The effect of ABCB1 polymorphism on sirolimus in renal transplant recipients: a meta-analysis
Introduction
Renal transplantation is one of the most effective treatments for end-stage renal disease (1). The emergence of immunosuppression drugs has dramatically improved the long-term survival of allografts and patients (2). Sirolimus (SRL), also known as rapamycin, which is a potent immunosuppressive drug used for prophylaxis of allograft rejection after renal transplantation (3). SRL shows substantial interindividual differences in pharmacokinetics (4). To achieve the desired efficacy and avoid the adverse reaction, monitoring the blood concentration of SRL is necessary (5). SRL is the substrate of P-glycoprotein (P-GP), an efflux transporter encoded by the ABCB1 gene (6). P-GP transports SRL from the intracellular to the extracellular domain and influencing SRL pharmacokinetics (7). The expression and production of ABCB1 are related to single nucleotide polymorphisms (SNPs) (8). The genetic polymorphisms of ABCB1 have been considered as significant determinants of SRL pharmacokinetic (9).
Increasing studies have been conducted to investigate the influence of genetic polymorphisms of ABCB1 on SRL trough blood concentrations and pharmacokinetic parameters in renal transplantation (4,10,11). While until now, the results of the ABCB1 genotype on SRL pharmacokinetics are contradictory (12). Miao et al. (10) evaluated the relationship between the ABCB1 3435C>T genotype and C/D (trough concentrations/dose ratios) of SRL, but no significant association was observed. However, Sam et al. (13) reported that ABCB1 3435C>T genotype was significantly associated with log C/D of SRL. More than 50 genotypes have been studied in ABCB1, but most widely studied are the 3435C>T in exon 26, 1236C>T in exon 12, and three alleles 2677G>T/A in exon 21 (14). Although there are various studies on the correlation between ABCB1 polymorphisms and dose-adjusted concentration of SRL, there is no systematical evidence about the effect of ABCB1 polymorphisms on the dosage adjusted concentration of SRL. Therefore, to explore the relationship between ABCB1 C3435T, C1236T, G2677T/A genotypes, and the SRL dose requirement in kidney transplant recipients, we performed the meta-analysis in related studies.
Methods
The report followed the guidelines set out in the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement (15).
Search strategy
The studies were searched in the databases of PubMed, Embase, and the Cochrane Library up to November 2019. To investigate the association between ABCB1 polymorphism and pharmacokinetics of sirolimus in renal transplant recipients, we combined search terms as (kidney transplantation or renal transplantation) and (sirolimus or rapamycin or rapamune or AY-22989 or I-2190A) and (multidrug resistance-1 or ABCB1 or MDR1 or p-glycoprotein or P-GP or C3435T or C1236T or G2677T or G2677A or G2677T/A or rs1045642 or rs1128503 or rs2032582) and (polymorphism* or variant or mutation or genotype).
Study selection
Two reviewers evaluated studies for the titles, abstracts, and the full texts of the candidate articles (n=138) independently and in duplicate. Studies were enrolled according to the following inclusion criteria: (I) studies that assessed the association between ABCB1 C3435T, C1236T or G2677T/A polymorphisms and sirolimus metabolism; (II) provided original data including sirolimus dosage adjusted concentration [C/D ratio = concentration (ng/mL)/dose (mg/kg)] after renal transplantation; (III) studies included detailed genotyping data of ABCB1. Exclusion criteria were (I) incomplete genotype data; (II) insufficient C/D data; (III) articles only with an abstract.
Data extraction
Two independent researchers extracted the following information from each study: lead author, publication year, country of origin, ethnicity, mean or range of age, sample size, sex, therapy time (the time of renal transplant recipients treated with SRL), weight-adjusted dosage of sirolimus [the daily dose of SRL (mg) divided by the weight (mg/kg/day)], target therapeutic window (rang of dosage adjusted trough steady-state blood levels of SRL), method of genotype measured, method of concentration measured. Furthermore, the C/D ratios were shown by the form of mean ± standard deviation (SD). If the studies only provided minimum and maximum; instead, the mean ± standard deviation was estimated by the method, which was reported by Jiang et al. (16).
