D-004 ameliorates phenylephrine-induced urodynamic changes and increased prostate and bladder oxidative stress in rats
Introduction
Benign prostatic hyperplasia (BPH), an enlargement of the prostate gland, may lead to troublesome lower urinary tract symptoms (LUTS), bladder outlet obstruction and reduced quality of life, common in aging men (1-3).
The pathogenesis of BPH/LUTS clinical entity involves both hormonal and non-hormonal factors. The increased conversion of testosterone (T) in dihydrotestosterone (DHT) mediated by the activity of prostate 5α-reductase is the key factor in prostate growth, the static component of this disease (1,2). In turn, stimulation of alpha1-adrenoreceptors (α1-ADR) that regulate bladder and prostatic smooth muscle tone triggers the enhanced contraction of these tissues and leads to LUTS and bladder outlet obstruction in BPH patients (4,5).
Hence, the pharmacological management of BPH/LUTS mainly includes the use of α1-ADR antagonists, 5α-reductase inhibitors and their combined therapy (6,7), although phosphodiesterase inhibitors and non-steroidal anti-inflammatory drugs (NSAIDs) have been also used in BPH patients (8,9). In addition, phytotherapeutic alternatives have been used to treat BPH/LUTS for decades, their efficacy being based in multiple mechanisms like the antagonism of α1-ADR, the inhibition of 5α-reductase activity, and beneficial anti-inflammatory and antioxidant pleiotropic effects (10,11).
On its side, BPH has been also linked with increased oxidative stress (OS) and free radicals have been claimed to be implicated in the micturition dysfunction observed in patients with BPH/LUTS. Also, it is suggested that the use of antioxidants would ameliorate micturition dysfunction in patients with BPH (12-15).
Sympathomimetic stimulation achieved by phenylephrine (PHE) administration (1-20 mg/kg) to rodents has been shown to induce atypical prostatic hyperplasia (PH), characterized by piling-up with papillary and cribriform patterns, and budding-out of epithelial cells, accompanied by urodynamic changes like reduced volume voided per micturition (VM) and micturition total volume (VT) (16). These PHE-induced changes seem to be mediated by the α1A-ADR, which predominate in the stroma of the rodent ventral prostate, so that PHE could directly modulate prostate stromal growth, and indirectly modulate epithelial growth in a paracrine fashion, although the contribution of other indirect effects, including the increased OS, have not been ruled out (17).
D-004, a lipid extract of Roystonea regia fruits that contains a mixture of fatty acids, mainly oleic, lauric, palmitic and myristic, has been effective in experimental models of PH (18-22). D-004 has been able to inhibit prostate 5α-reductase in vitro (23), and to prevent PH induced with T (18-20), not with DHT (19), in rodents. Also, D-004 effectively antagonizes ADR-mediated responses in vitro (24,25) and in vivo (21,22). The addition of D-004 inhibited PHE-induced contractions in preparations of isolated prostate strips and vas deferens (24,25), while its oral administration reduced significantly PHE-induced impairment of micturition and histological changes in rat prostate, indicating that, in vivo, D-004 effectively opposed these PHE-induced responses, mediated through urogenital α1A-ADR (21-24). Also, D-004 has demonstrated to produce antioxidant effects on normal and hyperplasic prostate tissue in rats (26,27), and on plasma oxidative variables in healthy and BPH men (28,29). Nevertheless, the coincidence of the effects of D-004 on OS variables in prostate and bladder tissues and PHE-induced urodynamic changes in rats have not been explored.
In light of this background, this study investigated whether D-004 produces antioxidant effects in rats treated with PHE.
Methods
Animals
Young adult male Sprague Dawley rats (250-270 g) acquired in the National Centre for Laboratory Animals Production (CENPALAB, Havana, Cuba) were adapted to laboratory conditions (temperature 20-25 °C, relative humidity 60%±5%, 12 hours light/dark cycles) for 7 days. Food (rodent chow from CENPALAB) and water were provided ad libitum.
