of Wilms tumor. Given the known association with a variety of cancer predisposition syndromes, guidelines have been developed for following these at-risk patients in a way that balances the benefits of early identification with safe and reasonable utilization of imaging. Specifically, renal USs are recommended every 3 months from the time the predisposition syndrome is diagnosed at least until the 7th birthday (23).
Bladder/prostate tumors
Pediatric bladder tumors are rare, and often present with voiding symptoms or urinary obstruction. The most common malignant bladder tumor in children is rhabdomyosarcoma, which is a high-grade soft tissue sarcoma arising from skeletal muscle precursors. In hollow organs like the bladder, uterus, or vagina, these tumors often grown intraluminally in a grapelike fashion characteristic of the botryoidal subtype. In contrast, rhabdomyosarcoma of the prostate often appears as a solitary growing mass creating an external indention on the bladder. However, many tumors are bulky at initial presentation, making it difficult to determine the precise site of origin. Most rhabdomyosarcomas of the urogenital tract have favorable features such as embryonal histology and absence of FOXO1 gene fusions, although historically tumors arising in the bladder/prostate area have had worse outcomes than other genitourinary sites. Tumors of the bladder/prostate are generally large (two-thirds are >5 cm) but nodal involvement and distant metastases are relatively uncommon (24).
Like renal tumors, the imaging evaluation of bladder tumors often begins with US, which is best performed with a full bladder. If a mass is suspected on US, MRI offers excellent soft tissue contrast resolution for bladder lesions, and T2-weighted images can often help bladder tumors stand out from the urine-filled bladder lumen, the hypointense bladder wall, and the neighboring prostate. Staging for bladder rhabdomyosarcoma involves assessment for regional adenopathy with cross-sectional imaging (CT or MRI), as well as assessment for bone and pulmonary metastases. Functional imaging with PET or PET/CT has now become standard for rhabdomyosarcoma given its utility in identifying nodal, bone, bone marrow, and soft tissue disease (25), and is often used in place of bone scans. For the assessment of pulmonary metastases, CT chest remains the best imaging modality. However, as seen with Wilms tumor, the finding of indeterminate small lung nodules is frustratingly common. One recent review from the European Soft Tissue Study Group proposed management for patients with indeterminate lung nodules (defined as <4 nodules <5 mm, or 1 nodule measuring ≥5 and <10 mm) to be similar to those without nodules provided there was no other evidence of metastatic disease. In that trial, 21% of patients met the definition for indeterminate lung nodules, and these patients had similar survival to those without nodules (26). This data would suggest that the presence of these indeterminate nodules in patients with otherwise localized rhabdomyosarcoma may not require surgical resection or treatment modification.
Other bladder tumor histologies are even more rare in pediatrics, and include inflammatory myofibroblastic tumors (IMTs), which are typically seen at the dome of the bladder. Plexiform neurofibromas may involve the bladder in patients with neurofibromatosis type I, and can present as either diffuse thickening or focal lesions. One characteristic imaging feature is the target sign on T2-weighted imaging, with low signal in the center of a lesion surrounded peripherally by high signal.
Although urothelial neoplasms are common in adults, they are quite rare in children. Lesions such as the papillary urothelial neoplasm of low malignant potential (PUNLMP) or low-grade superficial urothelial carcinoma can be found in adolescent and young adult patients, and often presents with hematuria (27). These neoplasms are best evaluated and treated with transurethral resection via cystoscopy.
In addition to staging, imaging is also used to assess response to therapy. For rhabdomyosarcoma, however, tumor response to induction therapy is not necessarily correlated to overall survival (28). This important finding may be related in some patients to the maturation (rather than disappearance) of rhabdomyoblasts seen as a response to therapy, and serves as a caution against early discontinuation of potentially curative therapy simply because a rapid decrease in tumor size was not achieved. Following completion of treatment for rhabdomyosarcoma, patients are generally followed with periodic cross-sectional imaging of the primary site, as well as CXR. A recent European study including only patients with localized rhabdomyosarcoma questioned the benefit of routine surveillance imaging (29). The majority (61%) of relapses were identified by symptoms rather than schedule surveillance of asymptomatic patients. Outcome for these two groups of patients were similar, challenging the assumption that surveillance imaging saves lives through earlier identification of relapse. For rhabdomyosarcoma as well as other pediatric solid tumors, it is likely that off-therapy surveillance strategies will continue to evolve, eventually utilizing more precise risk stratification or even biomarkers to identify patients in whom early identification of recurrence will improve outcomes.