Quality assessment
The quality assessment of included eligible studies was conducted by the Newcastle-Ottawa Scale (NOS) (17). It consisted of three parts: a selection of participants (four items), comparability of cases, and control groups (two items), adequacy of Outcome (three items). Thus, the quality assessment score ranged from zero to nine-point. The score seven points or more were expressed high quality and insignificant risk of bias, and if less than seven points represent low or moderate quality, considered as high or moderate risk of bias.
Statistical analysis
Statistical analyses were conducted with Stata (release 15.0; Stata Corporation, College Station, TX, USA) software. The weighted mean difference (WMD) and 95% confidence intervals (CIs) on forest plots of sirolimus C/D ratio among different C1236T, C3435T and G2677T/A genotypes were evaluated. We examined the value of WMD for the allelic model, homozygous model, heterozygous model, dominant model, recessive model, over-dominant model. I-squared (I2) statistics estimated the heterogeneity. The fixed effects model was initially applied. When heterogeneity existed as I2>50%, and the random effects model was used. To evaluate the influence of ethnicity and therapy time differences in heterogeneity, subgroup analysis based on ethnicity and therapy time was performed. Moreover, deviation from the Hardy-Weinberg equilibrium (HWE) of each eligible study was assessed, and if P<0.05 was considered as disequilibrium. Studies not in HWE were subjected to sensitivity analysis. We performed a sensitivity analysis for the influence of each study on the stability of the results. Publication bias was examined by the symmetry of the funnel plot and evaluated by Egger’s test (P<0.05 was considered as significant publication bias).
Results
Studies selection and characteristics
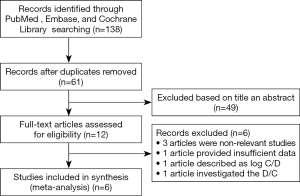
The flow diagram for the study selection process is shown in Figure 1. After a preliminary online search, a total of 138 potentially relevant articles, with 58 from PubMed, 36 from Embase, and 44 from the Cochrane Library, were named for further evaluations. There were 61 studies removed after duplicates. Then 49 studies were screened for inclusion by the titles and abstracts articles not associated with the ABCB1 polymorphisms and the C/D ratio of SRL. Six studies were excluded: 3 articles were non-relevant studies; 1 article supplied insufficient data; 1 article described as log C/D; 1 article investigated the D/C ratio. Thus, there were 6 eligible studies (10,11,18-21) described the association of ABCB1 polymorphism with the C/D ratio of SRL. These studies were conducted in different countries including China (10,18,19), Spain (20), Belgium (21), France (11). The detailed characteristics, ABCB1 genotype distributions, and dose-adjusted concentration of sirolimus of these included studies were shown in Tables 1 and 2.

Full table

Full table
Study quality assessment
The quality of the included eligible studies was evaluated according NOS. The scores of these studies were between 6 and 9, which represented high quality and minimal risk of bias. The results of the quality assessment were showed in Table 2. The distribution of the genotypes of all included studies was in HWE except for C1236T (P-HWE =0.042) of Lee et al. (18).
Association between ABCB1 C3435T polymorphism and C/D ratio of sirolimus
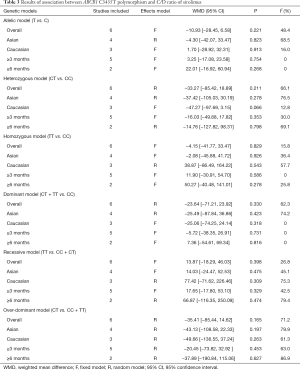
A total of six studies analyzed the association between ABCB1 C3435T polymorphism and the C/D ratio of SRL. As shown in Table 3, three studies were conducted in China (10,18,19), and the others were respectively in Spain (20), Belgium (21), and France (11). According to the statistical analysis in total populations via different genetic models, no significant association was observed between ABCB1 C3435T polymorphism and C/D ratio of SRL. The subgroup analyses were performed according to the ethnicity of recipients (grouped as Asian or Caucasian) and the interval after transplantation (grouped as over 3 months or over 6 months). No significant association was found in subgroups of ethnicity and the interval after transplantation. Overall, there was no significant effect of ABCB1 C3435T polymorphism on the dose-adjusted trough level of SRL.
