
Syndromic Wilms tumor: a review of predisposing conditions, surveillance and treatment
Introduction
Wilms tumor (WT) is the most common renal malignancy in pediatric populations. Approximately 9–17% of all WTs are associated with a predisposing syndrome (1). The most common syndromes associated with WT are WAGR (Wilms-Aniridia-Genitourinary-mental Retardation), Denys-Drash syndrome (DDS), Beckwith-Wiedemann syndrome (BWS), isolated hemihypertrophy, and Perlman syndrome. At least 100 other syndromes have been associated with WT (2), and that number seems likely to continue growing. Recent case reports persist in describing new syndromes associated with an increased incidence of WT (3,4).
In addition to an increased likelihood of developing malignancy, children are typically diagnosed with syndromic WT at an earlier age than those with non-syndromic WT, at 1 versus 3–4 years old, respectively (2). Despite this earlier presentation, in a study of 12 patients who presented with WT prior to diagnosis of Beckwith Wiedemann, 58% presented at stage 3–5 disease which is similar to that of sporadic WT (5). In general, bilateral WTs account for about 5% of all WT. As they are associated with germline mutations of WT1, bilateral tumors occur at higher rates than in non-syndromic WT. There is also a greater likelihood of developing metachronous bilateral tumors and having nephrogenic rests (6).
The risk of developing malignancy varies by syndrome. Scott et al. assigned risk categories to various conditions. Syndromes with a high risk, or greater than 20%, of developing WT include: WAGR, DDS, familial Wilms, Perlman syndrome, mosaic variegated aneuploidy, Fanconi anemia/biallelic BRCA2. Those with a moderate risk, or 5–20%, include Frasier syndrome, BWS, and Simpson-Golabi-Behmel syndrome (SGBS). Finally, syndromes where less than 5% develop WT, or low risk, include: isolated hemihypertrophy, Bloom syndrome, Li-Fraumeni, Hereditary hyperparathyroidism-jaw tumor syndrome, mulibrey nanism, Trisomy 18, and 2q37 microdeletion syndrome (2).
Due to the differences mentioned above, including increased frequency, earlier age at diagnosis, and higher incidence of bilateral disease, approaches to management differ when compared to sporadic WT when an underlying predisposing syndrome is present. These patients should be offered nephron-sparing surgeries, cautious chemotherapeutic and radiation therapy, and familial genetic counseling. We will review the various syndromes, current surveillance strategies, and treatment recommendations for the most common syndromic WT.
Syndromes
Each of the various syndromes associated with WT has various characteristic findings. Generally, diagnoses are made in infancy, but some may not present until they develop WT or renal failure. If a patient is referred with a new renal mass, a thorough family history and physical exam to investigate for stigmata of various syndromes (Table 1) is imperative. Similarly, patients with bilateral disease or imaging findings suggestive of renal dysplasia and lab abnormalities are also more likely to have an underlying syndrome. When there is suspicion of a cancer predisposing syndrome, consider prompt referral to genetics, as preoperative chemotherapy and nephron-sparing may be preferable to radical nephrectomy. The geneticist can also counsel the family about their risks in the event that a syndrome is identified. Table 2 presents some of the genes known to be associated with the syndromes discussed below.

Full table
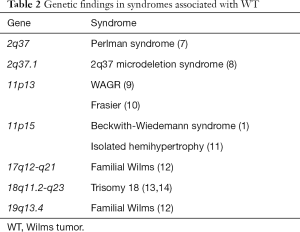
Full table
High risk syndromes
WAGR is due to a germline deletion encompassing one allele of WT1. The severity of phenotypes depends upon the size of deletion, followed by a mutation event in the second WT1 allele (6). Patients with WAGR have a 50% risk of developing WT with median age at 22 months (2). Approximately 17% of patients with WAGR and WT will have bilateral disease (6). A study of 54 patients with WAGR found that 70% had three or four of the classic findings. All but one patient had aniridia. Genitourinary anomalies included undescended testes, abnormalities of the female internal genital organs, and “ambiguous genitalia”. They also noted 70% of the patients had an IQ <74 (9).
DDS presents as a triad of disorders: genital abnormalities, congenital nephropathy leading to end stage renal disease, and WT. The genital abnormalities can vary, ranging from ambiguous genitalia and proximal hypospadias, to undescended testes. Also associated with the WT1 gene, renal histologic findings are typically diffuse mesangial sclerosis or focal segmental glomerulosclerosis. Patients with DDS have as high as 95% chance of developing WT with median age of occurrence at 12 months (15). Of children with DDS and WT, 20% will have bilateral masses (6). They are also at risk of developing gonadoblastoma (16).
Of patients with WT, 2% will have a family history. As with other predisposing conditions, familial Wilms occurs at a younger age and with a higher prevalence of bilateral disease, which can occur in up to 16% of patients. Most patients with familial Wilms do not exhibit features of the other syndromes. Genetic studies would suggest that there is great variability in WT features, including age and laterality, among affected individuals with familial Wilms, with unaffected carriers of the genetic trait, FWT1 and FWT2 (12).
Perlman syndrome is a rare, autosomal recessive condition involving the DIS3L2 gene, where 75% of patients will have nephroblastomatosis, predisposing them to WT. Antenatal studies often show polyhydramnios, macrosomia, fetal ascites, and large kidneys. Postnatally, this overgrowth syndrome should be distinguished from BWS and SGBS. Besides WT, these children present with macrosomia, nephromegaly, hepatomegaly, hypotonia, and characteristic facies. Approximately 75% of male infants will also have cryptorchidism (7). Infant mortality is high, from respiratory distress, pulmonary hypoplasia, and renal failure (17). While only 28% of children with Perlman syndrome ultimately develop WT, it is estimated that up to two-thirds of those who survive infancy will be diagnosed with malignancy (7), with 55% of them having bilateral WT (6).