The study was conducted according to the Cuban Guidelines for Animal Handling and the Cuban Code of Good Laboratory Practices (GLP). An independent institutional board of the centre approved the study protocol and the use of the animals in the experiments.
Chemical and test substances
The batch of D-004 used in the experiments, supplied by the Plants of Natural Products (National Center for Scientific Research, Havana, Cuba), had the following composition: caprylic 0.2%, capric 0.5%, lauric 25.1%, myristic 10.9%, palmitic 11.3%, palmitoleic 0.2%, stearic 2.8%, oleic 42.9%, linoleic 9.5% and linolenic 0.1%). Purity (total content of these fatty acids) was 93.9%.
Grape seed extract (GSE) (95% of proanthocyanidins) was from New Directions (Sydney, Australia), vitamin E (VE) from Carlson Health (Australia), tamsulosin from ULTRA® Laboratories (México) and PHE from the Cuban Pharmaceutical Industry (QUIMEFA, Cuba).
Treatment methods and dosage
D-004, GSE, VE and tamsulosin were suspended in Tween-65/H2O vehicle (2%). All treatments (including the vehicle) were given as single oral doses by gastric gavage (5 mL/kg of body wt) one hour before inducing PHE-urodynamic impairment.
PHE was diluted in saline solution (5 mg/mL) and administered by subcutaneous injection (s.c).
Rats were randomized into eight groups (ten rats/group): a negative vehicle control and seven groups treated with PHE: a positive control, three treated with D-004 (200, 400 and 800 mg/kg), three with tamsulosin (0.4 mg/kg), one with GSE (250 mg/kg) and one with VE (250 mg/kg).
After treatment completion, rats were weighed, then anesthetized under ether atmosphere and sacrificed by complete bleeding from the abdominal aorta. Prostate was immediately separated from bladder and both of them were removed and weighed in analytical balance Mettler Toledo.
Effects on urodynamic variables
One hour after administering the oral treatments, PHE-treated groups were injected with PHE (5 mg/kg, s.c). All groups (including the negative control) received a fluid loading dose (distilled water, 5 mL s.c + 5 mL p.o) for increasing the VM (22). Thirty min later, rats were placed unrestrained in metabolic cages for one hour and the urinary total volume (UTV) and VM were measured.
The amelioration of the reduction of VM induced by PHE was the main study outcome.
Effects on oxidative variables
Aliquots of whole prostate and bladder tissue were taken and gently homogenized in an ice-cold bath, with an Ultra-Turrax homogenizer. Tissue samples were homogenized in 150 mmol/L Tris/HCl buffer (pH 7.4) and 50 mmol/L phosphate buffer (pH 7.4) for determining the TBARS and carbonyl groups (CG) levels, respectively.
Effect on malondialdehyde (MDA) levels
Concentration of TBARS was determined according to Ohkawa (30). For that, the reaction mixture (prostate and bladder homogenates) was treated with 0.2 mL of sodium dodecyl sulfate (8.1%), 1.5 mL of acetic acid (20%, pH 3.5), and 1.5 of thiobarbituric acid (TBA) (0.8%), and heated to 95 °C for one hour. To prevent the production of TBA reactants 50 µL of butylated hydroxytoluene (1 mmol/L) were added to the mixtures. After cooling, 5 mL of n-butanol:pyridine (15:1 v/v) mixture was added, stirring vigorously with vortex, and centrifuged at 4,000 rpm for 20 min. The absorbance of the organic layer was measured at 534 nm using a spectrophotometer (Genesys 10 UV). Concentrations of TBARS were determined from a standard curve of MDA bis-(dimethyl acetal) and reported as nmol MDA/mg protein.
Protein concentrations were assessed by a modified Lowry method (31).