Full table
Association between ABCB1 C1236T polymorphism and C/D ratio of sirolimus
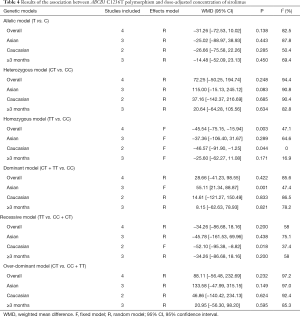
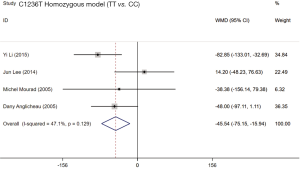
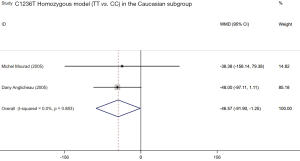
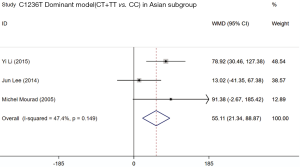
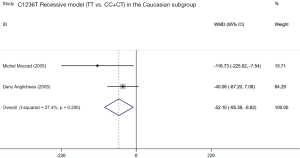
Four included studies evaluated the association between ABCB1 C1236T polymorphism and C/D ratio of SRL. As shown in Table 4, two studies were conducted in China (18,19), 1 in Belgium (21), and 1 in France (11). According to the statistical analysis, significant association were observed in the homozygous model of all patients (TT vs. CC; WMD: −45.54; 95% CI: −75.15, −15.94; P=0.003), subgroup of Caucasian in the homozygous model (TT vs. CC; WMD: −46.57; 95% CI: −91.90, −1.25; P=0.044), subgroup of Asian in the dominant model (CT + TT vs. CC; WMD: 55.11; 95% CI: 21.34, 88.87; P=0.001), and subgroup of Caucasian in the recessive model (TT vs. CC + CT; WMD: −52.10; 95% CI: −95.38, −8.82; P=0.018). The forest plots were shown in Figures S1-S4. The subjects with TT genotype in Caucasian subgroup ABCB1 C1236T had a lower C/D ratio and needed higher sirolimus dose than those with CC genotype.
Full table
Association between ABCB1 G2677T/A polymorphism and C/D ratio of sirolimus
The ABCB1 2677G>T/A mutation could lead to two changes of an amino acid (from alanine to serine or threonine) (22). The genotypes for the ABCB1 2677G> T/A SNP were classified as follows: wild type (G/G), heterozygous (G/T or G/A) and homozygous for the variant (T/T, T/A or A/A). Due to the diversity of this genotype, data can not be merged simply.
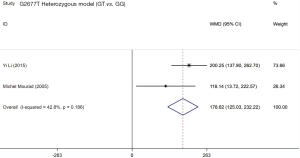
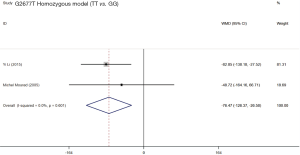
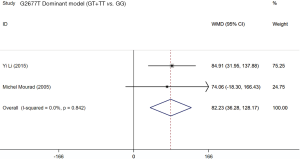
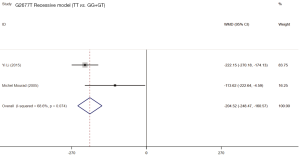
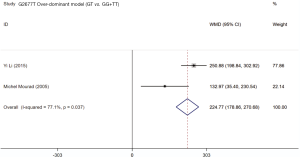
Two studies (19,21) assessed the influence of ABCB1 G2677T polymorphism on the dose-adjusted trough level of SRL, and the summarized results were shown in Table 5. According to the statistical analysis, significant differences were found in association between the ABCB1 G2677T polymorphism and the C/D ratio of SRL in the heterozygous model (GT vs. GG,WMD: 178.62; 95% CI: 125.03, 232.22; P=0.000), the homozygous model (TT vs. GG; WMD: −76.47; 95% CI: −126.37, −26.58; P=0.003), the dominant model (GT + TT vs. GG; WMD: 82.23; 95% CI: 36.28, 128.17; P=0.000), the recessive model (TT vs. GG + GT; WMD: −179.38; 95% CI: −283.33, −75.42; P=0.001), and the over-dominant model (GT vs. GG + TT; WMD: 199.44; 95% CI: 84.84, 314.05; P=0.001). The forest plots were shown in Figures S5-S9.