Mosaic variegated aneuploidy is an autosomal recessive syndrome where multiple chromosomes are impacted, with mosaic aneuploidies. The BUB1B gene has been specifically implicated. In addition to an increased risk of WT, these patients often have growth deficiencies, microcephaly, intellectual disability, and anomalies of the central nervous system. Patients are also at increased risk of developing rhabdomyosarcoma and leukemia (18). Patients with premature chromatic separation/mosaic variegated aneuploidy have the additional risks of cataracts, seizures, and polycystic kidneys (19).
Fanconi anemia is a heterogeneous condition with numerous possible genetic mutations and a variable clinical picture. Patients specifically with BRCA2/FANCD1 mutations are at risk of developing a solid tumor, most commonly WT (20). This subtype is known as Fanconi anemia D1 (2). While diagnosis is sometimes delayed until after the cancer is identified, other clinical features include failure to thrive, abnormal skin pigmentation, skeletal anomalies, renal abnormalities, and dysmorphic facial features. Many patients will also have a strong family history of malignancy because of the BRCA2 association, most commonly breast, prostate, and/or pancreatic cancer. Solid tumors besides WT have been reported in children with Fanconi anemia, including rhabdomyosarcoma, neuroblastoma, and brain tumors, however myelodysplasia and acute myeloid leukemia are the most common malignancies seen in this population (20).
Moderate risk syndromes
Frasier syndrome is very similar to DDS in that renal failure, disorders of sex development, and gonadal tumors feature prominently. Their shared features are unsurprising, as both conditions are associated with aberrations in WT1 (10). Patients with Frasier have complete gonadal dysgenesis. While WT occurs in children with Frasier syndrome (21), gonadoblastoma appears more commonly, at rates of up to 60%. Renal impairment is often the initial sign; infants present with proteinuria, hypertension, and edema (10). Renal failure does not always begin in infancy but may be delayed until or beyond adolescence (22). Older children may be diagnosed during adolescence with delayed puberty (10).
BWS, another WT1-associated syndrome, is characterized by hemihypertrophy or lateralized overgrowth, macroglossia, macrosomia, organomegaly, hyperinsulinism, omphalocele or umbilical hernia, and ear creases and/or pits. BWS patients have a 4.1% risk of developing WT, with median age of occurrence at 24 months (23). Occasionally, WT may be the presenting symptom of BWS. In a single institution’s review of their experience with 12 BWS patients who presented with WT, all patients had favorable histology but presented at higher stages, more consistent with sporadic WT. The authors recommend thoroughly examining all pediatric patients with renal masses for subtle BWS findings, so that if diagnosed, partial nephrectomy may be offered (5). This is especially relevant as tumors are bilateral in 17.3% of patients diagnosed with WT (6). Children with BWS are also at risk of developing hepatoblastoma (24).
An x-linked overgrowth syndrome associated with the GPC3 gene, patients with SGBS exhibit macrosomia, macrocephaly, organomegaly, distinct facial features, and abnormalities across most organ systems, including genitourinary anomalies (25). Newborns may experience hypoglycemia or have airway issues secondary to micrognathia and glossoptosis. Genitourinary anomalies include renal dysplasia and nephromegaly, in addition to undescended testes and hypospadias in males (26). As with BWS, these children are at an increased risk of developing both WT and hepatoblastoma (25). Additionally, neuroblastoma, gonadoblastoma, and medulloblastoma have been reported (26).
Isolated hemihypertrophy is defined as an asymmetric regional body growth secondary to an abnormal cell proliferation, with no other syndromic features despite sharing WT1 aberrations. As hemihypertrophy is a feature of several other syndromes, including BWS, proteus syndrome, neurofibromatosis type 1, mosaic trisomy 8, as well as with vascular malformation syndromes, it is important that the diagnosis be made by a geneticist (11). The incidence of WT in isolated hemihypertrophy is thought to be around 3% (2). Other abdominal masses have also been noted to occur in patients with isolated hemihypertrophy, including hepatoblastoma, adrenal cell carcinoma, and leiomyosarcoma of the small bowel (27).
Low risk syndromes
Bloom syndrome is an autosomal recessive condition involving the BLM gene and characterized by small size, microcephaly, sun sensitivity, insulin resistance, and immunodeficiency, in addition to the increased risk of malignancies. As well as WT, patients are at risk of developing leukemia and lymphoma, along with oropharyngeal, gastrointestinal, and integumentary cancers. Other long-term manifestations include chronic obstructive pulmonary disease, myelodysplasia, and diabetes mellitus (28).
Li Fraumeni syndrome, which predisposes the affected individual to a host of various malignancies, has been associated with WT. The more commonly associated neoplasms include: sarcoma, osteosarcoma, breast cancer, brain tumors, adrenocortical carcinoma, and leukemias. The clinical diagnosis is made when an individual has a sarcoma diagnosed before age 45, has a first degree relative with any malignancy before age 45, or a sarcoma at any age (29).
Hyperparathyroidism-jaw tumor syndrome is a condition that typically presents in later adolescence or adulthood. The initial finding is hyperparathyroidism. Around a third of patients develop ossifying fibromas in the mandible or maxilla. In addition to an increased risk of WT, other renal findings include cysts or hamartomas. Women are at risk of developing uterine tumors, both benign and malignant (30).