Effect on protein-linked carbonyl groups (CG)
Protein-linked CG levels were determined according to Reznick and Packer (32). The prostate or bladder homogenates were measured at 280/260 nm to discard the presence of nucleic acids. In all cases streptomycin sulphate 1% was added to eliminate nucleic acids. A volume equivalent to 50 mg of protein was then added to 4 mL of dinitro-phenyl-hydrazine (DNPH) solution (10 mmol/L), dissolved in HCl 2.5 mol/L. The mixture was vigorously stirred and placed in the darkness for 1 h, 5 mL of trichloroacetic acid (TCA) at 10% were added, and the mixture centrifuged at 3,000 rpm for 15 minutes. The protein pellet was washed three times with a mixture of ethanol:ethyl acetate (1:1, v/v) to eliminate the excess of DNPH and then dissolved in 2 mL of guanidine 6 mol/L. Optical density was measured at 450 nm (coefficient of molar extinction: 22,000 M–1) and the concentration of CG was reported in nmol/mg of protein.
Statistical analysis
Comparisons among groups were performed with the Kruskal Wallis test and paired comparisons versus the control group with the Mann Whitney U test. The level of statistical significance was set at 0.05. All analyses were performed using statistic software for Windows (Release 6.0, Stat Soft, Inc. USA).
Dose-effect relationship was assessed by using linear regression and correlation tests using the Primer of Biostatistics Program [Stanton A, Glantz; copyright (c) 1992, McGraw-Hill, Inc. Versión 3.01].
Results
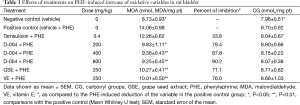
Decreased VM (P<0.001) and UTV (P<0.05) values were found in the positive control as compared to the negative control group, reductions that were significantly (P<0.001) and markedly attenuated by tamsulosin, the reference substance, which produced a 78% inhibition of the VM (Table 1).

Full table
Single oral doses of D-004 (200-800 mg/kg) dose-dependently (r=0.999; P<0.05) ameliorated the reduction of the VM induced by PHE. Meanwhile the doses of 400 and 800 mg/kg increased significantly the VM, the lowest dose was ineffective. The magnitude of the effect was moderate since the highest dose tested (800 mg/kg) increased VM by 48.1% as compared to the positive control. All doses of D-004, however, increased significantly the UTV. GSE and VE unchanged the UTV, whereas VE, not GSE, modestly (22.1%) but significantly attenuated the PHE-induced decrease of VM (Table 1).
Tables 2 and 3 show the effects of treatments on the oxidative variables (MDA and CG concentrations) in rat prostate and bladder, respectively. The s.c injection of PHE significantly increased MDA and CG concentrations in both tissues as compared to the negative controls, meanwhile tamsulosin failed to modify PHE-induced increases of MDA and GC in prostate (Table 2) and bladder tissues (Table 3) in rats.

Full table
Full table
Oral treatment with D-004 (200-800 mg/kg) reduced significantly the PHE-induced increase of prostate MDA and CG concentrations to about 60% and 100%, respectively. Likewise, significant reductions of both oxidative variables were achieved with GSE and VE at 250 mg/kg (Table 2).
All treatments prevented significantly the increase of MDA concentrations in bladder homogenates, but unchanged those of CG (Table 3).
None treatment modified body, bladder or prostate weight values (data not shown for simplicity).
Discussion
In this study, single oral doses of D-004 were able to ameliorate the PHE-induced impairment of urodynamic variables in rats, like the reductions of VM and UTV; and of the concomitant increases of prostate concentrations of both oxidative variables: MDA (a marker of lipid peroxidation) and GC (a marker of protein oxidation).
Rats of the positive control group exhibited PHE-induced significant reductions of VM (main efficacy outcome) and UTV as compared to the negative control group. These decreases were markedly attenuated in the group pre-treated with tamsulosin (0.4 mg/kg), which reduced such effect by 78% as compared to the positive control. These findings support the validity of the model in our experimental conditions. The effects of tamsulosin, the reference substance, on this model are attributable to its specific antagonistic action on the α1-ADR (24) that mediate the contractile function of prostate and bladder neck smooth muscle.
