Reactive oxygen species, inflammation and calcium oxalate nephrolithiasis
Kidney stones are comprised of mineral and organic components. Approximately 80% of the kidney stones contain calcium oxalate (CaOx) as the major mineral phase mixed mostly with calcium phosphate (CaP) and sometime uric acid (1). Stone formation involves crystal nucleation, growth, aggregation and their retention in the kidneys (2). These processes are modulated by a variety of urinary macromolecules which become incorporated in the growing crystals and stones and eventually constitute stones’ organic component or matrix. A better understanding of the pathogenesis of kidney stone formation has been developed through examination of clinical data and the use of animal models and tissue cultures. Based on clinical and experimental data, it is becoming obvious that stone formation is not a simple physicochemical disorder. Renal epithelial cells as well as others respond to changing urinary environment; dysregulated mineral metabolism and in the case of CaOx nephrolithiasis, abnormal calcium, citrate, oxalate, phosphate, and CaOx/CaP crystals, by increased production of a variety of crystallization modulating macromolecules, epithelial to mesenchymal transition (EMT), epithelial to osteoblast transformation (EOT), and remodeling of extracellular matrix (ECM). It appears that reactive oxygen species (ROS) are intimately involved as signaling molecules as well as agents of injury and inflammation during stone formation (3-6).
Reactive oxygen species (ROS)
ROS comprising free radicals, atoms or molecules with unpaired electrons, and their metabolites, are highly reactive and play a critical role as signaling molecules. But they can also produce chemical modifications of, and damage to proteins, lipids, carbohydrates and nucleotides (7,8). Major cellular ROS include superoxide anion (O2-•), nitric oxide radical (NO•), hydroxyl radical (OH•), and hydrogen peroxide (H2O2), which are generated by several pathways (Figure 1). O2-• anions are produced by NADPH oxidases, xanthine oxidase, lipooxigenase, cyclooxygenase, hemeoxygenase and as a byproduct of mitochondrial respiratory chain. Lipid radicals can also produce O2-•. NO• radicals are produced by the endothelial nitric oxide synthase (eNOS) mediated oxidation of L-arginine. In addition, eNOS can also produce O2-• rather than NO. The reaction between superoxide and nitric oxide can produce the highly reactive peroxynitrite ONOO-.
Cells are equipped with a number of scavenging systems to control ROS availability (Figure 1). These include superoxide dismutase (SOD) to eliminate O2-•, and glutathione (GSH) peroxidase (GPx) and catalase to detoxify H2O2 (Figure 1). Superoxide has a short half-life and spontaneously converts to H2O2 which is long-lasting and far more reactive than superoxide ions. The reaction is noticeably enhanced by SOD. Moreover, in a more complex transition metal catalyzed reaction called metal catalyzed Haber-Weiss reaction, H2O2 yields an even more reactive hydroxyl radical, which is however, short lived and works at short range. Initially, superoxide anions donate single electrons to ferric ions resulting in molecular oxygen and ferrous ions. The Fenton Reaction between ferrous ions and H2O2, leads to the formation of OH•. H2O2 is subsequently metabolized to water via catalase or by glutathione peroxidase in the presence of reduced glutathione.
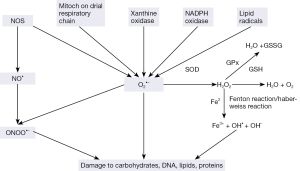
Under normal conditions the superoxide anions (O2-•), NO radicals (NO•) and their metabolites are generated by tightly controlled enzymes and serve as mediators in a variety of regulatory processes and signaling pathways including proliferation, activation or inactivation of regulatory biomolecules, and regulation of transcriptional activities. ROS regulate many calcium signals as well as such genes as c-fos, c-myc, and c-jun and transcription factor activation protein-1 (AP-1) and nuclear factor κB (NF-κB).
ROS and reactive nitrogen species (RNS) normally occur at steady state levels, generated when needed and then cleared by activities of various antioxidants and scavengers. But uncontrolled generation of the reactive oxygen or nitrogen species and/or a reduction in the endogenous antioxidant capacity creates oxidative stress (OS). Most cells respond to OS by boosting the levels of intracellular antioxidants such as glutathione. The oxidants can react with all the basic constituents of cells: lipids, carbohydrates, proteins and nucleic acids severely affecting their structure and function. Pathological changes may result from the damaging effects of ROS and from ROS-mediated changes in gene expression and signal transduction.
Sources of ROS in CaOx nephrolithiasis
ROS are produced through the involvement of both mitochondria (4,9-12) and NADPH oxidase (Figure 2) (4,13,14). NADPH oxidase is a major source of ROS in the kidneys (15,16), particularly in the presence of Angiotensin II (17). NADPH oxidase consists of six subunits, the two transmembrane units, p22phox and gp91phox; and four cytosolic units, p47phox, p67phox, p40phox and the small GTPase rac1 or rac2 (18). The two transmembrane units, gp91phox and p22phox and a flavin make cytochrome b558. The cytosolic units translocate to the membrane and assemble with the cytochrome to activate the enzyme.
ROS in response to oxalate and CaOx crystals are in part produced with the involvement of NADPH oxidase through the activation of the rennin angiotensin system (RAS). Reduction of angiotensin production, by inhibiting the angiotensin converting enzyme as well as blocking the angiotensin receptor, increased renin expression, reduced osteopontin (OPN) expression, crystal deposition and ameliorated the associated inflammatory response (Figure 2) (19-21). NADPH oxidase inhibition by apocynin treatment reduced the production of ROS, urinary excretion of kidney injury molecule (KIM) and renal deposition of CaOx crystals in hyperoxaluric rats (22). Atrovastatin, which has been shown to reduce the expression of gp91phox and p22phox subunits of NADPH oxidase (23), also inhibited crystal deposition in rats with experimentally induced hyperoxaluria (24).
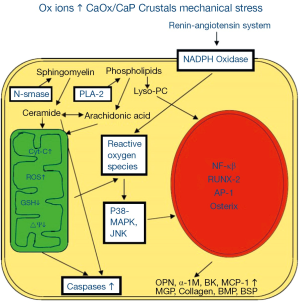
Mitochondria are generally the most common source of superoxide and H2O2 in most cells and tissues. Hyperoxaluria and CaOx crystal deposition in rat kidneys causes mitochondrial damage. Treatment with taurine which has been shown to prevent oxidative injury of the mitochondria, reversed mitochondrial changes in the hyperoxaluric rat kidneys and decreased crystal deposition (25). Selective probes, substrates and inhibitors show mitochondria to be significant site of CaOx crystal induced superoxide production and glutathione depletion in both LLC-PK1 and MDCK cells (9). Exposure of LLC-PK1 cells to oxalate significantly increased cellular ceramides (26), however, pretreatment with glutathione precursor N-acetylcysteine (NAC) blocked this increase. Isolated mitochondria responded to oxalate exposure by the accumulation of ROS, lipid peroxides and oxidized thiol proteins (11). Citrate is also involved in maintaining endogenous antioxidant defenses. Administration of exogenous citrate to LLC-PK1 and MDCK cells bolstered these defenses and diminished the cellular injury inflicted by exposure to increased Ox and CaOx crystals (27). The presence of citrate in the culture medium was associated with a significant increase in GSH peroxidase and a drop in the production of H2O2 and 8-isoprostane (8-IP), which is an end product of lipid breakdown. There was a significant improvement in cell viability as demonstrated by decreased LDH release and increased trypan blue exclusion.
Mitochondrial damage is suggested to be induced by the opening of mitochondrial permeability transition pore (mPTP). mPTP opening depends upon the activation of cyclophilin D in the mitochondrial matrix by ROS produced by NADPH oxidase and is inhibited by cyclosporine A (CSA) (28). CSA prevented the depolarization of mitochondrial membrane, decrease in SOD expression, increase in 4-hydroxy-2-nonenal (4HNE) and release of cytochrome-c into the cytosol in NR52E renal epithelial cells exposed to CaOx monohydrate crystals in vitro. CSA treatment of hyperoxaluric rats resulted in reduced mitochondrial damage, OS and CaOx crystal deposition in the kidneys.
Association of inflammation and injury with human stone formation
Most idiopathic stones are formed attached to Randall’s plaques (RPs), the sub-epithelial deposits of CaP on renal papillary surfaces (29). RPs are postulated to start as deposits of poorly crystalline biological apatite in the basement membrane of the loops of Henle (30,31) or collecting ducts (32) or vasa recta (33,34). The deposits, consisting of aggregated CaP spherules, grow through the interstitium towards the renal papillary epithelium, where they eventually ulcerate to the surface (35). Interestingly, all RPs are not connected to stones and kidneys of non-stone formers also contain interstitial plaques (36).
Stones such as cystine, brushite, CaOx in primary hyperoxaluria and after bariatric surgery, some idiopathic stones and CaP in primary hyperparathyroidism are found attached to Randall’s plugs (the tubular crystal deposits in the ducts of Bellini (31,37,38), which were most likely formed as a result of higher supersaturation with respect to the precipitating salt (39). Crystal deposition is associated with renal cell injury, cell loss, inflammation and fibrosis (4,40-45). The inflammation is generally localized to areas around crystal deposits in the renal papillae. In brushite stone formers, however, inflammation and fibrosis reach the cortex showing wide spread renal tubular atrophy and glomerular pathology (42).
It has been suggested that RPs are formed without causing renal injury and inflammation (31,46). But a close examination of published illustrations (30,32,36,47) clearly demonstrate the presence of necrotic tubules with thickened and layered basal lamina, along with perfectly normal ones in association with the CaP spherules embedded in a matrix of collagen and other fibers. Similarly we have found injured tubules associated with the interstitial deposits of apatitic mineral (35,38). The molecules generally involved in inflammatory pathways, such as OPN (48,49), heavy chain of inter-α- inhibitor (50,51), collagen (30,36,47), and zinc (52) have been seen in the interstitial plaques strongly suggesting that inflammation may have been an early and local participant (4), which was resolved by the time stone was discovered. Biopsies are taken at the time of stone removal, many months after stone formation. Moreover, only a very small amount of tissue from limited number of patients has so far been investigated. “It is important to emphasize that urolithiasis is merely a final manifestation of diverse and systemic etiological and pathogenic events” (53). Inflammation is a complex biological response to various irritants. Osteopotin and inter-α-inhibitor are protective mediators, which are most likely produced to inhibit crystallization and protect the kidneys. In normal human kidneys, OPN is localized primarily to the distal nephron and is strongly expressed in the thick ascending limbs of the loops of Henle and papillary surface epithelium. OPN expression is increased during inflammation and interstitial fibrosis (54).