MULIBREY (MUscle, LIver, BRain, EYe) nanism is associated with ectopic tissue, as well as benign and malignant neoplasms (31). It is most commonly found among those of Finnish descent (32). Affected individuals typically exhibit growth deficiencies, cardiomyopathies, characteristic facies, and a predisposition towards developing metabolic disorders, such as type II diabetes mellitus (31,33). Important diagnoses to exclude include 3-M and Silver-Russel syndromes, as they are not associated with the risks of malignancy and cardiac disease (32). Widespread tumors are encountered, including benign and malignant neoplasms of the liver, kidney, pancreas, thyroid, and central nervous system. Genitourinary masses include: renal cysts, renal hamartomas, angiomyolipomas, WT, renal cell carcinoma, ovarian cysts, ovarian carcinoma, endometrial adenocarcinoma, and epididymal cysts (31). A study of over 100 patients with mulibrey nanism found that 14% had renal masses, the majority of which were WT. In addition to renal masses, patients also may have structural or location anomalies of the kidney (33).
Trisomy 18, also known as Edwards syndrome, historically has a high neonatal mortality rate, primarily from congenital cardiac anomalies. These children also typically have dysmorphic facial features, clenched hands, and rocker-bottom feet, in addition to a host of other less common abnormalities. Hepatoblastoma has been identified most commonly, followed by WT. There are also case reports of children with a neuroblastoma and lymphoma (13).
2q37 microdeletion syndrome has varied findings, including: characteristic facial features, short stature, obesity, hypotonia, developmental and intellectual disabilities, and bony abnormalities including brachymetaphalangy, hypermobile joints, and scoliosis. They may also have anomalies of the cardiac, central nervous, and gastrointestinal systems. Renal cysts and horseshoe kidney are described. The risk of WT is less than 5% for patients without 2q37.1 deletions but is thought to be higher for those with that specific genetic finding (8).
Screening/surveillance
The first step in an effective screening protocol for syndromic WT is to identify who has a syndrome that predisposes patients to the malignancy. Cullinan et al. developed an eHealth decision support tool to assist physicians in identifying children at an elevated risk of having an underlying cancer predisposition syndrome (34). Recognizing the physical manifestations of the syndromes associated with WT is crucial to ensuring the patient receives prompt tumor detection. For example, children with WAGR and aniridia are diagnosed with WT sooner than those without (35). Proceed with caution before determining that a child with WT does not have a syndrome; there is a subset of WT with an underlying predisposition syndrome without phenotypic abnormalities. At a single institution in the Netherlands, 109 patients with WT were tested for WT1 and 11p15 aberrations. There were 12 patients found to have a WT1 aberration. Three of those patients were not found to have any phenotypic aberrations. A total of 8 patients were found to have 11p15 imprinting aberrations, four of which were only diagnosed with BWS after the discovery of WT. Two of these patients had very minor features. Due to their findings, authors suggest all WT should be offered genetic counseling (1). Similarly, variability has been described in the phenotype of mosaic variegated aneuploidy. A high index of suspicion is necessary on the part of pediatricians and pediatric specialists to ensure that patients are referred to geneticists (18,36). Dumoucel et al. propose a referral to genetics when there is bilateral disease, a family history of WT, a family history of cancer, or a major malformation or two or more minor malformations on physical or radiologic examination (37).
Because of the increased risks of WT associated with various syndromes, screening is recommended to identify tumors earlier. Prior to instituting screening protocols, one must consider if it is cost-effective, accurate, and low risk. Other factors to consider include timing, end-point, and modality (29). Screening has been shown to be effective in children with BWS and isolated hemihypertrophy. In a series including 15 patients who underwent quarterly ultrasound and 59 who did not, no screened patients were diagnosed with stage III or higher WT, while 42% of the tumors diagnosed with non-screened children were stage III or higher (P<0.003) (38). Ultrasound is an optimal screening tool, as it is widely available, lacks ionizing radiation, and can be performed without sedation (24). Magnetic resonance imaging (MRI) has been suggested as a screening modality for certain cancer predisposition syndromes. There are drawbacks, including cost, potential need for sedation or anesthesia, and anxiety (39). MRI is considered ideal for detection of multiple tumors and nephrogenic rests, including differentiating between active and inactive rests (40). Table 3 presents the various neoplasms associated with the previously discussed syndromes.
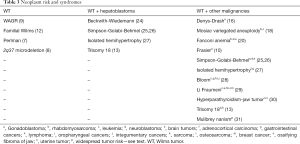
Full table
WT have a rapid growth rate, with a doubling time reported at 11–40 days. Interval tumor growths have been found at 4–6 months (41). While each individual syndrome does not necessarily have set surveillance protocols, general syndromic screening recommendations include renal ultrasound every 3–4 months to address this doubling time. When determining who should be surveilled, it is important to first have the patient evaluated by a geneticist. Children at a greater risk of developing WT should undergo screening ultrasound until the age of 5, with some exceptions that should be surveilled until the age of 7, including: BWS, SGBS, and some familial WT (42). Others also recommend surveilling isolated/idiopathic hemihypertrophy, Perlman, WAGR, DDS, Frasier, mulibrey nanism, and trisomy 18 until the age of 7 (24). Several syndromes with more specific surveillance recommendations will be discussed further.
WAGR has the highest reported rates of WT, with one report of 54 patients describing 57% with the malignancy (9). It is recommended that patients undergo serial renal ultrasound until the age of 7, approximately every 3 months (24). It is also recommended that patients with a personal history of WT be surveilled indefinitely, although formal guidelines are lacking. Families should also be educated about the importance of prompt evaluation of hematuria and hypertension, and some may also perform abdominal exams. Because of the prevalence of renal insufficiency, patients should also undergo blood pressure measurements, urine analyses to assess for proteinuria, and serum blood urea nitrogen and creatinine levels (9). Of children with WAGR who develop bilateral WT, 90% ultimately are diagnosed with end stage renal disease (35).