Because both bladder and renal tumors often arise in young children, consideration of the logistical issues and risks associated with imaging modalities is important. These topics were recently reviewed by Callahan and colleagues (30), who comprehensively contrasted the advantages and disadvantages of CT vs. MRI. CT scans can be performed quickly even in patients who are not fully cooperative, and this modality is superior for assessment of lung metastases. However, the ionizing radiation used in CT scans has raised concerns about the development of second malignancies despite recent attempts to substantially reduce the amount of radiation used with routine scans. On the other hand, MRI does not have ionizing radiation, but the longer study times usually require sedation or anesthesia in young children for an adequate study to be completed. Although it is feasible to provide sedation for these studies, there are short-term risks of procedure-related complications as well as growing concern for long-term cognitive effects of repeated administration of sedation medications in young children. Given questions about whether CT using current pediatric protocols truly increase cancer risk (31), and acknowledging that patients with medical comorbidities (commonly seen in oncology patients) are at increased risk of complications from sedation, some authors suggest that CT without sedation may be the safest course of action in situations when CT and MRI can provide equivalent clinical information (30).
Ongoing work in this area involves several themes, including further improvements in iterative reconstruction algorithms and detection technologies for CT, as well as further reductions in radiation exposures. Increasing the number of patients who can undergo MRI without sedation would help address both of the major safety concerns. This could be accomplished through patient-specific measures such as manipulating the feeding and sleeping schedules of very young patients, creation of an accommodating and friendly environment in the scanner room, and the use of child life specialists who can incorporate movie goggles or use educational videos to better distract or prepare patients (32). In addition, motion artifact can be reduced though the use of mechanical “papoose” devices as well as imaging protocols using more rapidly acquired T2-weighted images and specific motion-sensitive acquisition sequences.
Testicular and paratesticular tumors
Scrotal masses in pediatric and adolescent patients may have a variety of malignant and benign causes. While germ cell tumors are the most common malignant testicular tumor in this age group, the histologic findings and clinical behavior of these tumors are distinct when comparing prepubescent vs. adolescent patients. In young boys, yolk sac tumors are the primary histology seen in tumor registries (33), although reporting bias may result in under-identification of benign lesions such as mature teratoma or epidermoid cysts (34). Other less common and usually benign lesions in younger patients include stromal tumors such as Sertoli cell, Leydig cell, and granulosa cell tumors. Because of the greater chance of benign pathology in younger patients, careful attention to imaging findings and consideration for testicular-sparing procedures is of particular importance in this age group. In contrast, testicular tumors in adolescents are more likely to be malignant, with histologies and biological features that are similar to adults (35).
In adolescents and young adults, seminomas and mixed malignant germ cell tumors are more common, and up to 30% of patients in this age range will have metastatic disease at initial presentation. In addition to germ cell tumors, rhabdomyosarcoma arising in the paratestis from the muscles of the distal spermatic cord can present similarly with a scrotal mass. Unlike rhabdomyosarcomas arising in the bladder/prostate, paratesticular lesions are associated with a better prognosis, and often have excellent outcomes even with more limited therapy.
Most neoplasms in this area will present with unilateral painless swelling noted by the patient or the parent. US with flow Doppler is the initial imaging procedure of choice to help rule out structural issues like hernia and hydrocele, which can sometimes obscure an underlying mass. In addition, scrotal US can differentiate testicular masses from paratesticular lesions (36), and also evaluate the contralateral testis. The appearance of the lesion on US can suggest particular diagnoses, such as cystic changes with teratomas, low flow patterns with other benign masses, and solid, uniform, and hypoechoic features with malignant lesions. Serum tumor markers including alpha-fetoprotein and beta-human chorionic gonadotropin are routinely part of the evaluation of patients with testicular lesions, and can help not only with diagnosis but also with assessing response to therapy.