Single oral doses of D-004 (200-800 mg/kg) ameliorated the PHE-induced impairment of both urodynamic variables in a dose-dependent fashion, consistent with previous data on this model (21,22), and with its antagonism of α1-ADR-mediated responses in vitro (24,25). The highest effect observed, differently from that of tamsulosin, was moderate (≈48% of inhibition versus the positive control) and the lowest dose was not effective.
The novelty of this study, however, resides on the demonstration of the ability of D-004 for lowering the PHE-induced increases of the concentrations of oxidative variables (MDA and GC) in the rat prostate and of MDA in the rat bladder. In addition, the increase of markers of OS in this model had not been demonstrated previously (Entrez PubMed, review up to August 2013). Nevertheless, keeping in mind that OS has been implicated in the damage to the detrusor musculature following a period of chronic intravesical obstruction in rats and that such effect was attenuated by the antioxidant galangin (15), we assumed that a similar situation may be present in the PHE-induced urodynamic dysfunction in the rat.
In this study, we used two markers for demonstrating the occurrence of OS increase in rat prostate and bladder tissues (MDA and CG). MDA, the final product of free radical-induced lipid peroxidation, has been implicated in the attack to the polyunsaturated fatty acids of cell membranes, with consequent changes in membrane fluidity and permeability, increased protein degradation and rates of cell lysis (33). In turn, GC concentrations are used as a marker of protein oxidation (34), another indicator of the levels of OS in tissues.
Prostate and bladder MDA and GC concentrations in the positive controls were greater than in the negative controls. When taken together, these findings support that high levels of lipid peroxidation and protein oxidation are linked with PHE-induced urodynamic dysfunction. We believe that the damage induced by PHE (proven by the reduction of VM and UTV) is related, disregarding its effects on α1-ADR, to the increased OS on prostate and bladder.
The coexistence of the ameliorating effects of D-004 on PHE-induced urodynamic impairment and increased OS markers (MDA and CG) demonstrates, for the first time, the efficacy of D-004 for lowering OS in conditions that mimics the stimulation of α1-ADR at the same doses reported as effective for preventing T-induced PH in rats (18-20).
Although this result seems to reinforce the link between urinary dysfunction and OS on this model, it should be noted that proven antioxidants like GSE and VE at 250 mg/kg, effective for lowering oxidative markers, did not modify UTV, and that tamsulosin, highly effective for reducing urodynamic dysfunction, was devoid of effect on the oxidative variables. These facts support that the main efficacy of treatments on this model is related with the antagonism of α1-ADR, not with changes on oxidative variables. Nevertheless, since a modest effect of VE on VM was seen, a door for the contribution of antioxidant effects for alleviating urodynamic dysfunction remains open, mainly in light of the important role of the reactive oxygen species-reactive nitrogen species (ROS-RNS) on the bladder contraction in rats (35) and rabbits (36).
The fact that D-004, GSE and VE were effective to lower prostate and bladder MDA concentrations, but only the concentrations of CG in the prostate, not in the bladder, remains intriguing, since we have no conclusive explanation of this differential effect on both tissues. This result, therefore, merits further studies on this target.
From a wider perspective, since several experimental and clinical evidences have demonstrated the role of ROS-RNS on the stimulation of lower urinary tract contractions and the benefits of some antioxidant agents on the progression of BPH (12-14,35,36) it is very interesting to find that D-004 not only ameliorates the PHE-induced urodynamic dysfunction in rats, but also decrease the oxidative damage induced by PHE on target tissues related with the BPH/LUTS clinical entity. These results suggest that the use of antioxidants would have a protective role against micturition dysfunction due to PHE-induced stimulation of α1-ADR.