There exists a paucity of literature that specifically addresses surveillance of DDS for WT, presumably as these patients reach end stage renal disease status prior to WT diagnosis. As discussed previously, the first step remains diagnosis of a cancer-predisposing syndrome. In a retrospective study of 12 patients with DDS and Frasier at a single institution, authors found that of eight 46XY patients with “ambiguous genitalia”, six had nephropathy and four had WT. The authors thus advocate for systematic genetic analysis of WT1 gene in XY patients with XY disorders of sex development or pure gonadal dysgenesis, so that DDS and Frasier might be diagnosed, as those with a WT1 mutation are at risk of developing renal disease and WT (43). There are several case reports of tragic presentations of acute renal failure in patients with a previous diagnosis of gonadal dysgenesis. Based on these case reports, authors conclude that all patients with gonadal dysgenesis should undergo genetic screen for WT1 mutation in order to make the diagnosis early (16,43,44). General screening recommendations of serial ultrasound every 3 months until the age of 7 apply (24), although it should be noted that DDS patients, on average, are diagnosed just over a year of age (21).
As mentioned previously, BWS is associated with an increased risk of hepatoblastoma, as well as WT. While all surveillance regimens include serial abdominal ultrasounds and serum alpha-fetoprotein (AFP) measurements to evaluate for hepatoblastoma, there exists a divergence in recommendations for surveillance for WT in BWS. The four main BWS molecular subgroups include: IC2-LOM (centromeric imprinting center 2, loss of methylation), IC1-GOM (telomeric imprinting center 1, gain of methylation), pUPD (paternal uniparental disomy), and CKDN1C gene mutations. Telomeric domain dysregulation (IC1-GOM and pUPD) subgroups have a higher predisposition for tumor development than the centromeric domain dysregulation (IC2-LOM and CKDN1C) subgroups (45).
Mussa et al. performed a meta-analysis of 7 studies with a combined total of 1,370 epigenotyped patients with BWS. Only 1 patient of the 836 patients (0.1%) with ICR2-LOM developed a WT whereas 6.2% (21/341) of pUPD and 21.1% (26/123) ICR1-GOM developed WT. There were no reported WT in those with CDKN1C mutation. The authors therefore recommend renal ultrasound in the telomeric subgroups every 3 months through the age of 8–10 years old and WT screening per clinical discretion for the centromeric subgroups (46).
The American Association for Cancer Research (AACR) acknowledged these findings and admits a distinction in practice environments between the United States and Europe. Kalish et al. presents the recommendations from the 2016 AACR Workshop which agrees with a broad, uniform screening protocol for any syndrome associated with a 1% or greater risk of developing WT. In Europe, the threshold is a 2% risk of WT. This entails renal ultrasounds every 3 months from birth or the time of diagnosis through the 7th birthday which covers the age range in which 90–95% of tumors present. They also recommend physical exam by a specialist (geneticist or pediatric oncologist) twice yearly. They present these recommendations with the acknowledgement that uniform recommendations may result in some patients being screened more frequently and for longer duration. Therefore, families should be counseled in detail regarding weighing the risk of false positives with the benefit of earlier tumor detection (47).
Children with SGBS syndrome should undergo screening abdominal ultrasound every 3 months until the age of 7 because of the risk of WT and hepatoblastoma. Additionally, they should undergo serum AFP testing every 3 months until the age of 4. Relatives should also be offered genetic counseling, as apparently unaffected individuals may benefit from tumor surveillance (26).
Due to isolated hemihypertrophy being a poorly defined disease, limited data exists regarding tumor risk. Hemihypertrophy has been shown to have similar molecular aberrations to BWS, suggesting isolated hemihypertrophy and BWS may be related on a spectrum (48). Because of the difficulty in diagnosis, all children with isolated hemihypertrophy should be followed by a geneticist. Until the age of 7, they should undergo quarterly abdominal ultrasounds. Because of the increased risk of hepatoblastoma, they should also undergo quarterly AFP measurements until the age of 4. Conversations with the family can determine if the parents are interested in learning how to perform an abdominal exam, as determined by the physician (11). After the age of 7, a physician should perform an abdominal exam ever 6 months, indefinitely (14).
Because of the malignancy risks besides WT, screening recommendations for Bloom and Li Fraumeni syndromes are more inclusive than most of the other predisposing conditions. For Bloom syndrome, abdominal ultrasounds are recommended quarterly until the age of 8. Leukemia and lymphoma clinical screening should occur at every visit, as should thorough skin exams. Whole body MRI is recommended every 1 to 2 years, starting from the age of 12, while a focus on breast MRI should occur annually from age 18. Annual colonoscopies should be scheduled starting from ages 10 to 12 (28). The tumors associated with Li Fraumeni syndrome are so vast, whole body and brain MRI are recommended annually. It is also recommended that they undergo abdominal ultrasound every 3–4 months (24).
From the age of diagnosis, patients with hyperparathyroidism-jaw tumor syndrome are recommended to undergo renal ultrasound at least every 5 years. This is more likely because of the other renal findings than WT, including end stage renal disease from polycystic kidney disease. However, one patient was diagnosed with bilateral WT at the age of 53 years. Additionally, patients undergo screening for jaw and uterine tumors, along with serum creatinine, calcium, intact parathyroid hormone, and 25-(OH) vitamin D levels (39).
In syndromes associated with a high mortality rate, screening is more controversial, such as with trisomy 18. The wishes of the family, along with the individual patient factors, must be respected. Current recommendations mimic those seen in BWS and hemihypertrophy, as those patients are also at an increased risk of hepatoblastoma: serum AFP levels quarterly until the age of 4, abdominal ultrasounds quarterly until the age of 4, then quarterly renal ultrasounds until the age of 7 (13). Patients with partial trisomy 18 may also benefit from screening, particularly with large duplications of the q arm of chromosome 18 (49).
Patients with 2q37.1 deletions may benefit from ultrasound screening for WT, although set protocols are not established. All children with 2q37 deletions should undergo screening for renal cysts at age 4 and then again at puberty with renal ultrasound (8).