Renal CaOx crystal deposits have been seen in a variety of disorders with increased production and excretion of oxalate. In biopsies from a patient with primary hyperoxaluria, crystals were seen within tubular epithelial cells as well as in the interstitium of the transplanted kidney (55) and were associated with vascular and interstitial inflammation, cell proliferation and multinucleated giant cells. Similar observations have been made in other cases of increased urinary excretion of Ox secondary to enteric hyperoxaluria, Crohn’s disease, and after intestinal bypass (56,57).
Higher than normal levels of renal enzymes, gamma-glutamyl transpeptidase (GGTP), angiotensin 1 converting enzyme (ACE), β-galactosidase (GAL), and N-acetyl-β-glucoseaminidase (NAG) were found in the urine of idiopathic CaOx stone formers (58), indicative of renal proximal tubular injury. The urine had significantly increased NAG, β-GAL, α-glutathione S-transferase (α-GST), malondialdehyde (MDA) and thiobarbituric acid-reactive substances (TBARS) (59), suggesting that stone-associated injury was most likely caused by the production of ROS. Urinary 8-hydroxydeoxyguanosine (8-OHdG), a marker of oxidative damage of DNA, was increased in stone patients and was positively correlated with tubular damage as assessed by urinary excretion of NAG (60). Recurrent idiopathic calcium stone formers with and without stones, exhibited antioxidant deficiency. Investigators concluded that lithiasis started with oxidatively damaged cells (61). Anti-inflammatory proteins calgranulin, α-defensin, and myeloperoxidase (62), were increased in urine of stone patients and were also found in the inner core of the CaOx stones.
Members of IαI family of proteins, which are important participants in wound healing, were significantly increased in the urine of male stone formers (63), and found in the stone matrix as well as the matrix of CaOx and CaP crystals induced in the urine (64,65). Hyaluronan, which plays an important role in renal injury and repair, was a major constituent of the organic matrix of stones (66). Prothrombin fragment-1, a member of thrombin family of proteins which are extensively involved in tissue repair, was also excreted in urine, present in stone matrix and preferentially bound to CaOx crystals (67). Kidneys of stone formers expressed mRNA for MCP-1 as well as IL-6 (68).
Nephrolithiasis and chronic kidney diseases (CKDs)
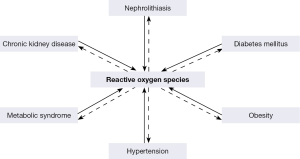
Kidney stone formation has been linked with a number of chronic diseases (69), such as obesity (OB) (70), diabetes mellitus (DM) (71), hypertension (HTN) (70), metabolic syndrome (MS) (72), and CKD (Figure 3) (73). Nephrolithiasis is a risk factor for the development of hypertension (74), while similarly hypertensive patients are at greater risk to develop nephrolithiasis (75). There is an association between stone disease and DM (71,76-81), as diabetics persistently produce acidic pH and have a greater risk to form uric acid stones (82). Kidney stone formers are also at greater risk for coronary artery disease (83), myocardial infarction (84) and CKD (83,85). Not surprisingly, stone formation is common in adults with metabolic syndrome and the frequency of stone formation is directly correlated with weight and BMI (86-88).

Both clinical and experimental investigations indicate that OS and inflammation play a significant role in the development of cardiovascular diseases (89). OS is a common feature of HTN, DM, atherosclerosis, and myocardial infarct (Figure 4) (69). An increase in the production of ROS/RNS, and/or decrease in the extra and intracellular antioxidants has been demonstrated in both clinical and experimental HTNs (90) and leads to OS which may not only initiate HTN but also be developed by the hypertensive state (91). Experimental studies have shown the involvement of NADPH oxidase in the development of hypertension (91-93). OB associated OS eventually leads to systemic inflammation and endothelial cell dysfunction (94,95). Proper endothelial performance requires NO which acts on pericytes, and is depleted during OS because of its inactivation by O2-• (96,97). Oxidation of the NO also results in the formation of highly active ONOO- and enhancement of OS. NADPH oxidase is a major source of ROS in the kidneys and is activated by Ang II, mostly through the AT1 receptor. Both NO and O2-• are produced by the renal epithelial cells, in addition, NO is also produced by endothelial cells. There is a tubulovascular cross talk, whereby NO produced by the epithelial cells of the medullary thick ascending limb affect the interstitial pericytes and endothelial cells.
NADPH oxidase is the major source of ROS in the kidneys and cardiovascular system (93). The kidneys of spontaneously hypertensive rats (SHR) showed increased production of O2-• and upregulation of p47phox subunit (98). Administration of SOD mimetic tempol produced a reduction of blood pressure and renal vascular resistance (99). Significantly higher p22phox mRNA levels and NADPH oxidase driven O2–• production were found in the aorta of SHR which were ameliorated by treatment with irbesartan, an angiotensin II receptor antagonist (100). The importance of p47phox is also shown by moderate hypertensive response to angiotensin II in mice lacking the p47phox (101,102). Inhibition by membrane permeable gp91ds-tat of p47phox assembly with gp91phox in Dahl salt sensitive (DS) rats fed a 4% salt diet, normalized ROS production and endothelium dependent relaxation as well as expression of LOX-1 and MCP-1 (103). Administration of apocynin, an antioxidant and an inhibitor of the p47phox assembly with gp91phox, to DS rats on high salt diet produced significant reductions in the mRNA expression of gp91phox, p47phox, p22phox, and p67phox subunits in addition to significantly reducing insterstitial superoxide and mean arterial pressure (104). Apocynin also reduced NADPH oxidase activity, renal cortical O2-•, monocyte/macrophage infiltration and glomerular and interstitial damage (105). Experimental studies involving other animal models of hypertension have similarly shown the involvement of NADPH oxidase in the development of hypertension (91-93).
NaDPH oxidase also plays an important role in diabetic nephropathy (106-108), particularly in the presence of high glucose. Levels of Nox 4 as well as p22phox mRNA were increased in kidneys of rats with STZ-induced diabetes along with an increase in immunostaining of 8-OHdG (109). Insulin treatment reduced them to control levels. STZ induced diabetes also increased excretion of H2O2, lipid peroxidation (LPO) products, and nitric oxide products (Nox) (110). Kidneys showed increased expression of gp91phox and p47phox and endothelial eNOS, increased mesangial matrix, fibronectin and type I collagen. The treatment with apocynin, which inhibits assembly of the cytosolic p47phox with the membranous gp91phox, inhibited the increases in membrane fraction of p47phox, and excretion of H2O2, LPO and Nox.
Dietary Approaches to Stop Hypertension (DASH) diet, which reduces the risks for stroke and cardiovascular diseases, also reduced the risk for stone formation by up to 45% (111). The relationship between DASH type diet and the incident symptomatic kidney stones was examined in a prospective analysis of data from Health Professionals Follow up Study (n=45,821), Nurses’ Health Study-1 (n=94,108) and 2 (n=101,837) and found that men and women with higher DASH scores were significantly less likely to develop kidney stones than those with lower DASH scores. Low sodium DASH diet reduces OS and improves vascular functions, lowers blood pressure (112,113). Analysis of data from National Health and Nutrition Examination Survey (NHANESIII) showed that serum levels of antioxidants alpha-carotene, beta-carotene and beta-cryptoxanthin were significantly lower in stone patients. Lower levels of these antioxidants were associated with decreasing incidents of stone disease (114).
The association between nephrolithiasis, CKD, DM, OB, HTN, and MS is most likely a result of common pathophysiological mechanisms (70). ROS and OS are common feature of CKD, nephrolithiasis, DM, OB, HTN, and MS. It is conceivable that ROS produced by one disease may lead to another under appropriate circumstances (Figure 4) (69,115,116). For example mild hypercalciuria, hyperoxaluria, hypocitraturia which under normal conditions may just be a curiosity or nuisance, can promote crystallization and stone formation when cells are injured by ROS produced through another disorder.
Epithelial exposure to oxalate or calcific crystals, inflammation and injury
Tissue culture studies in which renal epithelial cells are exposed to Ox and/or CaOx or CaP crystals have provided new insights into mechanisms involved in stone formation. Cell response is time and concentration dependent and cell specific. Exposure to high concentrations of Ox as well as CaOx and CaP crystals for longer duration is injurious to renal epithelial cells (117-121). Crystals bind rapidly to the surface of epithelial cells and are internalized (122-127). Cells of proximal tubular origin are more susceptible to injury then cells originating from distal sections of the renal tubules. Lower Ox levels induce expression of immediate early genes, stimulate DNA synthesis and promote cellular proliferation, while higher Ox levels induce cell damage and death.
The response of renal epithelial cells to COM crystals is characterized by increased expression of specific genes that encode transcriptional activators, regulators of the ECM, and growth factors (118,128), and production of pro and anti-inflammatory molecule, such as OPN, monocyte chemoattractant-1 (MCP-1), prostaglandin E2 (PGE2), bikunin and components of inter-α-inhibitor (IαI), α-1 microglobulin, CD-44, calgranulin, heparin sulfate, osteonectin, fibronectin and matrix-gla-protein (MGP) (14,21,129-134). Even though many of these molecules are integral to inflammation and fibrosis, they are also modulators of calcification (135,136). Gene expression of vimentin, a mesenchymal marker, is also increased (137).
Tissue culture studies have also provided the evidence for the involvement of free radicals in toxicity, production of various crystallization modulators, and inflammatory and anti-inflammatory macromolecules (14,138-141). Renal cells exposed to CaOx crystals secrete superoxide (142) and cellular injury could be ameliorated by antioxidants and free radical scavenger (5,14,138-140,143). Free radical scavengers, catalase and SOD provided protection from oxalate induced injury to LLC-PK1 and MDCK cells (138). Catalase treatment also reduced MCP-1 mRNA as well as protein in the oxalate, CaOx, CaP and uric acid treated NRK52E renal epithelial cells (132,133).