Conclusions
Single oral administration of D-004 (200-800 mg/kg), not of tamsulosin, VE or GSE, was the only treatment able to ameliorate simultaneously PHE-induced urodynamic dysfunction and increased prostate OS in rats.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Briganti A, Capitanio U, Suardi N, et al. Benign Prostatic Hyperplasia and Its Aetiologies. Eur Urol 2009;8:865-71.
- Tang J, Yang J. Etiopathogenesis of benign prostatic hypeprlasia. Indian J Urol 2009;25:312-7. [PubMed]
- Barry MJ, Avins AL, Meleth S, et al. Performance of the American Urological Association Symptom Index with and without an additional urge incontinence item. Urology 2011;78:550-4. [PubMed]
- Bhargava S, Canda AE, Chapple CR. A rational approach to benign prostatic hyperplasia evaluation: recent advances. Curr Opin Urol 2004;14:1-6. [PubMed]
- Schwinn DA, Roehrborn CG. Alpha1-adrenoceptor subtypes and lower urinary tract symptoms. Int J Urol 2008;15:193-9. [PubMed]
- Marberger M. Drug Insight: 5alpha-reductase inhibitors for the treatment of benign prostatic hyperplasia. Nat Clin Pract Urol 2006;3:495-503. [PubMed]
- Roehrborn CG, Siami P, Barkin J, et al. The effects of dutasteride, tamsulosin and combination therapy on lower urinary tract symptoms in men with benign prostatic hyperplasia and prostatic enlargement: 2-year results from the CombAT study. J Urol 2008;179:616-21; discussion 621. [PubMed]
- Kahokehr A, Vather R, Nixon A, et al. Non-steroidal anti-inflammatory drugs for lower urinary tract symptoms in benign prostatic hyperplasia: systematic review and meta-analysis of randomized controlled trials. BJU Int 2013;111:304-11. [PubMed]
- Wong P, Lawrentschuk N, Bolton DM. Phosphodiesterase 5 inhibitors in the management of benign prostatic hyperplasia and erectile dysfunction: the best of both worlds. Curr Opin Urol 2009;19:7-12. [PubMed]
- Curtis Nickel J, Shoskes D, Roehrborn CG, et al. Nutraceuticals in Prostate Disease: The Urologist’s Role. Rev Urol 2008;10:192-206. [PubMed]
- Abe M, Ito Y, Suzuki A, et al. Isolation and pharmacological characterization of fatty acids from saw palmetto extract. Anal Sci 2009;25:553-7. [PubMed]
- Merendino RA, Salvo F, Saija A, et al. Malondialdehyde in benign prostate hypertrophy: a useful marker? Mediators Inflamm 2003;12:127-8. [PubMed]
- Aydin A, Arsova-Sarafinovska Z, Sayal A, et al. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin Biochem 2006;39:176-9. [PubMed]
- Aryal M, Pandeya A, Bas BK, et al. Oxidative stress in patients with benign prostate hyperplasia. JNMA J Nepal Med Assoc 2007;46:103-6. [PubMed]
- Bisogni S, Ferreira FT, Amstalden Neto A, et al. Influence of oxidative stress on inducing micturition dysfunction following chronic infravesical obstruction and the protective role of an antioxidant diet - association of in vivo and in vitro studies in rats. Int Braz J Urol 2012;38:552-60. [PubMed]
- Golomb E, Kruglikova A, Dvir D, et al. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate 1998;34:214-21. [PubMed]
- Marinese D, Patel R, Walden PD. Mechanistic investigation of the adrenergic induction of ventral prostate hyperplasia in mice. Prostate 2003;54:230-7. [PubMed]
- Arruzazabala ML, Carbajal D, Más R, et al. Preventive effects of D-004, a lipid extract from Cuban royal palm (Roystonea regia) fruits, on testosterone-induced prostate hyperplasia in intact and castrated rodents. Drugs Exp Clin Res 2004;30:227-33. [PubMed]