Treatment
Treatment for syndromic WT has several major differences from unilateral non-syndromic WT, where open radical nephrectomy with lymph node sampling is the gold standard. Nephrogenic rests are a known precursor to WT (50). Nephroblastomatosis, or the presence of numerous rests, has been noted in some syndromes, thereby increasing the risk of bilateral or metachronous WT. Thus, efforts should be made to preserve renal tissue in these patients (6). In other conditions where renal failure, including end stage renal disease, is common, bilateral nephrectomy may be more appropriate. On the medical oncology and radiation side, chemotherapeutic and radiation regimens must be tailored in some syndromes to avoid more significant side effects.
There are some occasions when children with non-syndromic WT, typically with bilateral disease, are offered nephron-sparing surgery, either by way of radical nephrectomy and partial nephrectomy, or bilateral partial nephrectomies. The results of AREN0534, a study focused on bilateral WT and unilateral high-risk tumors, found improved long-term survival when compared to previous studies. Their goal was to maximize overall survival, while at the same time preserving renal function. Although it is not as common as in some syndromes, children with bilateral WT are more likely to develop end stage renal disease than those with unilateral disease (51). Children with metachronous bilateral WT, as opposed to synchronous bilateral WT, develop end stage renal disease at greater rates (52). End stage renal disease that develops in non-syndromic children with progressive, bilateral WT is highly morbid, with only half of children living 5 years after diagnosis (53). In AREN0534, children received 3-agent chemotherapy for 6 to 12 weeks, followed by surgery and adjuvant chemotherapy. Radiation was added to adjuvant chemotherapy when at least one kidney has stage III or IV disease. They achieved an 82% event-free survival and 95% overall survival, much improved over the previously reported National Wilms Tumor Study 5 results (51). This would suggest a similar protocol could potentially be successful in children with syndromic WT.
In 2012, Romão et al. described eight unilateral WT diagnosed in patients with predisposing syndromes. Partial nephrectomy was attempted in seven, and successfully accomplished in six. They used surface cooling in three patients, and found intraoperative ultrasound helpful in five patients. Five patients underwent neoadjuvant chemotherapy, and all patients underwent post-operative chemotherapy. At a mean follow-up of 36 months, no recurrences were documented and all had normal creatinine levels (54).
The techniques of nephron-sparing surgery have been additionally demonstrated in case reports of presumed multifocal unilateral WT with an increased risk of developing bilateral or metachronous WT, in a patient with isolated hemihypertrophy (55). Scalabre et al. aimed to provide indications for nephron-sparing surgery in patients with BWS and isolated hemihypertrophy. BWS and isolated hemihypertrophy are associated with a higher incidence of nephrogenic rests. The International Society of Pediatric Oncology (SIOP) defines the presence of nephroblastomatosis as an indication for chemotherapy. In a retrospective study of all cases of BWS or isolated hemihypertrophy in France, authors conclude that nephron-sparing surgery with neoadjuvant chemotherapy can have excellent oncological outcomes (56).
Partial nephrectomy has also been described in patients with DDS, Frasier syndrome, or other WT1-related malignancies and nephropathy, when patients had bilateral disease. At a median of 104 months, none had recurrences (21). Nephron-sparing is also recommended for patients with SGBS syndrome. This is likely not only because of concerns for metachronous cancers, but also to preserve renal function as patients may have concomitant renal dysplasia (26).
The robotic-assisted laparoscopic approach to partial nephrectomy for syndromic WT has also been described. Quarterly screening ultrasound identified a 3 cm renal mass in an 18-month-old, which responded to neoadjuvant chemotherapy and was 2 cm prior to surgery. The procedure was notable for a prolonged urine leak. Importantly, the authors do not mention either performing a lymph node dissection in their description of the procedure, nor do they mention nodal status in the pathology (57). Even in cases of open partial nephrectomy, lymphadenectomy is more likely to be overlooked than in open radical nephrectomy (58). Further, decreased lymph node sampling during minimally invasive nephrectomy for WT has also been noted (59). As newer technologies are employed, it will be important to meet the standard of care and include lymph node sampling.
Nephron-sparing surgery does appear to have a higher complication rate than radical nephrectomy (60). Complication rates between 23–36% have been reported for children undergoing partial nephrectomy for malignancy (60,61). A study including 54 children undergoing radical nephrectomy and 55 undergoing partial nephrectomy found that those undergoing partial nephrectomy were nearly three times as likely to have a complication. The mean blood loss was greater during surgery, and 16.4% experienced a prolonged urine leak. There was no difference in the rates of the other complications they encountered: post-operative infection, temporary renal insufficiency, or intussusception (60).
Some populations may be better served by bilateral nephrectomies. In DDS it is generally accepted that patients who have developed end stage renal disease should undergo bilateral nephrectomy to eliminate the risk of WT. An alternative approach would be prophylactic bilateral nephrectomy prior to the progression to irreversible renal failure, thus avoiding WT development and potentially shortening the total duration of dialysis. Arguments proposed in favor of bilateral prophylactic nephrectomy include reducing the complications of WT and avoiding the recommendation of waiting 1 year following completion of WT treatment prior to transplantation. Following nephrectomies, they could be transplanted within 6–8 weeks, avoiding the inherent decrease in quality of life and higher morbidity/mortality from prolonged dialysis. One potential con is the higher mortality rate infants on dialysis face than if they were older, aged 5–20 years (16). Prior to prophylactic nephrectomy in a child who does not require renal replacement therapy, eligibility for transplant should be considered, such as the patient’s size. Other considerations include availability of organs for transplant, wait time prior to transplantation, and whether a living donor is available or appropriate, depending on the donor’s risk factors for renal disease. Children besides those with DDS may also benefit from bilateral nephrectomies and dialysis, as opposed to nephron-sparing. Long-term remission was described for a boy with premature chromatid separation/mosaic variegated aneuploidy following bilateral nephrectomy. This became his only option after a severe response to chemotherapy (19). Finally, children who undergo nephron-sparing or unilateral nephrectomy with WT1-related nephropathy should undergo removal of remaining renal tissue when there is no further function to prevent later development of metachronous WT (21).