Inflammation and injury in animal models of CaOx nephrolithiasis
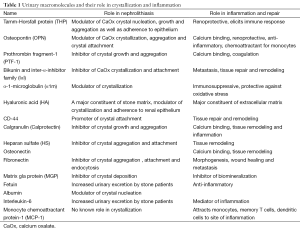
A number of rat and mice models (144,145), have been developed to investigate the pathogenesis of kidney stones. None of the models completely replicate the process of idiopathic stone formation and develop stones on papillary surface attached to the RPs. Instead the crystals are intraluminal, reminiscent of Randall’s plugs. CaOx nephrolithiasis is produced by inducing hyperoxaluria through the administration of oxalate or its precursors such as glyoxylate, ethylene glycol (EG) and hydroxyl-L-proline (HLP). Both hyperoxaluria and CaOx crystal deposition trigger morphological and pathophysiological changes in the kidneys and alter urinary composition (22,146). Renal expression of OPN (21,147), Tamm-Horsfall Protein (THP) (148-150), bikunin (130,131), IαI (151), α-1microglobulin (152), prothrombin fragment-1 (PTF-1) (153), calgranulin, heparin sulfate (HS) (154), matrix gla protein (MGP) (155,156), are generally increased (Table 1) and often found at locations not normally seen. For example THP is specifically produced by epithelial cells lining the thick ascending limbs of the loops of Henle. However, in the rat model of CaOx nephrolithiasis THP is seen associated with crystals throughout the kidneys including the cortex (150,157). In addition the expression of NFκB, KIM, proliferating cell nuclear antigen (PCNA), and CD 44, e-cadherin, is also increased indicating both injury and proliferation (22,158). Urinary excretion of many of these molecules is increased as well (22,131,146,147,151,153,159). There is migration of monocyte and macrophages to the sites of crystal deposition. We examined kidneys at different times after induction of acute hyperoxaluria in male Sprague-Dawley rats, and found that CaOx crystals appeared first in the tubular lumen, then moved to inter- and intracellular locations, eventually relocating into the interstitium, where they became surrounded by macrophages. After a few weeks, the interstitial crystals disappeared, indicating the existence of a mechanism to remove the CaOx crystals (160). Multinucleated giant cells were also identified in the interstitium (161).
Full table
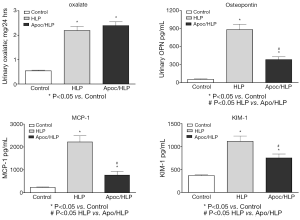
Lipid peroxides increased in both the renal tissue and urine in rats with hyperoxaluria and CaOx nephrolithiasis (162). Additionally, treatment with antioxidant vitamin E improved the tissue levels of antioxidant enzymes, reduced injury and totally eliminated CaOx crystal deposition in the kidneys (163). Deposition of CaOx crystals in the kidneys was associated with reduction of total renal cellular glutathione and an increase in lipid peroxides (19). Rats who received ACE inhibitor losartan, known to reduce OS, showed a significant increase in glutathione concentration and a decrease in the thiobarbituric acid reactive substances in the kidneys. The activities of catalase and MnSOD increased in kidneys while α- and μ-glutathione-S-transferase (GST) levels increased in the urine of hyperoxaluric rats (164). Microarray analysis of the kidneys of hyperoxaluric rats also revealed the development of OS during hyperoxaluria and CaOx crystals deposition. Expression of genes for SOD, GPx, GST, aldehyde dehydrogenase, mitochondrial uncoupling protein and ceruloplasmin was increased in hyperoxaluric rats (165). Administration of apocyanin, an antioxidant and inhibitor of NADPH oxidase, to rats with hydroxypropline induced hyperoxaluria nearly completely reversed the effects of hyperoxaluria (146), in addition, the deposition of CaOx crystals in the kidneys was also significantly reduced (Figure 5), and the urinary excretion of OPN, KIM, MCP-1 was significantly reduced without any effect on hyperoxaluria (Figure 6).
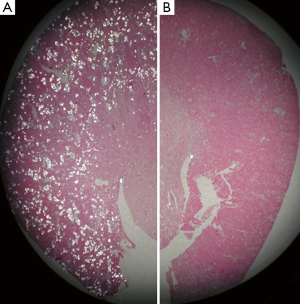
CaOx crystal deposition caused inflammation and attracted many inflammatory cells including leukocytes, monocytes, and macrophages (146,161,166,167), and multinucleated giant cells were identified around the crystals. The mechanism by which inflammatory cells enter the renal interstitium is not known, but chemotactic factors and adhesion molecules are involved. Leukocytes (neutrophils, monocytes, and lymphocytes) infiltrate the kidneys during a variety of inflammatory diseases. They mediate renal injury and subsequent sclerosis induced by such pathologies. Chemotactic factors are produced by renal cells and are found in the kidney and urine during inflammation. Results show that approximately 70-80% of monocyte chemotactic activity produced by cultured human mesangial cells (168), renal cortical epithelial cells (169), proximal tubular epithelial cells (170), and bovine glomerular endothelial cells (171), is accounted for by MCP-1. As discussed earlier, exposure to oxalate and both the CaP and CaOx crystals is associated with the production of MCP-1 by rat kidney cells culture (14,132,133).
Epithelial to osteoblast transformation (EOT)
Vascular calcification, which plays a major role in the development of CKD, was considered to occur by a passive, unregulated physicochemical mechanism as an irreversible degenerative process. Now however, it is considered to be an actively regulated process in which vascular smooth cells (VSMC) acquire osteogenic phenotype (172-174). Exposure of VSMC to elevated levels of calcium and phosphate triggers osteogenic transformation of VSMC (175-178), which involves an increased expression of osteoblast specific genes and a decrease in smooth muscle cell markers (179,180). Bone morphogenetic proteins, BMP 2 and BMP 4, and Wnt signaling pathways are activated through up-regulation of transcription factor, Runt-related transcription factor 2 (RUNX2)/msh homeobox 2 (MSX-2). Cells produce matrix proteins. Crystallization starts in membrane bound matrix vesicles produced by the viable transformed vascular smooth muscle cells or apoptotic bodies produced on their death (178,181,182). The vesicles are similar in composition to the matrix vesicles derived from chondrocytes and provide sites for the nucleation of CaP crystals (176). Once mineralized, the crystals poke through the limiting membrane of the vesicles and help mineralize the nearby ECM which sustains calcification. In addition to abnormal mineral metabolism, OS, inflammation and aberrant crystallization inhibition play significant role in vascular calcification. ROS are likely involved in the VSMC transformation to osteogenic phenotype by regulating RUNX-2 transcription factor (183,184). Advanced glycation end-products commonly seen in blood and arteries of diabetic patients and older individuals can promote vascular calcification mediated by NADPH oxidase induced ROS (185). Cytokines such as interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β produced by macrophages induce transformation of VSMCs (186). Inflammatory cells also produce proteolytic enzymes such as metalloproteinases (MMP)-2 and -9 which degrade matrix and promote calcification (187-190).
Calcification of vascular smooth cell is inhibited by MGP, pyrophosphate, OPN and Fetuin-A (179). MGP is a vitamin K-dependent protein functioning primarily as an inhibitor of vascular calcification (191). MGP also regulates BMP-2 activity (192). Mutations in the MGP gene lead to keutel syndrome, a disorder associated with extensive soft tissue and vascular calcification (193). MGP knockout mice die within two months as a result of arterial calcification and blood vessel rupture (194) while restoration of MGP in these mice prevents arterial calcification (195). Polymorphism of MGP may play a role in vascular calcification (196) and has shown an association with myocardial infarction (197).
Fetuin A, a member of cystatin family of protease inhibitors, is a serum protein, produced by the liver and specifically enriched in mineralized tissues (198-200). Irrespective of its origin and posttranslational modifications fetuin-A prevents precipitation of hydroxyapatite in vitro (201). In vivo, serum fetuin A levels are lower in patients with CKD (202), and ectopic calcification is seen in fetuin A -/- mice (200).
Cardiovascular complications are significantly increased in patients with CKD (203,204), and coronary artery calcification is considered an independent predictor of future cardiac event (205). Carotid atherosclerotic lesions of CKD patients frequently become calcified (206,207). The calcified plaques are more unstable and contain significantly lower amounts of collagen. Serum levels of MMPs are significantly increased (206-209). Enhanced calcification and reduced collagen, perhaps through the activities of MMPs, lead to plaque instability and rupture (206).
It is our hypothesis that stone formation is yet another case of pathological biomineralization. Renal epithelial cells under OS may become osteogenic (210) as happens to vascular smooth muscle cells during vascular calcification (177). The production of OPN (147,149), MGP (155,211), collagen, fribronectin, osteonectin and fetuin (unpublished results) by renal epithelial cells of rats with experimentally induced CaOx nephrolithiasis are indicative of such a transition. The presence of OPN, osteocalcin, fibronectin, and collagen (212) in stone matrices also suggests their increased production and excretion into the urine. Renal crystals in a CaOx stone patient were also associated with bone sialoprotein (BSP) (213). EMT (214) as well as endothelial to mesenchymal transition (215,216) are regularly seen in the diseased kidneys. Mesenchymal stromal cells have the ability to differentiate into osteoblast. Interestingly, perivascular cells or pericyte were heavily stained for MGP in kidneys of hyperoxaluric rats (211).
Stone patients excrete lower amounts of fetuin-A (217), and more BMP-2 (218). Single nucleotide polymorphism of MGP gene is associated with CaOx kidney stones disease in the Japanese (219) and Chinese populations (220). Brush border membrane vesicles of renal epithelium promote nucleation of CaOx and CaP crystals in vivo as well as in vitro (221-223).
Discussion and concluding remarks
Supersaturation is the driving force behind crystallization and in most idiopathic CaOx stone formers hypercalciuria, hyperoxaluria and hypocitraturia alone or in combination are the main abnormalities. As a result, most stone therapies attempt to lower urinary supersaturation with respect to the crystallizing salt, yet 30% to 50% stone patients still continue to form stones (224). The risk of stone recurrence increases with each new episode (225), and nearly all stone formers are expected to form another provided they live long enough after the first episode (226). Even initial interventions do not stop stone recurrence in about fifty percent of the patients (227,228). Apparently stone formation does not occur by a passive unregulated physicochemical mechanism, but by a regulated process, similar to pathological biomineralization at other sites in the body including kidneys during vascular calcification (172, 229,230).