- Carbajal D, Arruzazabala Mde L, Rosa M, et al. Effects of D-004, a lipid extract from Cuban royal palm fruit, on inhibiting prostatic hypertrophy induced with testosterone or dihydrotestosterone in a rat model: A randomized, controlled study. Curr Ther Res Clin Exp 2004;65:505-14. [PubMed]
- Noa M, Arruzazabala ML, Carbajal D, et al. Effect of D-004, a lipid extract from Cuban royal palm fruit, on histological changes of prostate hyperplasia induced with testosterone in rats. Int J Tissue React 2005;27:203-11. [PubMed]
- Arruzazabala ML, Más R, Molina V, et al. Effect of D-004, a lipid extract from the Cuban royal palm fruit, on atypical prostate hyperplasia induced by phenylephrine in rats. Drugs R D 2006;7:233-41. [PubMed]
- Arruzazabala Mde L, Molina V, Más R, et al. Effects of D-004, a lipid extract from the royal palm (Roystonea regia) fruits, tamsulosin and their combined use on urodynamic changes induced with phenylephrine in rats. Arzneimittelforschung 2008;58:81-5. [PubMed]
- Pérez LY, Menéndez R, Má R, et al. In vitro effect of D-004, a lipid extract of the fruit of the cuban royal palm (Roystonea regia), on prostate steroid 5α-reductase activity. Curr Ther Res Clin Exp 2006;67:396-405. [PubMed]
- Arruzazabala ML, Más R, Carbajal D, et al. Effect of D-004, a lipid extract from the Cuban royal palm fruit, on in vitro and in vivo effects mediated by alpha-adrenoceptors in rats. Drugs R D 2005;6:281-9. [PubMed]
- Arruzazabala ML, Molina V, Carbajal D, et al. Effect of D-004, a lipid extract from royal palm (Roystonea regia) fruits, on phenylephrine-induced contractions of isolated rat prostate. Rev Cubana Farm 2009;43:1-9.
- Menéndez R, Más R, Pérez Y, et al. In Vitro effect of D-004, a lipid extract of the ground fruits of the Cuban royal palm (Roystonea regia), on rat microsomal lipid peroxidation. Phytother Res 2007;21:89-95. [PubMed]
- Perez Y, Molina V, Mas R, et al. Ex vivo antioxidant effects of D-004, a lipid extract from Roystonea regia fruits, on rat prostate tissue. Asian J Androl 2008;10:659-66. [PubMed]
- López E, Molina V, Illnait J, et al. Antioxidant effects of D-004, a lipid extract from the Roystonea regia fruit, on the plasma of healthy men. Asian J Androl 2009;11:385-92. [PubMed]
- Guzmán R, Illnait J, Mas R, et al. Comparative Effects of Roystonea Regia (D-004) and Saw Palmetto Lipid Extracts On Blood Oxidative Variables in Men with Benign Prostate Hyperplasia (BPH). IOSRPHR 2013;3:1-8.
- Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95:351-8. [PubMed]
- Markwell MA, Haas SM, Bieber LL, et al. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem 1978;87:206-10. [PubMed]
- Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol 1994;233:357-63. [PubMed]
- Guichardant M, Lagarde M. Analysis of biomarkers from lipid peroxidation: A comparative study. Eur J Lipid Sci Technol 2009;111:75-82.
- Dalle-Donne I, Aldini G, Carini M, et al. Protein carbonylation, cellular dysfunction, and disease progression. J Cell Mol Med 2006;10:389-406. [PubMed]
- Aikawa K, Leggett R, Levin RM. Effect of age on hydrogen peroxide mediated contraction damage in the male rat bladder. J Urol 2003;170:2082-5. [PubMed]
- Matsumoto S, Hanai T, Matsui T, et al. Eviprostat suppresses urinary oxidative stress in a rabbit model of partial bladder outlet obstruction and in patients with benign prostatic hyperplasia. Phytother Res 2010;24:301-3. [PubMed]