Most children with WT undergo adjuvant chemotherapy. Patients who undergo resection of WT with known contralateral nephroblastomatosis may benefit from maintenance chemotherapy with vincristine and dactinomycin to decrease the likelihood of developing a metachronous tumor (62).
Various syndromes have other characteristics that place them at increased risk of treatment side effects than the general population. The genetic anomalies seen in patients with mosaic variegated aneuploidy may complicate chemotherapeutic regimens, increasing their risk of intolerable side effects or secondary malignancy. Similarly, radiation damage may be more difficult for these individuals to repair (19). Children with WT and Fanconi anemia are likewise difficult to treat. In addition to severe myelosuppression, they are more likely to experience more significant mucositis, and pulmonary and renal toxicity from certain chemotherapeutic agents and radiotherapy (20). Radiation therapy should be used in caution for patients with Li Fraumeni syndrome, as patients are at greater risk of secondary malignancies (29).
In addition to treatment for WT, some patients may require other urologic interventions related to their underlying diagnosis. Boys with Frasier syndrome face a 60% chance of gonadoblastoma, thus gonadectomy is recommended (10). Similarly, in DDS, bilateral gonadectomy is recommended (16).
Should a patient develop end stage renal disease, either from nephrectomy(ies), dysplasia, or chemotherapy toxicity, he or she should expect similar outcomes to transplant recipients who do not have a history of malignancy (53,63). Typically, transplant is delayed at least 1 year after treatment for WT is completed (16,53,63-66). Although post-transplant malignancies occur in WT patients rarely (53,63,65), special consideration should be given to those with syndromes that also predispose to other malignancies. Recurrences in native kidneys have been reported following renal transplantation, thus completion nephrectomy prior to transplant and immunosuppression is recommended for patients who previously underwent nephron-sparing (65,67). Prior to a family member considering organ donation, the potential donor should be thoroughly evaluated to ensure there is not a familial increased risk of renal insufficiency.
Conclusions
In conclusion, numerous syndromes are associated with WT, and new syndromes are still being discovered. Pediatricians should refer infants where there is concern for a predisposing syndrome to a geneticist for confirmation. Those children should then be surveilled every 3 to 4 months with abdominal or renal ultrasounds to enable early detection. Given other risks to renal health, such as renal dysplasia and nephropathy, as well as concern for synchronous or metachronous WT, nephron-sparing surgery should be considered. If renal failure is eminent, bilateral nephrectomy is also an option. As other signs and symptoms may be subtle, pediatric surgeons, urologists, and oncologists should evaluate patients referred with renal masses thoroughly prior to treatment so that the opportunity for nephron-sparing is not missed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John Wiener, Jonathan Routh and Nicholas Cost) for the series “Pediatric Urologic Malignancies” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.03.27). The series “Pediatric Urologic Malignancies” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Segers H, Kersseboom R, Alders M, et al. Frequency of WT1 and 11p15 constitutional aberrations and phenotypic correlation in childhood Wilms tumour patients. Eur J Cancer 2012;48:3249-56. [Crossref] [PubMed]
- Scott RH, Stiller CA, Walker L, et al. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 2006;43:705-15. [Crossref] [PubMed]
- Peterman CM, Fevurly RD, Alomari AI, et al. Sonographic screening for Wilms tumor in children with CLOVES syndrome. Pediatr Blood Cancer 2017;64:e26684. [Crossref] [PubMed]
- Cayrol J, Nightingale M, Challis J, et al. Wilms Tumor associated with the 9q22.3 microdeletion syndrome: 2 new case reports and a review of the literature. J Pediatr Hematol Oncol 2019;41:e517-20. [Crossref] [PubMed]
- MacFarland SP, Duffy KA, Bhatti TR, et al. Diagnosis of Beckwith-Wiedemann syndrome in children presenting with Wilms tumor. Pediatr Blood Cancer 2018;65:e27296. [Crossref] [PubMed]
- Charlton J, Irtan S, Bergeron C, et al. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 2017;19:e8. [Crossref] [PubMed]
- Morris MR, Astuti D, Maher ER. Perlman syndrome: overgrowth, Wilms tumor predisposition and DIS3L2. Am J Med Genet C Semin Med Genet 2013;163C:106-13. [Crossref] [PubMed]
- Doherty ES, Lacbawan FL. 2q37 Microdeletion Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews®. Seattle: University of Washington, 2007.