Renal epithelial cells are exposed to high oxalate and/or CaOx and/or CaP crystals during stone formation. Experimental studies suggest that renal cellular exposure to high oxalate and/or CaOx or CaP crystals results in increased gene expression and production of molecules involved in tissue remodeling, inflammation and biomineralization (Figure 2). Hyperoxaluria and CaOx crystal deposition induce rennin upregulation and generation of angiotensin II (21). Non phagocytic NADPH oxidase is activated (14,22,231,232) leading to the production of ROS (5,231,233) which is mediated by protein kinase C (PKC) (232). The activation involves phosphorylation of p47phox and translocation of Rac1 (234) and p47 phox to the membrane. P-38 MAPK/JNK transduction pathway is turned on (235,236), in addition to a variety of transcriptional and growth factors, including NFκB, AP-1, TGFβ, become involved (19,20,237). There is the generation of secondary mediators such as isoprostanes, cytoplasmic phospholipase A2 and prostaglandins (4,5,238), and an increased production of chemoattractants such as MCP-1 (132-134) and crystallization modulators OPN (134), bikunin (130,131), α1-microglobulin (152), IαI (151) and prothrombin fragment-1 (153). Macrophages infiltrate the renal interstitium around the crystal deposits and phagocytic NADPH oxidase is also activated producing additional ROS resulting in inflammation, ECM production and fibrosis. Clinical data also provide the evidence of ROS generation and byproducts of their activities have been detected in both the kidneys and urine of the patients who form CaOx kidney stones.
The macromolecules, produced on exposure to oxalate and or various types of crystals through ROS dependent pathways, have dual functions (Table 1). They regulate crystallization and are also involved in inflammatory processes. For example, HS, an inhibitor of crystal aggregation and attachment, regulates ECM production (3,239). Bikunin, a constituent of ITI, an inhibitor of CaOx crystal formation and attachment, is a proteinase inhibitor, and stabilizes the ECM (240-242). THP a modulator of CaOx crystal nucleation, growth and aggregation, is renoprotective and present in the renal interstitium during many tubulointerstitial diseases (243). OPN, an inhibitor of crystal nucleation, growth and aggregation, is also a chemoattractant involved in inflammation and fibrosis (244,245). Prothrombin is the precursor of thrombin and fragments 1 and 2 and plays a major role in the recruitment and activation of infiltrating immune cells. Fragment-1 is inhibitor of CaOx crystal growth and aggregation. Inflammation is primarily a protective response, therefore in the presence of impending mineralization, the body responds by producing macromolecules to inhibit crystallization and once crystal are formed to attract the inflammatory cells for their elimination (4,161,246). Crystals are phagocytosed and eliminated (246) or fenced in by a “wall” of macromolecules adsorbed to crystal surfaces rendering them harmless (247). This is likely the case with the interstitial plaques which are not attached to kidney stones and are common in the kidneys (32,36,47,248).
In summary, disturbance in the physiochemical milieu leads to the production of ROS and development of OS. ROS start a signaling cascade culminating in the production of macromolecules to inhibit crystal nucleation, growth and aggregation. In case of transitory disorder, either no crystal will form or crystals formed will stay small, well dispersed and passed out as crystalluria particles. In the face of persistent disorder, for example, hyperoxaluria, hypercalciuria and hypocitraturia, there is a loss of the balance between oxidative and antioxidative forces. ROS induced damage to the cells leads to cell death and the formation of membrane bound vesicles which support crystal nucleation (221,249). As a result crystallization inhibitors which are produced may be defective or damaged by exposure to the free radicals and thus not able to provide adequate protection resulting in crystal growth and aggregation. Cell death also leads to the formation of new cells to repopulate the epithelium. The surfaces of the new cells as well as the exposed basement membrane are conducive to crystal attachment and retention (250). Crystals retained in the terminal collecting ducts produce Randall’ plugs (38,251) which will act as stone nidus when exposed to the pelvic urine. A recent study has shown that plugging is quite common in stones patients (37).
As far as interstitial RPs are concerned, CaP crystals may originate in the tubular lumen, endocytose on the luminal side and exocytose on the basolateral side (213,251,252), to initiate the formation of the plaque. Alternatively renal epithelial cells under OS may become osteogenic (210). In addition to epithelium to osteoblast transformation, vascular endothelial cells may also become osteogenic (216). The transformed epithelial or endothelial cells will produce a membrane bound vesicle on the basolateral side of the epithelium. Renal epithelial cells have been shown to produce in vitro, CaP crystal deposits in the basement membrane under a variety of growing conditions (213,253,254).
Calcification of the membrane vesicles and their aggregation produces calcified RP’s in the basement membrane of the renal tubules. ROS induced inflammation results in the formation of collagen which is deposited during fibrosis (255), and is an excellent nucleator of CaP (182,256) playing a critical role in biomineralization processes in the body (182,257). Calcification which starts with membrane bound vesicles is propagated through the mineralization of collagen (35). Mineralization of collagen leads to the growth of the plaque, which eventually reaches the papillary epithelium, ulcerates to the surface and develops into a stone nidus. Once exposed to the pelvic urine, the plaque is overgrown by CaOx crystals, and promotes the formation of an idiopathic CaOx kidney stone attached to the sub-epithelial RP (258).
Acknowledgements
Funding: This study was supported in part by the National Institutes of Health (NIH) grant 5R01-DK 078602 and R01-DK092311.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Finlayson B. Symposium on renal lithiasis. Renal lithiasis in review. Urol Clin North Am 1974;1:181-212. [PubMed]
- Finlayson B. Physicochemical aspects of urolithiasis. Kidney Int 1978;13:344-60. [PubMed]
- Khan SR. Role of renal epithelial cells in the initiation of calcium oxalate stones. Nephron Exp Nephrol 2004;98:e55-60. [PubMed]
- Khan SR. Crystal-induced inflammation of the kidneys: results from human studies, animal models, and tissue-culture studies. Clin Exp Nephrol 2004;8:75-88. [PubMed]
- Khan SR. Hyperoxaluria-induced oxidative stress and antioxidants for renal protection. Urol Res 2005;33:349-57. [PubMed]
- Khan SR. Renal tubular damage/dysfunction: key to the formation of kidney stones. Urol Res 2006;34:86-91. [PubMed]
- Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 2002;82:47-95. [PubMed]
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 1999;11:1-14. [PubMed]
- Khand FD, Gordge MP, Robertson WG, et al. Mitochondrial superoxide production during oxalate-mediated oxidative stress in renal epithelial cells. Free Radic Biol Med 2002;32:1339-50. [PubMed]
- Meimaridou E, Jacobson J, Seddon AM, et al. Crystal and microparticle effects on MDCK cell superoxide production: oxalate-specific mitochondrial membrane potential changes. Free Radic Biol Med 2005;38:1553-64. [PubMed]
- Cao LC, Honeyman TW, Cooney R, et al. Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int 2004;66:1890-900. [PubMed]
- Meimaridou E, Lobos E, Hothersall JS. Renal oxidative vulnerability due to changes in mitochondrial-glutathione and energy homeostasis in a rat model of calcium oxalate urolithiasis. Am J Physiol Renal Physiol 2006;291:F731-40. [PubMed]
- Khan SR, Khan A, Byer KJ. Temporal changes in the expression of mRNA of NADPH oxidase subunits in renal epithelial cells exposed to oxalate or calcium oxalate crystals. Nephrol Dial Transplant 2011;26:1778-85. [PubMed]
- Umekawa T, Byer K, Uemura H, et al. Diphenyleneiodium (DPI) reduces oxalate ion- and calcium oxalate monohydrate and brushite crystal-induced upregulation of MCP-1 in NRK 52E cells. Nephrol Dial Transplant 2005;20:870-8. [PubMed]
- Li N, Yi FX, Spurrier JL, et al. Production of superoxide through NADH oxidase in thick ascending limb of Henle’s loop in rat kidney. Am J Physiol Renal Physiol 2002;282:F1111-9. [PubMed]
- Geiszt M, Kopp JB, Varnai P, et al. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A 2000;97:8010-4. [PubMed]
- Hanna IR, Taniyama Y, Szocs K, et al. NAD(P)H oxidase-derived reactive oxygen species as mediators of angiotensin II signaling. Antioxid Redox Signal 2002;4:899-914. [PubMed]
- Joshi S, Peck AB, Khan SR. NADPH oxidase as a therapeutic target for oxalate induced injury in kidneys. Oxid Med Cell Longev 2013;2013:462361.