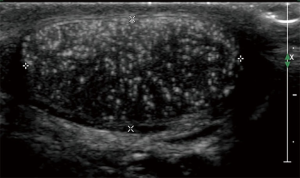
One feature of testicular US that has been studied recently is the appearance of microlithiasis, defined as 5 or more small areas of calcification in the testicle (Figure 3). These calcifications are thought to arise from degenerated intratubular cells in the seminiferous tubules. There has been a concern that the presence of microlithiasis may be related to the development of malignancy. In a retrospective review of over 37,000 pediatric patients under 18 years of age who underwent testicular US for any indication, 2.9% of patients had the confirmed finding of microlithiasis, which in their study was associated with a 22 times greater chance of developing malignant germ cell tumor (37). This report is similar to an adult study which also showed an increased risk of testicular neoplasia in individuals having microlithiasis (38). However, correlation does not necessarily prove causation, and limitations of retrospective studies with potential selection bias make the formulation of recommendations difficult. To date, surveillance strategies in adults have not been especially productive or cost-effective (39,40) in a disease with a very high cure rate, leading many experts in adult testicular cancer to recommend that the isolated finding of testicular microlithiasis without other risk features may not warrant additional evaluation other than recommendations for monthly testicular self-examination (41-43). Given the absence of prospective studies to better characterize incidence and benefit of follow-up, the true risk of developing testicular cancer in pediatric patients after discovery of isolated microlithiasis is unknown. Some pediatric groups such as the European Society of Pediatric Radiology have recommended considering regular surveillance US for isolated microlithiasis (44), even while acknowledging the limitations in our knowledge. A recent international survey of physicians caring for adolescents with microlithiasis demonstrated a range of approaches, with extent of follow-up varying with geographical location, physician specialty, and years in practice (45). Patient education and individualization of decision making remain key principles until guidelines based on prospective data are available.
For patients with testicular masses, cross-sectional imaging of the abdomen and pelvis with contrast-enhanced CT or MRI is necessary to identify retroperitoneal nodal metastases. In paratesticular rhabdomyosarcoma, patients over age 10 years have a higher rate of nodal involvement, which is not always associated with increased nodal size. Therefore, while imaging is still important for staging purposes, cooperative oncology groups in the United States have endorsed the use of staging ipsilateral retroperitoneal lymph node dissection (RPLND) to help identify otherwise occult disease which should be treated with radiotherapy in addition to the planned chemotherapy (46). With both germ cell tumors and paratesticular rhabdomyosarcoma, chest CT can help identify lung metastases. Imaging of bones or brain should be considered in symptomatic germ cell tumor patients, and assessment for skeletal metastases with PET is now routine for staging of paratesticular rhabdomyosarcoma.
For adolescent and adult testicular germ cell tumor patients with retroperitoneal adenopathy, treatment usually includes multiple cycles of platinum-based chemotherapy. Following this treatment, many patients are left with smaller but still measurable residual retroperitoneal masses. In patients with non-seminomatous tumors, the differential diagnosis for these lesions includes viable tumor, benign teratoma, or necrosis/fibrosis. Surgical resection is necessary for optimal management of patients with either viable tumor or benign teratoma, which may continue to grow unless removed. The preferred surgical intervention is a RPLND, which unfortunately carries with it a measurable rate of permanent life-affecting complications such as retrograde ejaculation or other sexual difficulties. Determining which patients with residual retroperitoneal masses should undergo this procedure has been difficult, even with the use of algorithms incorporating various clinical and radiographic features. Functional imaging with PET has not been able to improve diagnostic accuracy in assessment of residual masses, in part because mature teratomas are not PET avid and may mimic the appearance of fibrosis. Current guidelines recommend surgery for patients with residual lesions >1 cm (47). With this approach, up to 40% of patients have only fibrosis identified histologically, and thus underwent surgery that may have been unnecessary (48). In an effort to improve the predictive accuracy of residual mass assessment, Lewin et al. used radiomics, which utilizes computer programs to obtain quantitative data from conventional imaging studies like CT (48). The hypothesis is that otherwise imperceptible imaging differences in radiographic features such as coarseness or density can be identified by the computer and correlated with the different histologies and clinical behaviors. In their study of 102 patients who had undergone RPLND for residual masses, combining clinical features with the radiomics algorithm improved the predictive accuracy. Further work in this area, including the use of completely automated deep learning programs, is underway.