- Fischbach BV, Trout KL, Lewis J, et al. WAGR syndrome: a clinical review of 54 cases. Pediatrics 2005;116:984-8. [Crossref] [PubMed]
- Ezaki J, Hashimoto K, Asano T, et al. Gonadal tumor in Frasier syndrome: a review and classification. Cancer Prev Res (Phila) 2015;8:271-6. [Crossref] [PubMed]
- Clericuzio CL, Martin RA. Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med 2009;11:220-2. [Crossref] [PubMed]
- Ruteshouser EC, Huff V. Familial Wilms tumor. Am J Med Genet C Semin Med Genet 2004;129C:29-34. [Crossref] [PubMed]
- Farmakis SG, Barnes AM, Carey JC, et al. Solid tumor screening recommendations in trisomy 18. Am J Med Genet A 2019;179:455-66. [Crossref] [PubMed]
- Tan TY, Amor DJ. Tumour surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: a critical review of the evidence and suggested guidelines for local practice. J Paediatr Child Health 2006;42:486-90. [Crossref] [PubMed]
- Mueller RF. The Denys-Drash syndrome. J Med Genet 1994;31:471-7. [Crossref] [PubMed]
- Gariépy-Assal L, Gilbert RD, Žiaugra A, et al. Management of Denys-Drash syndrome: a case series based on an international survey. Clin Nephrol Case Stud 2018;6:36-44. [Crossref] [PubMed]
- Alessandri JL, Cuillier F, Ramful D, et al. Perlman syndrome: report, prenatal findings and review. Am J Med Genet A 2008;146A:2532-7. [Crossref] [PubMed]
- García-Castillo H, Vásquez-Velásquez AI, Rivera H, et al. Clinical and genetic heterogeneity in patients with mosaic variegated aneuploidy: delineation of clinical subtypes. Am J Med Genet A 2008;146A:1687-95. [Crossref] [PubMed]
- Ochiai K, Yamada A, Kimoto Y, et al. Long-term remission of bilateral Wilms tumors that developed from premature separation of chromatids/mosaic variegated aneuploidy syndrome due to bilateral nephrectomy and peritoneal dialysis. Pediatr Blood Cancer 2019;66:e27804. [Crossref] [PubMed]
- Malric A, Defachelles AS, Leblanc T, et al. Fanconi anemia and solid malignancies in childhood: a national retrospective study. Pediatr Blood Cancer 2015;62:463-70. [Crossref] [PubMed]
- Auber F, Jeanpierre C, Denamur E, et al. Management of Wilms tumors in Drash and Frasier syndromes. Pediatr Blood Cancer 2009;52:55-9. [Crossref] [PubMed]
- Barbosa AS, Hadjiathanasiou CG, Theodoridis C, et al. The same mutation affecting the splicing of WT1 gene is present on Frasier syndrome patients with or without Wilms' tumor. Hum Mutat 1999;13:146-53. [Crossref] [PubMed]
- Maas SM, Vansenne F, Kadouch DJ, et al. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A 2016;170:2248-60. [Crossref] [PubMed]
- Srinivasan AS, Saade-Lemus S, Servaes SE, et al. Imaging surveillance for children with predisposition to renal tumors. Pediatr Radiol 2019;49:1453-62. [Crossref] [PubMed]
- Vuillaume ML, Moizard MP, Rossignol S, et al. Mutation update for the GPC3 gene involved in Simpson-Golabi-Behmel syndrome and review of the literature. Hum Mutat 2018;39:790-805. [Crossref] [PubMed]
- Sajorda BJ, Gonzalez-Gandolfi CX, Hathaway ER, et al. Simpson-Golabi-Behmel Syndrome Type 1. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews®. Seattle: University of Washington, 2006.
- Hoyme HE, Seaver LH, Jones KL, et al. Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet 1998;79:274-8. [Crossref] [PubMed]
- Flanagan M, Cunniff CM. Bloom Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews®. Seattle: University of Washington, 2006.
- Schneider K, Zelley K, Nichols KE, et al. Li-Fraumeni Syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews®. Seattle: University of Washington, 1999.
- Hyde SM, Rich TA, Waguespack SG, et al. CDC73-Related Disorders. In: Adam MP, Ardinger HH, Pagon RA, et al. editors. GeneReviews®. Seattle: University of Washington, 2008.
- Karlberg N, Karlberg S, Karikoski R, et al. High frequency of tumours in Mulibrey nanism. J Pathol 2009;218:163-71. [Crossref] [PubMed]
- Hämäläinen RH, Mowat D, Gabbett MT, et al. Wilms' tumor and novel TRIM37 mutations in an Australian patient with Mulibrey nanism. Clin Genet 2006;70:473-9. [Crossref] [PubMed]
- Sivunen J, Karlberg S, Lohi J, et al. Renal findings in patients with Mulibrey nanism. Pediatr Nephrol 2017;32:1531-6. [Crossref] [PubMed]
- Cullinan N, Villani A, Mourad S, et al. An eHealth decision-support tool to prioritize referral practices for genetic evaluation of patients with Wilms tumor. Int J Cancer 2020;146:1010-7. [Crossref] [PubMed]
- Breslow NE, Collins AJ, Ritchey ML, et al. End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol 2005;174:1972-5. [Crossref] [PubMed]
- Akasaka N, Tohyama J, Ogawa A, et al. Refractory infantile spasms associated with mosaic variegated aneuploidy syndrome. Pediatr Neurol 2013;49:364-7. [Crossref] [PubMed]
- Dumoucel S, Gauthier-Villars M, Stoppa-Lyonnet D, et al. Malformations, genetic abnormalities, and Wilms tumor. Pediatr Blood Cancer 2014;61:140-4. [Crossref] [PubMed]
- Choyke PL, Siegel MJ, Craft AW, et al. Screening for Wilms tumor in children with Beckwith-Wiedemann syndrome or idiopathic hemihypertrophy. Med Pediatr Oncol 1999;32:196-200. [Crossref] [PubMed]
- Saade-Lemus S, Degnan AJ, Acord MR, et al. Whole-body magnetic resonance imaging of pediatric cancer predisposition syndromes: special considerations, challenges and perspective. Pediatr Radiol 2019;49:1506-15. [Crossref] [PubMed]