- Toblli JE, Ferder L, Stella I, et al. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol 2002;168:1550-5. [PubMed]
- Toblli JE, Ferder L, Stella I, et al. Protective role of enalapril for chronic tubulointerstitial lesions of hyperoxaluria. J Urol 2001;166:275-80. [PubMed]
- Umekawa T, Hatanaka Y, Kurita T, et al. Effect of angiotensin II receptor blockage on osteopontin expression and calcium oxalate crystal deposition in rat kidneys. J Am Soc Nephrol 2004;15:635-44. [PubMed]
- Zuo J, Khan A, Glenton PA, et al. Effect of NADPH oxidase inhibition on the expression of kidney injury molecule and calcium oxalate crystal deposition in hydroxy-L-proline-induced hyperoxaluria in the male Sprague-Dawley rats. Nephrol Dial Transplant 2011;26:1785-96. [PubMed]
- Wassmann S, Laufs U, Muller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol 2002;22:300-5. [PubMed]
- Tsujihata M, Momohara C, Yoshioka I, et al. Atorvastatin inhibits renal crystal retention in a rat stone forming model. J Urol 2008;180:2212-7. [PubMed]
- Li CY, Deng YL, Sun BH. Taurine protected kidney from oxidative injury through mitochondrial-linked pathway in a rat model of nephrolithiasis. Urol Res 2009;37:211-20. [PubMed]
- Cao LC, Honeyman T, Jonassen J, et al. Oxalate-induced ceramide accumulation in Madin-Darby canine kidney and LLC-PK1 cells. Kidney Int 2000;57:2403-11. [PubMed]
- Byer K, Khan SR. Citrate provides protection against oxalate and calcium oxalate crystal induced oxidative damage to renal epithelium. J Urol 2005;173:640-6. [PubMed]
- Niimi K, Yasui T, Hirose M, et al. Mitochondrial permeability transition pore opening induces the initial process of renal calcium crystallization. Free Radic Biol Med 2012;52:1207-17. [PubMed]
- Miller NL, Gillen DL, Williams JC Jr, et al. A formal test of the hypothesis that idiopathic calcium oxalate stones grow on Randall’s plaque. BJU Int 2009;103:966-71. [PubMed]
- Evan AP, Lingeman JE, Coe FL, et al. Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 2003;111:607-16. [PubMed]
- Coe FL, Evan AP, Lingeman JE, et al. Plaque and deposits in nine human stone diseases. Urol Res 2010;38:239-47. [PubMed]
- Weller RO, Nester B, Cooke SA. Calcification in the human renal papilla: an electron-microscope study. J Pathol 1972;107:211-6. [PubMed]
- Stoller ML, Low RK, Shami GS, et al. High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J Urol 1996;156:1263-6. [PubMed]
- Stoller ML, Meng MV, Abrahams HM, et al. The primary stone event: a new hypothesis involving a vascular etiology. J Urol 2004;171:1920-4. [PubMed]
- Khan SR, Rodriguez DE, Gower LB, et al. Association of Randall plaque with collagen fibers and membrane vesicles. J Urol 2012;187:1094-100. [PubMed]
- Haggitt RC, Pitcock JA. Renal medullary calcifications: a light and electron microscopic study. J Urol 1971;106:342-7. [PubMed]
- Linnes MP, Krambeck AE, Cornell L, et al. Phenotypic characterization of kidney stone formers by endoscopic and histological quantification of intrarenal calcification. Kidney Int 2013;84:818-25. [PubMed]
- Khan SR, Finlayson B, Hackett R. Renal papillary changes in patient with calcium oxalate lithiasis. Urology 1984;23:194-9. [PubMed]
- Randall A. Recent Advances in Knowledge Relating to the Formation, Recognition and Treatment of Kidney Calculi. Bull N Y Acad Med 1944;20:473-84. [PubMed]
- Evan AP, Coe FL, Lingeman JE, et al. Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int 2006;69:2227-35. [PubMed]
- Evan AP, Lingeman J, Coe F, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int 2007;71:795-801. [PubMed]
- Evan AP, Lingeman JE, Coe FL, et al. Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 2005;67:576-91. [PubMed]
- Evan AP, Lingeman JE, Worcester EM, et al. Renal histopathology and crystal deposits in patients with small bowel resection and calcium oxalate stone disease. Kidney Int 2010;78:310-7. [PubMed]
- Evan AP, Coe FL, Gillen D, et al. Renal intratubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec (Hoboken) 2008;291:325-34. [PubMed]
- Evan AE, Lingeman JE, Coe FL, et al. Histopathology and surgical anatomy of patients with primary hyperparathyroidism and calcium phosphate stones. Kidney Int 2008;74:223-9. [PubMed]
- Evan AP, Lingeman JE, Coe FL, et al. Role of interstitial apatite plaque in the pathogenesis of the common calcium oxalate stone. Semin Nephrol 2008;28:111-9. [PubMed]
- Cooke SA. The site of calcification in the human renal papilla. Br J Surg 1970;57:890-6. [PubMed]
- Evan AP. Physiopathology and etiology of stone formation in the kidney and the urinary tract. Pediatr Nephrol 2010;25:831-41. [PubMed]
- Evan AP, Coe FL, Rittling SR, et al. Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localization. Kidney Int 2005;68:145-154. [PubMed]
- Evan AP, Bledsoe S, Worcester EM, et al. Renal inter-alpha-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Int 2007;72:1503-11. [PubMed]
- Evan A, Lingeman J, Coe FL, et al. Randall’s plaque: pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 2006;69:1313-8. [PubMed]
- Carpentier X, Bazin D, Combes C, et al. High Zn content of Randall’s plaque: a µ-X-ray fluorescence investigation. J Trace Elem Med Biol 2011;25:160-5. [PubMed]
- Moe OW, Pearle MS, Sakhaee K. Pharmacotherapy of urolithiasis: evidence from clinical trials. Kidney Int 2011;79:385-92. [PubMed]
- Xie Y, Sakatsume M, Nishi S, et al. Expression, roles, receptors, and regulation of osteopontin in the kidney. Kidney Int 2001;60:1645-57. [PubMed]
- Lieske JC, Spargo BH, Toback FG. Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J Urol 1992;148:1517-9. [PubMed]
- Mandell I, Krauss E, Millan JC. Oxalate-induced acute renal failure in Crohn’s disease. Am J Med 1980;69:628-32. [PubMed]
- Wharton R, D’Agati V, Magun AM, et al. Acute deterioration of renal function associated with enteric hyperoxaluria. Clin Nephrol 1990;34:116-21. [PubMed]
- Baggio B, Gambaro G, Ossi E, et al. Increased urinary excretion of renal enzymes in idiopathic calcium oxalate nephrolithiasis. J Urol 1983;129:1161-2. [PubMed]
- Huang HS, Ma MC, Chen CF, et al. Lipid peroxidation and its correlations with urinary levels of oxalate, citric acid, and osteopontin in patients with renal calcium oxalate stones. Urology 2003;62:1123-8. [PubMed]
- Boonla C, Wunsuwan R, Tungsanga K, et al. Urinary 8-hydroxydeoxyguanosine is elevated in patients with nephrolithiasis. Urol Res 2007;35:185-91. [PubMed]
- Schwille PO, Manoharan M, Schmiedl A. Is idiopathic recurrent calcium urolithiasis in males a cellular disease? Laboratory findings in plasma, urine and erythrocytes, emphasizing the absence and presence of stones, oxidative and mineral metabolism: an observational study. Clin Chem Lab Med 2005;43:590-600. [PubMed]
- Mushtaq S, Siddiqui AA, Naqvi ZA, et al. Identification of myeloperoxidase, alpha-defensin and calgranulin in calcium oxalate renal stones. Clin Chim Acta 2007;384:41-7. [PubMed]
- Marengo SR, Resnick MI, Yang L, et al. Differential expression of urinary inter-alpha-trypsin inhibitor trimers and dimers in normal compared to active calcium oxalate stone forming men. J Urol 1998;159:1444-50. [PubMed]
- Atmani F, Glenton PA, Khan SR. Identification of proteins extracted from calcium oxalate and calcium phosphate crystals induced in the urine of healthy and stone forming subjects. Urol Res 1998;26:201-7. [PubMed]
- Dawson CJ, Grover PK, Kanellos J, et al. Inter-alpha-inhibitor in calcium stones. Clin Sci (Lond) 1998;95:187-93. [PubMed]
- Roberts SD, Resnick MI. Glycosaminoglycans content of stone matrix. J Urol 1986;135:1078-83. [PubMed]
- Stapleton AM, Dawson CJ, Grover PK, et al. Further evidence linking urolithiasis and blood coagulation: urinary prothrombin fragment 1 is present in stone matrix. Kidney Int 1996;49:880-8. [PubMed]
- Boonla C, Hunapathed C, Bovornpadungkitti S, et al. Messenger RNA expression of monocyte chemoattractant protein-1 and interleukin-6 in stone-containing kidneys. BJU Int 2008;101:1170-7. [PubMed]
- Khan SR. Is oxidative stress, a link between nephrolithiasis and obesity, hypertension, diabetes, chronic kidney disease, metabolic syndrome? Urol Res 2012;40:95-112. [PubMed]
- Obligado SH, Goldfarb DS. The association of nephrolithiasis with hypertension and obesity: a review. Am J Hypertens 2008;21:257-64. [PubMed]
- Lieske JC, de la Vega LS, Gettman MT, et al. Diabetes mellitus and the risk of urinary tract stones: a population-based case-control study. Am J Kidney Dis 2006;48:897-904. [PubMed]
- Jeong IG, Kang T, Bang JK, et al. Association between metabolic syndrome and the presence of kidney stones in a screened population. Am J Kidney Dis 2011;58:383-8. [PubMed]
- Saucier NA, Sinha MK, Liang KV, et al. Risk factors for CKD in persons with kidney stones: a case-control study in Olmsted County, Minnesota. Am J Kidney Dis 2010;55:61-8. [PubMed]
- Tibblin G. A population study of 50-year-old men. An analysis of the non-participation group. Acta Med Scand 1965;178:453-9. [PubMed]
- Strazzullo P, Barba G, Vuotto P, et al. Past history of nephrolithiasis and incidence of hypertension in men: a reappraisal based on the results of the Olivetti Prospective Heart Study. Nephrol Dial Transplant 2001;16:2232-5. [PubMed]
- Madore F, Stampfer MJ, Willett WC, et al. Nephrolithiasis and risk of hypertension in women. Am J Kidney Dis 1998;32:802-7. [PubMed]
- Domingos F, Serra A. Nephrolithiasis is associated with an increased prevalence of cardiovascular disease. Nephrol Dial Transplant 2011;26:864-8. [PubMed]
- Akoudad S, Szklo M, McAdams MA, et al. Correlates of kidney stone disease differ by race in a multi-ethnic middle-aged population: the ARIC study. Prev Med 2010;51:416-20. [PubMed]
- Meydan N, Barutca S, Caliskan S, et al. Urinary stone disease in diabetes mellitus. Scand J Urol Nephrol 2003;37:64-70. [PubMed]
- Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int 2005;68:1230-5. [PubMed]
- Daudon M, Jungers P. Diabetes and nephrolithiasis. Current diabetes reports 2007;7:443-448. [PubMed]
- Daudon M, Traxer O, Conort P, et al. Type 2 diabetes increases the risk for uric acid stones. J Am Soc Nephrol 2006;17:2026-33. [PubMed]
- Rule AD, Bergstralh EJ, Melton LJ 3rd, et al. Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 2009;4:804-11. [PubMed]