Conclusions
Urologic tumors comprise an important part of pediatric oncology. A thorough working knowledge of the differential diagnosis of presenting symptoms such as flank masses, scrotal swelling, or voiding difficulties facilitates an appropriate initial radiographic work-up. The radiologist is critical in helping with decisions for surgical interventions, as well in identifying features such as tumor rupture or metastatic disease that warrant changes in oncologic management. In addition, the radiologist can also help direct surveillance imaging after completion of therapy in a way that balances identification of recurrence with risks of radiation exposure. Strategies to shorten the time of scanning may be particularly helpful with younger children, and could reduce the need for procedural sedation. Functional imaging with PET has already become standard for rhabdomyosarcoma, and going forward it is possible that new approaches like radiomics can further enhance the diagnostic accuracy and clinical utility of radiology evaluations for these patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John Wiener, Jonathan Routh and Nicholas Cost) for the series “Pediatric Urologic Malignancies” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau-19-839). The series “Pediatric Urologic Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Steliarova-Foucher E, Colombert M, Rues LA, et al. International incidence of childhood cancer, 2001-10: a population-based registry. Lancet Oncol 2017;18:719-31. [Crossref] [PubMed]
- Breslow N, Olshan A, Beckwith JB, et al. Epidemiology of Wilms tumor. Med Pediatr Oncol 1993;21:172-81. [Crossref] [PubMed]
- Jehangir S, Kurian JJ, Selvarajah D, et al. Recurrent and metastatic congenital mesoblastic nephroma: where does the evidence stand? Pediatr Surg Int 2017;33:1183-8. [Crossref] [PubMed]
- Chung EM, Lattin GE Jr, Fagen KE, et al. Renal tumors of childhood: radiologic-pathologic correlation part 2. The 2nd decade: from the radiologic pathology archives. Radiographics 2017;37:1538-58. [Crossref] [PubMed]
- Scott RH, Stiller CA, Walker L, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 2006;43:705-15. [Crossref] [PubMed]
- Servaes SE, Hoffer FA, Smith EA, et al. Imaging of Wilms tumor: an update. Pediatr Radiol 2019;49:1441-52. [Crossref] [PubMed]
- Charlton J, Irtan S, Bergeron C, et al. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 2017;19:e8. [Crossref] [PubMed]
- McDonald K, Duffy P, Chowdhury T, et al. Added value of abdominal cross-sectional imaging (CT or MRI) in staging of Wilms' tumours. Clin Radiol 2013;68:16-20. [Crossref] [PubMed]
- Shamberger RC, Ritchey ML, Haase GM, et al. Intravascular extension of Wilms tumor. Ann Surg 2001;234:116-21. [Crossref] [PubMed]
- Emir S. Wilms tumor with intravascular tumor thrombus. Transl Pediatr 2014;3:29-33. [PubMed]
- Servaes S, Khanna G, Naranjo A, et al. Comparison of diagnostic performance of CT and MRI for staging of pediatric renal tumors: a report from the Children’s Oncology Group. Pediatr Radiol 2015;45:166-72. [Crossref] [PubMed]
- Geller JI, Ehrlich PF, Cost NG, et al. Characterization of adolescent and pediatric renal cell carcinoma: a report from the Children's Oncology Group study AREN03B2. Cancer 2015;121:2457-64. [Crossref] [PubMed]
- Ehrlich PF, Hamilton TE, Grundy P, et al. The value of surgery in directing therapy for patients with Wilms' tumor with pulmonary disease. A report from the National Wilms' Tumor Study Group (National Wilms' Tumor Study 5). J Pediatr Surg 2006;41:162-7. [Crossref] [PubMed]