- Gylys-Morin V, Hoffer FA, Kozakewich H, et al. Wilms tumor and nephroblastomatosis: imaging characteristics at gadolinium-enhanced MR imaging. Radiology 1993;188:517-21. [Crossref] [PubMed]
- Craft AW. Growth rate of Wilms' tumour. Lancet 1999;354:1127. [Crossref] [PubMed]
- Scott RH, Walker L, Olsen Ø, et al. Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 2006;91:995-9. [Crossref] [PubMed]
- Auber F, Lortat-Jacob S, Sarnacki S, et al. Surgical management and genotype/phenotype correlations in WT1 gene-related diseases (Drash, Frasier syndromes). J Pediatr Surg 2003;38:124-9. [Crossref] [PubMed]
- Weaver J, Rove KO, Meenakshi-Sundaram B, et al. Genetic testing proves crucial in case of ambiguous genitalia and renal masses. Urology 2019;129:194-6. [Crossref] [PubMed]
- Rump P, Zeegers MP, van Essen AJ. Tumor risk in Beckwith-Wiedemann syndrome: A review and meta-analysis. Am J Med Genet A 2005;136:95-104. [Crossref] [PubMed]
- Mussa A, Molinatto C, Baldassarre G, et al. Cancer risk in Beckwith-Wiedemann syndrome: a systematic review and meta-analysis outlining a novel (epi)genotype specific histotype targeted screening protocol. J Pediatr 2016;176:142-9.e1. [Crossref] [PubMed]
- Kalish JM, Doros L, Helman LJ, et al. Surveillance recommendations for children with overgrowth syndromes and predisposition to Wilms tumors and hepatoblastoma. Clin Cancer Res 2017;23:115-22. [Crossref] [PubMed]
- Martin RA, Grange DK, Zehnbauer B, et al. LIT1 and H19 methylation defects in isolated hemihyperplasia. Am J Med Genet A 2005;134A:129-31. [Crossref] [PubMed]
- Starr LJ, Sanmann JN, Olney AH, et al. Occurrence of nephroblastomatosis with dup(18)(q11.2-q23) implicates trisomy 18 tumor screening protocol in select patients with 18q duplication. Am J Med Genet A 2014;164A:1079-82. [Crossref] [PubMed]
- Coppes MJ, Arnold M, Beckwith JB, et al. Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer 1999;85:1616-25. [Crossref] [PubMed]
- Ehrlich P, Chi YY, Chintagumpala MM, et al. Results of the first prospective multi-institutional treatment study in children with bilateral Wilms tumor (AREN0534): a report from the Children's Oncology Group. Ann Surg 2017;266:470-8. [Crossref] [PubMed]
- Lange J, Peterson SM, Takashima JR, et al. Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol 2011;186:378-86. [Crossref] [PubMed]
- Grigoriev Y, Lange J, Peterson SM, et al. Treatments and outcomes for end-stage renal disease following Wilms tumor. Pediatr Nephrol 2012;27:1325-33. [Crossref] [PubMed]
- Romão RL, Pippi Salle JL, Shuman C, et al. Nephron sparing surgery for unilateral Wilms tumor in children with predisposing syndromes: single center experience over 10 years. J Urol 2012;188:1493-8. [Crossref] [PubMed]
- Romero NG, Walker J, Cost NG, et al. Partial nephrectomy for multifocal, unilateral Wilms tumor in a patient with hemihypertrophy. Urology 2019;133:243-4. [Crossref] [PubMed]
- Scalabre A, Bergeron C, Brioude F, et al. Is nephron sparing surgery justified in Wilms tumor with Beckwith-Wiedemann syndrome or isolated hemihypertrophy? Pediatr Blood Cancer 2016;63:1571-7. [Crossref] [PubMed]
- Yadav P, Mahajan A, Kandpal DK, et al. Nephron-sparing surgery for syndromic Wilms' tumor: robotic approach. Urology 2018;116:172-5. [Crossref] [PubMed]
- Wang HH, Abern MR, Cost NG, et al. Use of nephron sparing surgery and impact on survival in children with Wilms tumor: a SEER analysis. J Urol 2014;192:1196-202. [Crossref] [PubMed]
- Warmann SW, Godzinski J, van Tinteren H, et al. Minimally invasive nephrectomy for Wilms tumors in children - data from SIOP 2001. J Pediatr Surg 2014;49:1544-8. [Crossref] [PubMed]
- Spiegl HR, Murphy AJ, Yanishevski D, et al. Complications following nephron-sparing surgery for Wilms tumor. J Pediatr Surg 2020;55:126-9. [Crossref] [PubMed]
- Suson KD, Wolfe-Christensen C, Elder JS, et al. Practice patterns and outcomes of pediatric partial nephrectomy in the United States: comparison between pediatric urology and general pediatric surgery. J Pediatr Urol 2015;11:171.e1-5. [Crossref] [PubMed]
- Ortiz MV, Fernandez-Ledon S, Ramaswamy K, et al. Maintenance chemotherapy to reduce the risk of a metachronous Wilms tumor in children with bilateral nephroblastomatosis. Pediatr Blood Cancer 2019;66:e27500. [Crossref] [PubMed]
- Serrano OK, Gannon A, Olowofela AS, et al. Long-term outcomes of pediatric kidney transplant recipients with a pretransplant malignancy. Pediatr Transplant 2019;23:e13557. [Crossref] [PubMed]
- Pais E, Pirson Y, Squifflet JP, et al. Kidney transplantation in patients with Wilms' tumor. Transplantation 1992;53:782-5. [Crossref] [PubMed]
- Kubiak R, Gundeti M, Duffy PG, et al. Renal function and outcome follow¬ing salvage surgery for bilateral Wilms tumor. J Pediatr Surg 2004;39:1667-72. [Crossref] [PubMed]
- Knoll G, Cockfield S, Blydt-Hansen T, et al. Canadian Society of Transplantation: consensus guidelines on eligibility for kidney transplantation. CMAJ 2005;173:S1-25. [Crossref] [PubMed]
- Roca N, Muñoz M, Cruz A, Vilalta R, Lara E, Ariceta G. Long-term outcome in a case series of Denys-Drash syndrome. Clin Kidney J 2019;12:836-9. [Crossref] [PubMed]