- Rule AD, Roger VL, Melton LJ 3rd, et al. Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 2010;21:1641-4. [PubMed]
- Vupputuri S, Soucie JM, McClellan W, et al. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol 2004;14:222-8. [PubMed]
- Curhan GC, Willett WC, Rimm EB, et al. Body size and risk of kidney stones. J Am Soc Nephrol 1998;9:1645-52. [PubMed]
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005;293:455-62. [PubMed]
- West B, Luke A, Durazo-Arvizu RA, et al. Metabolic syndrome and self-reported history of kidney stones: the National Health and Nutrition Examination Survey (NHANES III) 1988-1994. Am J Kidney Dis 2008;51:741-7. [PubMed]
- Manea A. NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 2010;342:325-39. [PubMed]
- Wilcox CS. Reactive oxygen species: roles in blood pressure and kidney function. Curr Hypertens Rep 2002;4:160-6. [PubMed]
- Wilcox CS, Welch WJ. Oxidative stress: cause or consequence of hypertension. Exp Biol Med (Maywood) 2001;226:619-20. [PubMed]
- Rodríguez-Iturbe B, Vaziri ND, Herrera-Acosta J, et al. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 2004;286:F606-16. [PubMed]
- Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep 2010;12:135-42. [PubMed]
- Furukawa S, Fujita T, Shimabukuro M, et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 2004;114:1752-61. [PubMed]
- Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal 2011;15:1911-26. [PubMed]
- Evans RG, Fitzgerald SM. Nitric oxide and superoxide in the renal medulla: a delicate balancing act. Curr Opin Nephrol Hypertens 2005;14:9-15. [PubMed]
- Schreck C, O’Connor PM. NAD(P)H oxidase and renal epithelial ion transport. Am J Physiol Regul Integr Comp Physiol 2011;300:R1023-9. [PubMed]
- Chabrashvili T, Tojo A, Onozato ML, et al. Expression and cellular localization of classic NADPH oxidase subunits in the spontaneously hypertensive rat kidney. Hypertension 2002;39:269-74. [PubMed]
- Schnackenberg CG, Welch WJ, Wilcox CS. Normalization of blood pressure and renal vascular resistance in SHR with a membrane-permeable superoxide dismutase mimetic: role of nitric oxide. Hypertension 1998;32:59-64. [PubMed]
- Zalba G, Beaumont FJ, San Jose G, et al. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension 2000;35:1055-61. [PubMed]
- Grote K, Ortmann M, Salguero G, et al. Critical role for p47phox in renin-angiotensin system activation and blood pressure regulation. Cardiovasc Res 2006;71:596-605. [PubMed]
- Landmesser U, Cai H, Dikalov S, et al. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 2002;40:511-5. [PubMed]
- Zhou MS, Hernandez Schulman I, Pagano PJ, et al. Reduced NAD(P)H oxidase in low renin hypertension: link among angiotensin II, atherogenesis, and blood pressure. Hypertension 2006;47:81-6. [PubMed]
- Taylor NE, Glocka P, Liang M, et al. NADPH oxidase in the renal medulla causes oxidative stress and contributes to salt-sensitive hypertension in Dahl S rats. Hypertension 2006;47:692-8. [PubMed]
- Tian N, Moore RS, Phillips WE, et al. NADPH oxidase contributes to renal damage and dysfunction in Dahl salt-sensitive hypertension. Am J Physiol Regul Integr Comp Physiol 2008;295:R1858-65. [PubMed]
- Kanwar YS, Sun L, Xie P, et al. A glimpse of various pathogenetic mechanisms of diabetic nephropathy. Annu Rev Pathol 2011;6:395-423. [PubMed]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058-70. [PubMed]
- Kakehi T, Yabe-Nishimura C. NOX enzymes and diabetic complications. Semin Immunopathol 2008;30:301-14. [PubMed]
- Etoh T, Inoguchi T, Kakimoto M, et al. Increased expression of NAD(P)H oxidase subunits, NOX4 and p22phox, in the kidney of streptozotocin-induced diabetic rats and its reversibity by interventive insulin treatment. Diabetologia 2003;46:1428-37. [PubMed]
- Asaba K, Tojo A, Onozato ML, et al. Effects of NADPH oxidase inhibitor in diabetic nephropathy. Kidney Int 2005;67:1890-8. [PubMed]
- Taylor EN, Fung TT, Curhan GC. DASH-style diet associates with reduced risk for kidney stones. J Am Soc Nephrol 2009;20:2253-9. [PubMed]
- Lopes HF, Martin KL, Nashar K, et al. DASH diet lowers blood pressure and lipid-induced oxidative stress in obesity. Hypertension 2003;41:422-30. [PubMed]
- Al-Solaiman Y, Jesri A, Zhao Y, et al. Low-Sodium DASH reduces oxidative stress and improves vascular function in salt-sensitive humans. J Hum Hypertens 2009;23:826-35. [PubMed]
- Holoch PA, Tracy CR. Antioxidants and self-reported history of kidney stones: the National Health and Nutrition Examination Survey. J Endourol 2011;25:1903-8. [PubMed]
- Khan SR. Reactive oxygen species as the molecular modulators of calcium oxalate kidney stone formation: evidence from clinical and experimental investigations. J Urol 2013;189:803-11. [PubMed]
- Khan SR. Stress oxidative: nephrolithiasis and chronic kidney diseases. Minerva medica 2013;104:23-30. [PubMed]
- Koul H, Kennington L, Nair G, et al. Oxalate-induced initiation of DNA synthesis in LLC-PK1 cells, a line of renal epithelial cells. Biochem Biophys Res Commun 1994;205:1632-7. [PubMed]
- Koul H, Kennington L, Honeyman T, et al. Activation of c-myc gene mediates the mitogenic effects of oxalate in LLC-PK1 cells, a line of renal epithelial cells. Kidney Int 1996;50:1525-30. [PubMed]
- Riese RJ, Mandel NS, Wiessner JH, et al. Cell polarity and calcium oxalate crystal adherence to cultured collecting duct cells. Am J Physiol 1992;262:F177-84. [PubMed]
- Scheid C, Koul H, Hill WA, et al. Oxalate toxicity in LLC-PK1 cells, a line of renal epithelial cells. J Urol 1996;155:1112-6. [PubMed]
- Verkoelen CF, van der Boom BG, Houtsmuller AB, et al. Increased calcium oxalate monohydrate crystal binding to injured renal tubular epithelial cells in culture. Am J Physiol 1998;274:F958-65. [PubMed]
- Lieske JC, Norris R, Swift H, et al. Adhesion, internalization and metabolism of calcium oxalate monohydrate crystals by renal epithelial cells. Kidney Int 1997;52:1291-301. [PubMed]
- Lieske JC, Norris R, Toback FG. Adhesion of hydroxyapatite crystals to anionic sites on the surface of renal epithelial cells. Am J Physiol 1997;273:F224-33. [PubMed]
- Lieske JC, Deganello S. Nucleation, adhesion, and internalization of calcium-containing urinary crystals by renal cells. J Am Soc Nephrol 1999;10 Suppl 14:S422-9. [PubMed]
- Lieske JC, Huang E, Toback FG. Regulation of renal epithelial cell affinity for calcium oxalate monohydrate crystals. Am J Physiol Renal Physiol 2000;278:F130-7. [PubMed]
- Lieske JC, Hammes MS, Toback FG. Role of calcium oxalate monohydrate crystal interactions with renal epithelial cells in the pathogenesis of nephrolithiasis: a review. Scanning Microsc 1996;10:519-33. [PubMed]
- Lieske JC, Farell G, Deganello S. The effect of ions at the surface of calcium oxalate monohydrate crystals on cell-crystal interactions. Urol Res 2004;32:117-23. [PubMed]
- Hammes MS, Lieske JC, Pawar S, et al. Calcium oxalate monohydrate crystals stimulate gene expression in renal epithelial cells. Kidney Int 1995;48:501-9. [PubMed]
- Lieske JC, Hammes MS, Hoyer JR, et al. Renal cell osteopontin production is stimulated by calcium oxalate monohydrate crystals. Kidney Int 1997;51:679-86. [PubMed]
- Iida S, Peck AB, Byer KJ, et al. Expression of bikunin mRNA in renal epithelial cells after oxalate exposure. J Urol 1999;162:1480-6. [PubMed]
- Iida S, Peck AB, Johnson-Tardieu J, et al. Temporal changes in mRNA expression for bikunin in the kidneys of rats during calcium oxalate nephrolithiasis. J Am Soc Nephrol 1999;10:986-96. [PubMed]
- Umekawa T, Chegini N, Khan SR. Oxalate ions and calcium oxalate crystals stimulate MCP-1 expression by renal epithelial cells. Kidney Int 2002;61:105-12. [PubMed]
- Umekawa T, Chegini N, Khan SR. Increased expression of monocyte chemoattractant protein-1 (MCP-1) by renal epithelial cells in culture on exposure to calcium oxalate, phosphate and uric acid crystals. Nephrol Dial Transplant 2003;18:664-9. [PubMed]
- Umekawa T, Iguchi M, Uemura H, et al. Oxalate ions and calcium oxalate crystal-induced up-regulation of osteopontin and monocyte chemoattractant protein-1 in renal fibroblasts. BJU Int 2006;98:656-60. [PubMed]
- Giachelli CM. Inducers and inhibitors of biomineralization: lessons from pathological calcification. Orthod Craniofac Res 2005;8:229-31. [PubMed]
- Khan SR, Kok DJ. Modulators of urinary stone formation. Front Biosci 2004;9:1450-82. [PubMed]
- Miyazawa K, Aihara K, Ikeda R, et al. cDNA macroarray analysis of genes in renal epithelial cells exposed to calcium oxalate crystals. Urol Res 2009;37:27-33. [PubMed]
- Thamilselvan S, Byer KJ, Hackett RL, et al. Free radical scavengers, catalase and superoxide dismutase provide protection from oxalate-associated injury to LLC-PK1 and MDCK cells. J Urol 2000;164:224-9. [PubMed]
- Thamilselvan S, Khan SR, Menon M. Oxalate and calcium oxalate mediated free radical toxicity in renal epithelial cells: effect of antioxidants. Urol Res 2003;31:3-9. [PubMed]
- Aihara K, Byer KJ, Khan SR. Calcium phosphate-induced renal epithelial injury and stone formation: involvement of reactive oxygen species. Kidney Int 2003;64:1283-91. [PubMed]
- Escobar C, Byer KJ, Khaskheli H, et al. Apatite induced renal epithelial injury: insight into the pathogenesis of kidney stones. J Urol 2008;180:379-87. [PubMed]
- Gáspár S, Niculiţe C, Cucu D, et al. Effect of calcium oxalate on renal cells as revealed by real-time measurement of extracellular oxidative burst. Biosens Bioelectron 2010;25:1729-34. [PubMed]
- Huang HS, Chen J, Chen CF, et al. Vitamin E attenuates crystal formation in rat kidneys: roles of renal tubular cell death and crystallization inhibitors. Kidney Int 2006;70:699-710. [PubMed]
- Khan SR. Nephrocalcinosis in animal models with and without stones. Urol Res 2010;38:429-38. [PubMed]
- Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol 2010;184:1189-96. [PubMed]
- Khan SR, Glenton PA, Byer KJ. Modeling of hyperoxaluric calcium oxalate nephrolithiasis: experimental induction of hyperoxaluria by hydroxy-L-proline. Kidney Int 2006;70:914-23. [PubMed]
- Khan SR, Johnson JM, Peck AB, et al. Expression of osteopontin in rat kidneys: induction during ethylene glycol induced calcium oxalate nephrolithiasis. J Urol 2002;168:1173-81. [PubMed]
- Katsuma S, Shiojima S, Hirasawa A, et al. Global analysis of differentially expressed genes during progression of calcium oxalate nephrolithiasis. Biochem Biophys Res Commun 2002;296:544-52. [PubMed]