- Dix DB, Seibel NL, Chi YY, et al. Treatment of stage IV favorable histology Wilms tumor with lung metastases: a report from the Children's Oncology Group AREN0533 Study. J Clin Oncol 2018;36:1564-70. [Crossref] [PubMed]
- Samim A, Littooij AS, van den Heuvel-Eibrink MM, et al. Frequency and characteristics of pulmonary nodules in children at computed tomography. Pediatr Radiol 2017;47:1751-8. [Crossref] [PubMed]
- McDaniel JD, Racadio JM, Patel MN, et al. CT-guided localization of pulmonary nodules in children prior to video-assisted thoracoscopic surgical resection utilizing a combination of two previously described techniques. Pediatr Radiol 2018;48:626-31. [Crossref] [PubMed]
- Misch D, Steffen IG, Schönberger S, et al. Use of positron emission tomography for staging, preoperative response assessment and posttherapeutic evaluation in children with Wilms tumour. Eur J Nucl Med Mol Imaging 2008;35:1642-50. [Crossref] [PubMed]
- Moinul Hossain AK, Shulkin BL, Gelfand MJ, et al. FDG positron emission tomography/computed tomography studies of Wilms' tumor. Eur J Nucl Med Mol Imaging 2010;37:1300-8. [Crossref] [PubMed]
- Littooij AS, Nikkels PG, Hulsbergen-van de Kaa CA, et al. Apparent diffusion coefficient as it relates to histopathology findings in post-chemotherapy nephroblastoma: a feasibility study. Pediatr Radiol 2017;47:1608-14. [Crossref] [PubMed]
- Malogolowkin M, Cotton CA, Green DM, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 2008;50:236-41. [Crossref] [PubMed]
- Mullen EA, Chi YY, Hibbitts E, et al. Impact of Surveillance imaging modality on survival after recurrence in patients with favorable-histology Wilms tumor: a report from the Children's Oncology Group. J Clin Oncol 2018;36:JCO1800076. [Crossref] [PubMed]
- Kaste SC, Brady SL, Yee B, et al. Is routine pelvic surveillance imaging necessary in patients with Wilms tumor? Cancer 2013;119:182-8. [Crossref] [PubMed]
- Kalish JM, Doros L, Helman LJ, et al. Surveillance recommendations for children with overgrowth syndromes and predisposition to Wilms tumors and hepatoblastoma. Clin Cancer Res 2017;23:e115-22. [Crossref] [PubMed]
- Rodeberg DA, Anderson JR, Arndt CA, et al. Comparison of outcomes based on treatment algorithms for rhabdomyosarcoma of the bladder/prostate: combined results from the Children's Oncology Group, German Cooperative Soft Tissue Sarcoma Study, Italian Cooperative Group, and International Society of Pediatric Oncology Malignant Mesenchymal Tumors Committee. Int J Cancer 2011;128:1232-9. [Crossref] [PubMed]
- Harrison DJ, Parisi MT, Shulkin BL. The role of 18F-FDG-PET/CT in pediatric sarcoma. Semin Nucl Med 2017;47:229-41. [Crossref] [PubMed]
- Vaarwerk B, Bisogno G, McHugh K, et al. Indeterminate pulmonary nodules at diagnosis in rhabdomyosarcoma: are they clinically significant? A report from the European Paediatric Soft Tissue Sarcoma Study Group. J Clin Oncol 2019;37:723-30. [Crossref] [PubMed]
- Saltsman JA, Malek MM, Reuter VE, et al. Urothelial neoplasms in pediatric and young adult patients: a large single-center series. J Pediatr Surg 2018;53:306-9. [Crossref] [PubMed]
- Vaarwerk B, van der Lee JH, Breunis WB, et al. Prognostic relevance of early radiologic response to induction chemotherapy in pediatric rhabdomyosarcoma: a report from the International Society of Pediatric Oncology Malignant Mesenchymal Tumor 95 study. Cancer 2018;124:1016-24. [Crossref] [PubMed]
- Vaarwerk B, Mallebranche C, Affinita MC, et al. Is surveillance imaging in pediatric patients treated for localized rhabdomyosarcoma useful? The European experience. Cancer 2020;126:823-31. [Crossref] [PubMed]