Full table
Two studies (11,18) assessed the influence of ABCB1 G2677mutant polymorphism on the C/D ratio of SRL. Mutant type included TT, TA or AA in both of these studies. The summarized results were shown in Table 6. The results of heterogeneity within all genetic models were 0. Moreover, no significant difference was found in association with the ABCB1 G2677mutant polymorphism with the C/D ratio of SRL.

Full table
Sensitivity analysis
As shown in Table 2, only one study (18) included in the meta-analysis was a departure from HWE (P<0.05). A sensitivity analysis was performed by sequential omission of each eligible study to assess the influence of the individual data on the pooled WMDs. The results revealed that the departure from HWE of study has no major impact. Sensitivity analysis to evaluate the ethnicity and therapy time showed that no individual study influenced the pooled estimate significantly. The results are shown in Figures S10-S13. None of the studies had an individually considerable influence on the impact of ABCB1 C3435T, C1236T, G2677T/A. Sensitivity analyses suggested that this meta-analysis was steady.
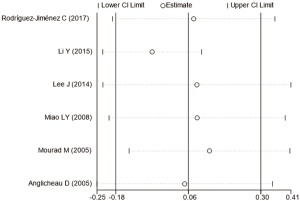
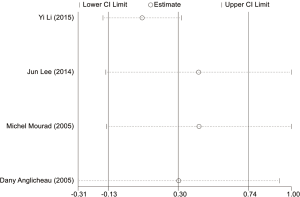
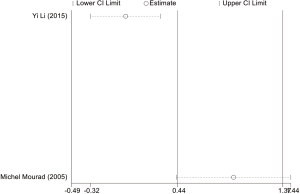
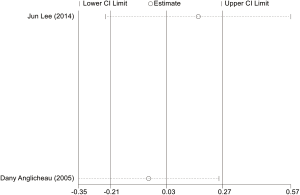
Estimation of publication bias
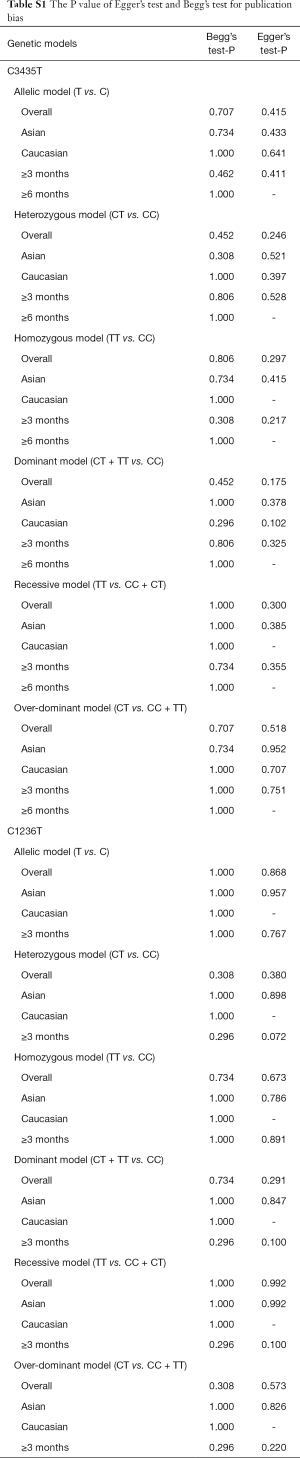
The potential publication bias of eligible studies was assessed by the funnel plot, Egger’s test, and Begg’s test. As shown in Figures S14-S17, the funnel plots did not provide evidence of obvious asymmetry. The Egger’s test and Begg’s test for publication bias were not statistically significant in all the genetic models of ABCB1 C3435T, C1236T (Table S1), because of the number of G2677T/A studies was small, the Egger’s test cannot be displayed.