- Gokhale JA, Glenton PA, Khan SR. Localization of tamm-horsfall protein and osteopontin in a rat nephrolithiasis model. Nephron 1996;73:456-61. [PubMed]
- Gokhale JA, Glenton PA, Khan SR. Characterization of Tamm-Horsfall protein in a rat nephrolithiasis model. J Urol 2001;166:1492-7. [PubMed]
- Moriyama MT, Glenton PA, Khan SR. Expression of inter-alpha inhibitor related proteins in kidneys and urine of hyperoxaluric rats. J Urol 2001;165:1687-92. [PubMed]
- Grewal JS, Tsai JY, Khan SR. Oxalate-inducible AMBP gene and its regulatory mechanism in renal tubular epithelial cells. Biochem J 2005;387:609-16. [PubMed]
- Suzuki K, Tanaka T, Miyazawa K, et al. Gene expression of prothrombin in human and rat kidneys: basic and clinical approach. J Am Soc Nephrol 1999;10 Suppl 14:S408-11. [PubMed]
- Iida S, Inoue M, Yoshii S, et al. Molecular detection of heparan sulfate proteoglycan mRNA in rat kidney during calcium oxalate nephrolithiasis. J Am Soc Nephrol 1999;10 Suppl 14:S412-6. [PubMed]
- Yasui T, Fujita K, Sasaki S, et al. Expression of bone matrix proteins in urolithiasis model rats. Urol Res 1999;27:255-61. [PubMed]
- Okada A, Yasui T, Fujii Y, et al. Renal macrophage migration and crystal phagocytosis via inflammatory-related gene expression during kidney stone formation and elimination in mice: Detection by association analysis of stone-related gene expression and microstructural observation. J Bone Miner Res 2010;25:2701-11. [PubMed]
- Gokhale JA, McKee MD, Khan SR. Immunocytochemical localization of Tamm-Horsfall protein in the kidneys of normal and nephrolithic rats. Urol Res 1996;24:201-9. [PubMed]
- Khan SR, Glenton PA, Byer KJ. Dietary oxalate and calcium oxalate nephrolithiasis. J Urol 2007;178:2191-6. [PubMed]
- Iida S, Ishimatsu M, Chikama S, et al. Protective role of heparin/heparan sulfate on oxalate-induced changes in cell morphology and intracellular Ca2+. Urol Res 2003;31:198-206. [PubMed]
- Khan SR, Shevock PN, Hackett RL. Acute hyperoxaluria, renal injury and calcium oxalate urolithiasis. J Urol 1992;147:226-30. [PubMed]
- McKee MD, Nanci A, Khan SR. Ultrastructural immunodetection of osteopontin and osteocalcin as major matrix components of renal calculi. J Bone Miner Res 1995;10:1913-29. [PubMed]
- Thamilselvan S, Hackett RL, Khan SR. Lipid peroxidation in ethylene glycol induced hyperoxaluria and calcium oxalate nephrolithiasis. J Urol 1997;157:1059-63. [PubMed]
- Thamilselvan S, Menon M. Vitamin E therapy prevents hyperoxaluria-induced calcium oxalate crystal deposition in the kidney by improving renal tissue antioxidant status. BJU Int 2005;96:117-26. [PubMed]
- Huang HS, Ma MC, Chen J, et al. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol 2002;167:2584-93. [PubMed]
- Chen DH, Kaung HL, Miller CM, et al. Microarray analysis of changes in renal phenotype in the ethylene glycol rat model of urolithiasis: potential and pitfalls. BJU Int 2004;94:637-50. [PubMed]
- de Bruijn WC, Boeve ER, van Run PR, et al. Etiology of calcium oxalate nephrolithiasis in rats. I. Can this be a model for human stone formation? Scanning Microsc 1995;9:103-14. [PubMed]
- de Water R, Noordermeer C, van der Kwast TH, et al. Calcium oxalate nephrolithiasis: effect of renal crystal deposition on the cellular composition of the renal interstitium. Am J Kidney Dis 1999;33:761-71. [PubMed]
- Kakizaki Y, Waga S, Sugimoto K, et al. Production of monocyte chemoattractant protein-1 by bovine glomerular endothelial cells. Kidney Int 1995;48:1866-74. [PubMed]
- Diamond JR, Kees-Folts D, Ding G, et al. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol 1994;266:F926-33. [PubMed]
- Wung BS, Cheng JJ, Chao YJ, et al. Cyclical strain increases monocyte chemotactic protein-1 secretion in human endothelial cells. Am J Physiol 1996;270:H1462-8. [PubMed]
- Satriano JA, Shuldiner M, Hora K, et al. Oxygen radicals as second messengers for expression of the monocyte chemoattractant protein, JE/MCP-1, and the monocyte colony-stimulating factor, CSF-1, in response to tumor necrosis factor-alpha and immunoglobulin G. Evidence for involvement of reduced nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidase. J Clin Invest 1993;92:1564-71. [PubMed]
- Moe SM, Chen NX. Mechanisms of vascular calcification in chronic kidney disease. J Am Soc Nephrol 2008;19:213-6. [PubMed]
- Shanahan CM. Vascular calcification. Curr Opin Nephrol Hypertens 2005;14:361-7. [PubMed]
- Briet M, Burns KD. Chronic kidney disease and vascular remodelling: molecular mechanisms and clinical implications. Clin Sci (Lond) 2012;123:399-416. [PubMed]
- Jono S, McKee MD, Murry CE, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 2000;87:E10-7. [PubMed]
- Kapustin AN, Davies JD, Reynolds JL, et al. Calcium regulates key components of vascular smooth muscle cell-derived matrix vesicles to enhance mineralization. Circ Res 2011;109:e1-12. [PubMed]
- Kapustin AN, Shanahan CM. Calcium regulation of vascular smooth muscle cell-derived matrix vesicles. Trends in cardiovascular medicine 2012;22:133-7. [PubMed]
- Shanahan CM, Crouthamel MH, Kapustin A, et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011;109:697-711. [PubMed]
- Shroff RC, Shanahan CM. The vascular biology of calcification. Seminars in dialysis 2007;20:103-9. [PubMed]
- Jono S, Shioi A, Ikari Y, et al. Vascular calcification in chronic kidney disease. Journal of bone and mineral metabolism 2006;24:176-81. [PubMed]
- Schoppet M, Shroff RC, Hofbauer LC, et al. Exploring the biology of vascular calcification in chronic kidney disease: what’s circulating? Kidney Int 2008;73:384-90. [PubMed]
- Murshed M, McKee MD. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr Opin Nephrol Hypertens 2010;19:359-65. [PubMed]
- Byon CH, Javed A, Dai Q, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem 2008;283:15319-27. [PubMed]
- Sun Y, Byon CH, Yuan K, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res 2012;111:543-52. [PubMed]
- Tada Y, Yano S, Yamaguchi T, et al. Advanced glycation end products-induced vascular calcification is mediated by oxidative stress: functional roles of NAD(P)H-oxidase. Horm Metab Res 2013;45:267-72. [PubMed]
- Watson KE, Bostrom K, Ravindranath R, et al. TGF-beta 1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J Clin Invest 1994;93:2106-13. [PubMed]
- Irwin CL, Guzman RJ. Matrix metalloproteinases in medial arterial calcification: potential mechanisms and actions. Vascular 2009;17 Suppl 1:S40-4. [PubMed]
- Pai A, Leaf EM, El-Abbadi M, et al. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol 2011;178:764-73. [PubMed]
- Basalyga DM, Simionescu DT, Xiong W, et al. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation 2004;110:3480-7. [PubMed]
- Vyavahare N, Jones PL, Tallapragada S, et al. Inhibition of matrix metalloproteinase activity attenuates tenascin-C production and calcification of implanted purified elastin in rats. Am J Pathol 2000;157:885-93. [PubMed]
- Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thrombosis and haemostasis 2008;100:593-603. [PubMed]
- Shanahan CM, Proudfoot D, Farzaneh-Far A, et al. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr 1998;8:357-75. [PubMed]
- Meier M, Weng LP, Alexandrakis E, et al. Tracheobronchial stenosis in Keutel syndrome. Eur Respir J 2001;17:566-9. [PubMed]
- Luo G, Ducy P, McKee M, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 1997;386:78-81. [PubMed]
- Murshed M, Schinke T, McKee M, et al. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol 2004;165:625-30. [PubMed]
- Farzaneh-Far A, Davies JD, Braam LA, et al. A polymorphism of the human matrix gamma-carboxyglutamic acid protein promoter alters binding of an activating protein-1 complex and is associated with altered transcription and serum levels. J Biol Chem 2001;276:32466-73. [PubMed]
- Herrmann SM, Whatling C, Brand E, et al. Polymorphisms of the human matrix gla protein (MGP) gene, vascular calcification, and myocardial infarction. Arterioscler Thromb Vasc Biol 2000;20:2386-93. [PubMed]
- Herrmann M, Kinkeldey A, Jahnen-Dechent W. Fetuin-A function in systemic mineral metabolism. Trends in cardiovascular medicine 2012;22:197-201. [PubMed]
- Jahnen-Dechent W, Heiss A, Schafer C, et al. Fetuin-A regulation of calcified matrix metabolism. Circ Res 2011;108:1494-509. [PubMed]
- Jahnen-Dechent W, Schäfer C, Ketteler M, et al. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med (Berl) 2008;86:379-89. [PubMed]
- Schinke T, Amendt C, Trindl A, et al. The serum protein alpha2-HS glycoprotein/fetuin inhibits apatite formation in vitro and in mineralizing calvaria cells. A possible role in mineralization and calcium homeostasis. J Biol Chem 1996;271:20789-96. [PubMed]
- Ketteler M, Wanner C, Metzger T, et al. Deficiencies of calcium-regulatory proteins in dialysis patients: a novel concept of cardiovascular calcification in uremia. Kidney Int Suppl 2003.S84-7. [PubMed]
- Leskinen Y, Lehtimaki T, Loimaala A, et al. Carotid atherosclerosis in chronic renal failure-the central role of increased plaque burden. Atherosclerosis 2003;171:295-302. [PubMed]
- Russo D, Morrone LF, Brancaccio S, et al. Pulse pressure and presence of coronary artery calcification. Clin J Am Soc Nephrol 2009;4:316-22. [PubMed]
- Qunibi WY. Cardiovascular calcification in nondialyzed patients with chronic kidney disease. Seminars in dialysis 2007;20:134-8. [PubMed]
- Pelisek J, Assadian A, Sarkar O, et al. Carotid plaque composition in chronic kidney disease: a retrospective analysis of patients undergoing carotid endarterectomy. Eur J Vasc Endovasc Surg 2010;39:11-6. [PubMed]
- Pelisek J, Hahntow IN, Eckstein HH, et al. Impact of chronic kidney disease on carotid plaque vulnerability. Journal of vascular surgery 2011;54:1643-9. [PubMed]
- Joghetaei N, Akhyari P, Rauch BH, et al. Extracellular matrix metalloproteinase inducer (CD147) and membrane type 1-matrix metalloproteinase are expressed on tissue macrophages in calcific aortic stenosis and induce transmigration in an artificial valve model. The Journal of thoracic and cardiovascular surgery 2011;142:191-8. [PubMed]
- Lenglet S, Mach F, Montecucco F. Role of matrix metalloproteinase-8 in atherosclerosis. Mediators Inflamm 2013;2013:659282.