- Callahan MJ, MacDougall RD, Bixby SD, et al. Ionizing radiation from computed tomography versus anesthesia for magnetic resonance imaging in infants and children: patient safety considerations. Pediatr Radiol 2018;48:21-30. [Crossref] [PubMed]
- American Association of Physicists in Medicine. AAPM position statement on radiation risks from medical imaging procedures. 2011. Available online: http://www.aapm.org/org/policies/details.asp?id=318&type=PP
- Janos S, Schooler GR, Ngo JS, Davis JT. Free-breathing unsedated MRI in children: Justification and techniques. J Magn Reson Imaging 2019;50:365-76. [Crossref] [PubMed]
- Maizlin II, Dellinger M, Gow KW, et al. Testicular tumors in prepubescent patients. J Pediatr Surg 2018;53:1748-52. [Crossref] [PubMed]
- Pohl HG, Shukla AR, Metcalf PD, et al. Prepubertal testis tumors: actual prevalence rate of histological types. J Urol 2004;172:2370-2. [Crossref] [PubMed]
- Taskinen S, Fagerholm R, Aronniemi J, et al. Testicular tumors in children and adolescents. J Pediatr Urol 2008;4:134-7. [Crossref] [PubMed]
- Annam A, Munden MM, Mehollin-Ray AR, et al. Extratesticular masses in children: taking ultrasound beyond paratesticular rhabdomyosarcoma. Pediatr Radiol 2015;45:1382-91. [Crossref] [PubMed]
- Trout AT, Chow J, McNamara ER, et al. association between testicular microlithiasis and testicular neoplasia: large multicenter study in a pediatric population. Radiology 2017;285:576-83. [Crossref] [PubMed]
- Heller HT, Oliff MC, Doubilet PM, et al. Testicular microlithiasis: prevalence and association with primary testicular neoplasm. J Clin Ultrasound 2014;42:423-6. [Crossref] [PubMed]
- Bennett HF, Middleton WD, Bullock AD, et al. Testicular microlithiasis: US follow-up. Radiology 2001;218:359-63. [Crossref] [PubMed]
- DeCastro BJ, Peterson AC, Costabile RA. A 5-year followup study of asymptomatic men with testicular microlithiasis. J Urol 2008;179:1420-3. [Crossref] [PubMed]
- Leblanc L, Lagrange F, Lecoanet P, et al. Testicular microlithiasis and testicular tumor: a review of the literature. Basic Clin Androl 2018;28:8. [Crossref] [PubMed]
- Winter TC, Kim B, Lowrance WT, et al. Testicular microlithiasis: what should you recommend? AJR Am J Roentgenol 2016;206:1164-9. [Crossref] [PubMed]
- Balawender K, Orkisz S, Wisz P. Testicular microlithiasis: what urologists should know. A review of the current literature. Cent European J Urol 2018;71:310-4. [PubMed]
- Riccabona M, Lobo ML, Augdal TA, et al. European Society of Paediatric Radiology Abdominal Imaging Task Force recommendations in paediatric uroradiology, part X: how to perform paediatric gastrointestinal ultrasonography, use gadolinium as a contrast agent in children, follow up paediatric testicular microlithiasis, and an update on paediatric contrast-enhanced ultrasound. Pediatr Radiol 2018;48:1528-36. [Crossref] [PubMed]
- Brodie KE, Saltzman AF, Cost NG. Adolescent testicular microlithiasis: a case-based, multinational survey of clinical management practices. J Pediatr Urol 2018;14:151.e1-8. [Crossref] [PubMed]
- Walterhouse DO, Pappo AS, Meza JL, et al. Shorter-duration therapy using vincristine, dactinomycin, and lower-dose cyclophosphamide with or without radiotherapy for patients with newly diagnosed low-risk rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol 2014;32:3547-52. [Crossref] [PubMed]
- Honecker F, Aparicio J, Berney D, et al. ESMO Consensus Conference on testicular germ cell cancer: diagnosis, treatment and follow-up. Ann Oncol 2018;29:1658-86. [Crossref] [PubMed]
- Lewin J, Dufort P, Halankar J, et al. Applying radiomics to predict pathology of postchemotherapy retroperitoneal nodal masses in germ cell tumors. JCO Clin Cancer Inform 2018;2:1-12. [Crossref] [PubMed]