Full table
Discussion
Sirolimus (SRL) is a necessary immunosuppressive drug after renal transplantation. Nevertheless, SRL exhibit significant interindividual variability in pharmacokinetics (23). It is necessary for therapeutic drug monitoring to avoid under or over-immunosuppression. It has been suggested that ABCB1 polymorphisms contribute to the variability of SRL pharmacokinetics and therapeutic outcome (24). Although the influence of ABCB1 polymorphisms on SRL metabolism has been studied focusing on C3435T, C1236T, and G2677T/A, the relationship between ABCB1 polymorphism and SRL metabolism in patients is still unclear. Therefore, our study was to explore the relationship between ABCB1 polymorphisms and the pharmacokinetics of SRL in renal transplantation by a meta-analysis of existing data. Our work is helpful to evaluate that whether ABCB1 genetic testing is expected to play a role in guiding the individualized treatment of SRL.
The AUC is challenging to apply in clinical practice, so other indicators such as trough concentration (C0) replace the AUC (25). That is why AUC is rarely reported in these included studies. To make a comparison between the different doses, the dosage adjusted trough concentration C/D ratio was adopted in our study.
ABCB1 C3435T, a silent SNP localized in exon 26, has been found to be associated with altered P-GP function. It was reported that the homozygosity for the T allele resulted in a 2-fold reduction in intestinal P-GP expression (26). However, our overall analysis of pooled results demonstrated no statistically significant association between the C/D ratio of SRL and ABCB1 C3435T polymorphism in different genetic models. In addition, relatively obvious heterogeneities existed in our study. With the aim of detecting the source of heterogeneity, we conducted stratified analysis according to the ethnicity and the interval after transplantation. The results were consistent with the overall analysis. Therefore, so far, there was no enough evidence showing the clinical relevance of the ABCB1 C3435T polymorphism and the dosage adjusted trough concentration of SRL in Caucasians or Asians.
Significant association were observed between ABCB1 C1236T polymorphism and C/D ratio of sirolimus in all patients via the homozygous model (TT vs. CC). The following subgroup analysis indicated the ethnicity of the renal transplant recipients might be one of the most critical covariates that could influence the dose adjusted concentration of SRL. The result showed that homozygous mutated genotype TT had a significant impact on the C/D ratio of sirolimus in Caucasians but nor in Asians. It was also found that the dose adjusted concentrations of SRL in Caucasian patients with ABCB1 C1236T CC carriers are significantly higher than TT carriers. Therefore, Caucasian renal transplant recipients ABCB1 C1236T TT carriers might need higher doses of SRL than CC carriers recipients.
The triallelic SNP G2677T/A results in a change of the amino acid alanine into serine or threonine (27) and may alter drug transport (28), whereas the synonymous SNP C3435T and C1236T are a silent mutation that do not lead to an amino acid change. The pooled analysis of studies focusing on G2677T polymorphism(alleles G and T) suggested that the polymorphism has significant influence on the C/D ratio of SRL. Patients carrying G2677T homozygous genotype TT would require higher doses of SRL to reach target levels compared with the wild genotype GG. However, The results of the pooled analysis about G2677mutant polymorphism (alleles G, A and T) showed no significant differences between ABCB1 G2677mutant and the C/D ratio of SRL within all the genetic models. The small sample size may limit the analysis.
While each of the polymorphisms in the ABCB1 haplotype may be independent, they may produce a much more salient phenotype when they appear together. One study was performed associated between ABCB1 C1236T/G2677T/C3435T haplotypes analyses and the C/D ratio of SRL. Among the haplotypes, TTT, TGC, and CGC were the most frequently observed (29). Lee et al. (18) showed that patients carrying the CGC/CGC diplotype had a significantly lower C/D ratio of SRL compared with those carrying the CGC/TTT and TTT/TTT diplotype (P<0.05).
This meta-analysis pooled available data from eligible studies and significantly increased the statistical reliability. Also, there are some advantages to this meta-analysis. Firstly, this research is the first one to estimate the association between ABCB1 polymorphism and the dosage adjusted concentration of SRL in renal transplant recipients. Secondly, the subgroup for the stratified analysis of potential sources of heterogeneity was performed based on ethnicity and the interval after transplantation. Thirdly, this study systematically analyzed the six genetic models to explore the association between the dosage adjusted concentration of SRL and ABCB1 polymorphism.