- Gambaro G, D’Angelo A, Fabris A, et al. Crystals, Randall’s plaques and renal stones: do bone and atherosclerosis teach us something? J Nephrol 2004;17:774-7. [PubMed]
- Khan A, Wang W, Khan SR. Calcium oxalate nephrolithiasis and expression of matrix GLA protein in the kidneys. World J Urol 2014;32:123-30. [PubMed]
- Canales BK, Anderson L, Higgins L, et al. Proteomic analysis of a matrix stone: a case report. Urol Res 2009;37:323-9. [PubMed]
- Kumar V, Farell G, Yu S, et al. Cell biology of pathologic renal calcification: contribution of crystal transcytosis, cell-mediated calcification, and nanoparticles. J Investig Med 2006;54:412-24. [PubMed]
- Liu Y. Epithelial to mesenchymal transition in renal fibrogenesis: pathologic significance, molecular mechanism, and therapeutic intervention. J Am Soc Nephrol 2004;15:1-12. [PubMed]
- Zeisberg M, Bonner G, Maeshima Y, et al. Renal fibrosis: collagen composition and assembly regulates epithelial-mesenchymal transdifferentiation. Am J Pathol 2001;159:1313-21. [PubMed]
- Shoshani O, Zipori D. Transition of endothelium to cartilage and bone. Cell stem cell 2011;8:10-1. [PubMed]
- Stejskal D, Karpisek M, Vrtal R, et al. Urine fetuin-A values in relation to the presence of urolithiasis. BJU Int 2008;101:1151-4. [PubMed]
- Salama RH, Alghasham A, Mostafa MS, et al. Bone morphogenetic protein-2 will be a novel biochemical marker in urinary tract infections and stone formation. Clin Biochem 2012;45:766-9. [PubMed]
- Gao B, Yasui T, Itoh Y, et al. A polymorphism of matrix Gla protein gene is associated with kidney stones. J Urol 2007;177:2361-5. [PubMed]
- Lu X, Gao B, Liu Z, et al. A polymorphism of matrix Gla protein gene is associated with kidney stone in the Chinese Han population. Gene 2012;511:127-30. [PubMed]
- Khan SR, Glenton PA, Backov R, et al. Presence of lipids in urine, crystals and stones: implications for the formation of kidney stones. Kidney Int 2002;62:2062-72. [PubMed]
- Khan SR, Shevock PN, Hackett RL. Membrane-associated crystallization of calcium oxalate in vitro. Calcif Tissue Int 1990;46:116-20. [PubMed]
- Fasano JM, Khan SR. Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: an in vitro study. Kidney Int 2001;59:169-78. [PubMed]
- Fink HA, Wilt TJ, Eidman KE, et al. Medical management to prevent recurrent nephrolithiasis in adults: a systematic review for an american college of physicians clinical guideline. Ann Intern Med 2013;158:535-43. [PubMed]
- Borghi L, Schianchi T, Meschi T, et al. Comparison of two diets for the prevention of recurrent stones in idiopathic hypercalciuria. N Engl J Med 2002;346:77-84. [PubMed]
- Coe FL, Keck J, Norton ER. The natural history of calcium urolithiasis. JAMA 1977;238:1519-23. [PubMed]
- Lipkin ME, Preminger GM. Demystifying the medical management of nephrolithiasis. Reviews in urology 2011;13:34-8. [PubMed]
- Raynal G, Petit J, Saint F. Which efficiency index for urinary stones treatment? Urol Res 2009;37:237-9. [PubMed]
- Shanahan CM. Mechanisms of vascular calcification in renal disease. Clin Nephrol 2005;63:146-57. [PubMed]
- Shanahan CM. Vascular calcification--a matter of damage limitation? Nephrol Dial Transplant 2006;21:1166-9. [PubMed]
- Umekawa T, Tsuji H, Uemura H, et al. Superoxide from NADPH oxidase as second messenger for the expression of osteopontin and monocyte chemoattractant protein-1 in renal epithelial cells exposed to calcium oxalate crystals. BJU Int 2009;104:115-20. [PubMed]
- Thamilselvan V, Menon M, Thamilselvan S. Oxalate-induced activation of PKC-alpha and -delta regulates NADPH oxidase-mediated oxidative injury in renal tubular epithelial cells. Am J Physiol Renal Physiol 2009;297:F1399-410. [PubMed]
- Rashed T, Menon M, Thamilselvan S. Molecular mechanism of oxalate-induced free radical production and glutathione redox imbalance in renal epithelial cells: effect of antioxidants. Am J Nephrol 2004;24:557-68. [PubMed]
- Thamilselvan V, Menon M, Thamilselvan S. Selective Rac1 inhibition protects renal tubular epithelial cells from oxalate-induced NADPH oxidase-mediated oxidative cell injury. Urol Res 2012;40:415-23. [PubMed]
- Koul HK, Menon M, Chaturvedi LS, et al. COM crystals activate the p38 mitogen-activated protein kinase signal transduction pathway in renal epithelial cells. J Biol Chem 2002;277:36845-52. [PubMed]
- Chaturvedi LS, Koul S, Sekhon A, et al. Oxalate selectively activates p38 mitogen-activated protein kinase and c-Jun N-terminal kinase signal transduction pathways in renal epithelial cells. J Biol Chem 2002;277:13321-30. [PubMed]
- Toblli JE, Cao G, Casas G, et al. NF-kappaB and chemokine-cytokine expression in renal tubulointerstitium in experimental hyperoxaluria. Role of the renin-angiotensin system. Urol Res 2005;33:358-67. [PubMed]
- Jonassen JA, Cao LC, Honeyman T, et al. Mechanisms mediating oxalate-induced alterations in renal cell functions. Crit Rev Eukaryot Gene Expr 2003;13:55-72. [PubMed]
- Celie JW, Reijmers RM, Slot EM, et al. Tubulointerstitial heparan sulfate proteoglycan changes in human renal diseases correlate with leukocyte influx and proteinuria. Am J Physiol Renal Physiol 2008;294:F253-63. [PubMed]
- Capon C, Mizon C, Lemoine J, et al. In acute inflammation, the chondroitin-4 sulphate carried by bikunin is not only longer, it is also undersulphated. Biochimie 2003;85:101-7. [PubMed]
- Mizon C, Piva F, Queyrel V, et al. Urinary bikunin determination provides insight into proteinase/proteinase inhibitor imbalance in patients with inflammatory diseases. Clin Chem Lab Med 2002;40:579-86. [PubMed]
- Balduyck M, Albani D, Jourdain M, et al. Inflammation-induced systemic proteolysis of inter-alpha-inhibitor in plasma from patients with sepsis. J Lab Clin Med 2000;135:188-98. [PubMed]
- Rampoldi L, Scolari F, Amoroso A, et al. The rediscovery of uromodulin (Tamm-Horsfall protein): from tubulointerstitial nephropathy to chronic kidney disease. Kidney Int 2011;80:338-47. [PubMed]
- Urtasun R, Lopategi A, George J, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin α(V)β(3) engagement and PI3K/pAkt/NFκB signaling. Hepatology 2012;55:594-608. [PubMed]
- Mazzali M, Kipari T, Ophascharoensuk V, et al. Osteopontin--a molecule for all seasons. QJM 2002;95:3-13. [PubMed]
- Okada A, Yasui T, Hamamoto S, et al. Genome-wide analysis of genes related to kidney stone formation and elimination in the calcium oxalate nephrolithiasis model mouse: detection of stone-preventive factors and involvement of macrophage activity. J Bone Miner Res 2009;24:908-24. [PubMed]
- Escobar C, Byer KJ, Khan SR. Naturally produced crystals obtained from kidney stones are less injurious to renal tubular epithelial cells than synthetic crystals. BJU Int 2007;100:891-7. [PubMed]
- Ryall RL. The future of stone research: rummagings in the attic, Randall’s plaque, nanobacteria, and lessons from phylogeny. Urol Res 2008;36:77-97. [PubMed]
- Talham DR, Backov R, Benitez IO, et al. Role of lipids in urinary stones: studies of calcium oxalate precipitation at phospholipid langmuir monolayers. Langmuir 2006;22:2450-6. [PubMed]
- Asselman M, Verhulst A, De Broe ME, et al. Calcium oxalate crystal adherence to hyaluronan-, osteopontin-, and CD44-expressing injured/regenerating tubular epithelial cells in rat kidneys. J Am Soc Nephrol 2003;14:3155-66. [PubMed]
- Khan SR. Experimental calcium oxalate nephrolithiasis and the formation of human urinary stones. Scanning Microsc 1995;9:89-100. [PubMed]
- Khan SR. Calcium oxalate crystal interaction with renal tubular epithelium, mechanism of crystal adhesion and its impact on stone development. Urol Res 1995;23:71-9. [PubMed]
- Naito Y, Ohtawara Y, Kageyama S, et al. Morphological analysis of renal cell culture models of calcium phosphate stone formation. Urol Res 1997;25:59-65. [PubMed]
- Senzaki H, Yasui T, Okada A, et al. Alendronate inhibits urinary calcium microlith formation in a three-dimensional culture model. Urol Res 2004;32:223-8. [PubMed]
- Zeisberg M, Strutz F, Muller GA. Renal fibrosis: an update. Curr Opin Nephrol Hypertens 2001;10:315-20. [PubMed]
- Gower LB. Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization. Chem Rev 2008;108:4551-627. [PubMed]
- Habibovic P, Bassett DC, Doillon CJ, et al. Collagen biomineralization in vivo by sustained release of inorganic phosphate ions. Adv Mater 2010;22:1858-62. [PubMed]
- Coe FL, Evan AP, Worcester EM, et al. Three pathways for human kidney stone formation. Urol Res 2010;38:147-60. [PubMed]