Although the meta-analysis conducted considerable retrieval and analysis, there are still several limitations existed. First of all, high heterogeneity existed in more than half of outcomes, and lots of factors could lead to heterogeneity, such as differences among various therapy regimens, disease staging, age, sex and method of genotype and concentration detecting. However, the complete data were hardly accessed to perform subgroup analysis. Some of these factors might further influenced the results. Second, several eligible studies are excluded due to the absence of available original data, which may have an impact on this meta-analysis. Third, the sample sizes of the included studies were relatively small. Further studies are expected to provide high-quality evidence.
Conclusions
In summary, this meta-analysis showed that no significant association exists between ABCB1 C3435T polymorphisms and the C/D ratio of SRL in renal transplant recipients. However, compared with ABCB1 C1236T CC carriers, those with TT genotype will require a higher dose of sirolimus to achieve target therapeutic concentrations in Caucasian renal transplant recipients. ABCB1 G2677T/A TT genotype will require a higher dose of sirolimus than wild type GG genotype. Performing ABCB1 C1236T and G2677T genotyping before transplantation may guide to improve the individual immunosuppressive therapy. Further studies with large sample size are expected to confirm the relationship of ABCB1 polymorphisms and the pharmacokinetics of SRL in renal transplant recipients.
Acknowledgments
Funding: This work was supported by the Beijing Municipal Natural Science Foundation (grant No. 7192218).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Webster AC, Lee VW, Chapman JR, et al. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation 2006;81:1234-48. [Crossref] [PubMed]
- Woodworth TG, Furst DE. Timely renal transplantation for scleroderma end-stage kidney disease patients can improve outcomes and quality of life. Ann Transl Med 2019;7:60. [Crossref] [PubMed]
- Haeri A, Osouli M, Bayat F, et al. Nanomedicine approaches for sirolimus delivery: a review of pharmaceutical properties and preclinical studies. Artif Cells Nanomed Biotechnol 2018;46:1-14. [Crossref] [PubMed]
- Renders L, Frisman M, Ufer M, et al. CYP3A5 genotype markedly influences the pharmacokinetics of tacrolimus and sirolimus in kidney transplant recipients. Clin Pharmacol Ther 2007;81:228-34. [Crossref] [PubMed]
- Liu YY, Li C, Cui Z, et al. The effect of ABCB1 C3435T polymorphism on pharmacokinetics of tacrolimus in liver transplantation: A meta-analysis. Gene 2013;531:476-88. [Crossref] [PubMed]
- Lampen A, Zhang Y, Hackbarth I, et al. Metabolism and transport of the macrolide immunosuppressant sirolimus in the small intestine. J Pharmacol Exp Ther 1998;285:1104-12. [PubMed]
- Ambudkar SV, Kim IW, Sauna ZE. The power of the pump: Mechanisms of action of P-glycoprotein (ABCB1). Eur J Pharm Sci 2006;27:392-400. [Crossref] [PubMed]
- Sakaeda T, Nakamura T, Okumura K. Pharmacogenetics of MDR1 and its impact on the pharmacokinetics and pharmacodynamics of drugs. Pharmacogenomics 2003;4:397-410. [Crossref] [PubMed]
- Rosso Felipe C, de Sandes TV, Sampaio ELM, et al. Clinical Impact of Polymorphisms of Transport Proteins and Enzymes Involved in the Metabolism of Immunosuppressive Drugs. Transplant Proc 2009;41:1441-55. [Crossref] [PubMed]
- Miao LY, Huang CR, Hou JQ, et al. Association study of ABCB1 and CYP3A5 gene polymorphisms with sirolimus trough concentration and dose requirements in Chinese renal transplant recipients. Biopharm Drug Dispos 2008;29:1-5. [Crossref] [PubMed]
- Anglicheau D, Le Corre D, Lechaton S, et al. Consequences of Genetic Polymorphisms for Sirolimus Requirements After Renal Transplant in Patients on Primary Sirolimus Therapy. Am J Transplant 2005;5:595-603. [Crossref] [PubMed]
- Cattaneo D, Baldelli S, Perico N. Pharmacogenetics of immunosuppressants: progress, pitfalls and promises. Am J Transplant 2008;8:1374-83. [Crossref] [PubMed]
- Sam WJ, Chamberlain CE, Lee SJ, et al. Associations of ABCB1 3435C>T and IL-10-1082G>A polymorphisms with long-term sirolimus dose requirements in renal transplant patients. Transplantation 2011;92:1342-7. [Crossref] [PubMed]
- Pauli-Magnus C, Kroetz DL. Functional implications of genetic polymorphisms in the multidrug resistance gene MDR1 (ABCB1). Pharm Res 2004;21:904-13. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Jiang ZP, Wang YR, Xu P, et al. Meta-analysis of the effect of MDR1 C3435T polymorphism on cyclosporine pharmacokinetics. Basic Clin Pharmacol Toxicol 2008;103:433-44. [Crossref] [PubMed]
- Zeng X, Zhang Y, Kwong JS, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2-10. [Crossref] [PubMed]
- Lee J, Huang H, Chen Y, et al. ABCB1 haplotype influences the sirolimus dose requirements in Chinese renal transplant recipients. Biopharm Drug Dispos 2014;35:164-72. [Crossref] [PubMed]
- Li Y, Yan L, Shi Y, et al. CYP3A5 and ABCB1 genotype influence tacrolimus and sirolimus pharmacokinetics in renal transplant recipients. Springerplus 2015;4:637. [Crossref] [PubMed]
- Rodríguez-Jiménez C, Garcia-Saiz M, Perez-Tamajon L, et al. Influence of genetic polymorphisms of CYP3A5 and ABCB1 on sirolimus pharmacokinetics, patient and graft survival and other clinical outcomes in renal transplant. Drug Metab Pers Ther 2017;32:49-58. [Crossref] [PubMed]
- Mourad M, Mourad G, Wallemacq P, et al. Sirolimus and tacrolimus trough concentrations and dose requirements after kidney transplantation in relation to CYP3A5 and MDR1 polymorphisms and steroids. Transplantation 2005;80:977-84. [Crossref] [PubMed]
- Sakurai A, Onishi Y, Hirano H, et al. Quantitative structure--activity relationship analysis and molecular dynamics simulation to functionally validate nonsynonymous polymorphisms of human ABC transporter ABCB1 (P-glycoprotein/MDR1). Biochemistry 2007;46:7678-93. [Crossref] [PubMed]
- Kahan BD, Napoli KL, Kelly PA, et al. Therapeutic drug monitoring of sirolimus: correlations with efficacy and toxicity. Clin Transplant 2000;14:97-109. [Crossref] [PubMed]
- Su L, Yin L, Yang J, et al. Correlation between gene polymorphism and blood concentration of calcineurin inhibitors in renal transplant recipients: An overview of systematic reviews. Medicine 2019;98:e16113. [Crossref] [PubMed]
- Mahalati K, Belitsky P, Sketris I, et al. Neoral monitoring by simplified sparse sampling area under the concentration-time curve: its relationship to acute rejection and cyclosporine nephrotoxicity early after kidney transplantation. Transplantation 1999;68:55-62. [Crossref] [PubMed]
- Singh R, Srivastava A, Kapoor R, et al. Do drug transporter (ABCB1) SNPs influence cyclosporine and tacrolimus dose requirements and renal allograft outcome in the posttransplantation period? J Clin Pharmacol 2011;51:603-15. [Crossref] [PubMed]
- Haerian BS, Lim KS, Tan CT, et al. Association of ABCB1 gene polymorphisms and their haplotypes with response to antiepileptic drugs: a systematic review and meta-analysis. Pharmacogenomics 2011;12:713-25. [Crossref] [PubMed]
- Loo TW, Clarke DM. Functional consequences of proline mutations in the predicted transmembrane domain of P-glycoprotein. J Biol Chem 1993;268:3143-9. [PubMed]
- Tang K, Ngoi SM, Gwee PC, et al. Distinct haplotype profiles and strong linkage disequilibrium at the MDR1 multidrug transporter gene locus in three ethnic Asian populations. Pharmacogenetics 2002;12:437-50. [Crossref] [PubMed]