
Transperineal prostate biopsy: a review of technique
Background
Prostate cancer is the second most frequently diagnosed cancer worldwide, and the fifth leading cause of cancer death in men (1). Most guidelines recommend screening for PCa for well-informed men with more than seven to ten years of life expectancy (2). The process involves performing a clinical history, digital rectal examination (DRE) and serum testing of prostate specific antigen (PSA). Prostate biopsy is performed on the basis of screening results, and remains the gold standard for diagnosis. This has recently been supplemented by the use of pre-biopsy multiparametric magnetic resonance imaging (mpMRI). mpMRI improves the sensitivity of prostate biopsy as well as the specificity for significant prostate cancer (3). It is estimated that over two million men undergo prostate biopsy world-wide each year. As such, it requires that the technique is as accurate and safe as possible for the patient’s well-being (4).
Tissue biopsy can be obtained using either transrectal ultrasound guided biopsy (TRUS-biopsy) or transperineal prostate biopsy (TPP-biopsy). TRUS-biopsy is the most commonly offered worldwide as it can be performed in a clinic setting with local anaesthesia. TPP-biopsy is typically a day procedure often requiring general anaesthesia (5). TPP biopsy was first described in the 1970s but has recently become more widely adopted as it has shown to be superior in sensitivity especially in detecting anterior cancers, as well as having a lower rate of sepsis compared to TRUS-biopsies (6-8).
Due to the indolent nature of insignificant (low risk) forms of prostate cancer and morbidity associated with treatment, avoiding the diagnosis of clinically insignificant disease is of increasing importance (9). The optimal prostate biopsy technique should aim to have a high detection rate of clinically significant PCa whilst also having a low detection rate of insignificant PCa (10). Given the high number of prostate biopsies performed each year, biopsy must be accessible, time-efficient and cost-effective to ensure feasibility for patients and health care systems (9,11).
This article offers a review of the brachytherapy grid technique used to perform a TPP-biopsy, as well as a discussion of possible variations in the procedure.
Selection criteria
Asymptomatic patients should be well informed of the potential for over-diagnosis and over-treatment when undergoing screening for prostate cancer. Patients undergoing screening should have a life expectancy of more than 10–15 years. If general anaesthesia is used, the patient’s fitness for this should be assessed before selecting patient for TPP-biopsy.
Set-Up
To perform the procedure, basic equipment required includes:
- Operating table and lithotomy stirrups;
- Stepper;
- Brachytherapy grid (if being used);
- Ultrasound (US) machine, transrectal ultrasound probe;
- Water balloon spacer;
- Core biopsy needle;
- Specimen container with formalin.
Procedure
Preparation
Anaesthesia can be general, spinal, regional or local. Prophylactic antibiotic should be administered up to 60 minutes prior to biopsy. For patients without sensitivity, current Australian therapeutic guidelines suggest 2 g intravenous cephazolin, a first-generation cephalosporin (6,12,13).
The patient is positioned in lithotomy on the operating table. A DRE is performed for clinical evaluation of the prostate, noting the size, consistency, any presence of nodules and clinical T stage if there is suspicion of malignancy.
The scrotum is elevated and held out of the way using tape to expose the perineum. Excessive hair is shaved off the perineum. The perineum is prepared using Betadine (7.5% povidone-iodine) or other equivalent antiseptic solutions.
A stepper is placed at the end of the operating table to allow for attachment of a sampling brachytherapy grid at the level of the perineum and an US probe at the level of the rectum.
Operative technique
A well lubricated ultrasound probe is inserted into the rectum. The gland is visualised fully in axial and sagittal views to allow for the identification of landmarks and estimation of volume. Prostate specific landmarks include the urethra, which can be further defined on imaging as being either at the apex or the base, the apex, mid gland of the prostate, the transitional and peripheral zones, and the verumontanum. Patient-specific landmarks can include calcifications, cysts and hypoechoic lesions which may or may not correlate to pre-operative prostate imaging. Prostate volume (mL) is calculated using the formula height × width × length × 0.52.
When no prior MRI has been obtained, or a prior MRI is negative, but the patient is deemed sufficiently at risk of harbouring significant prostate cancer, a systematic biopsy is performed with ultrasound guidance, with samples taken from the apex to the base and from posterior to anterior. The prostate is divided into left and right lobes. For each lobe, three to four cores are taken from anterior, middle and posterior zones. In larger prostates, additional biopsies may be taken to sample the base adequately. To avoid impairment of a target area on US images, targeted biopsies of any suspicious lesion on pre-biopsy mpMRI should be taken immediately prior to systematic biopsy. The number of cores taken should balance the detection of clinically significant prostate cancer whilst minimising side effects associated with increased sampling numbers (14). In a review by Shariat et al. (2008) the authors recommend that for initial biopsy, at least 10 biopsy cores should be taken (11).
The decision to take more cores is based on prostate size. For prostates larger than 50 mL, an extended sampling protocol of 12–14 cores must be taken to detect clinically significant prostate cancer. Taking more than 18 cores has not been found to improve the detection of prostate cancer, and a saturation technique involving 20 cores at initial biopsy is associated with a worse side effect profile, namely haematospermia and acute urinary retention (11,14,15). Using a solely sextant biopsy protocol is no longer considered adequate, additional cores should be taken from areas of suspicion (2,16). Various schemas are used to divide the prostate into zones to facilitate systematic biopsy of the whole gland. Barzell zones or its modified versions are examples. The prostate is divided into 20 zones and each zone is biopsied (17). Size of the prostate is taken into consideration. In large prostates, attention is also paid to the base and anterior zones to ensure adequate sampling. In the Ginsburg Study Group consensus (18) the prostate is divided into defined sectors or zones with preference placed on the peripheral zone and the anterior zone for biopsy. The group also suggested higher number of cores for bigger prostates.
Tissue samples are placed in formalin. Care must be taken when labelling to ensure that samples are correctly identified and correlate with the area of prostate from which they are taken.
The procedure usually takes 10–15 minutes.
Post-operative care
Due to the relatively short procedure time, the anaesthetic is generally well-tolerated. Patients should be advised of common complications (see detailed discussion below). Patients should void before being discharged. If patients develop acute urinary retention, a temporary urinary catheter is required. Patients should also be educated on the symptoms of sepsis and advised to seek medical attention if these occur. Non-opioid simple analgesia is usually adequate for pain. Some specialists may prescribe an alpha-blocker (prazosin, tamsulosin or similar) to reduce voiding symptoms. Typically, patients can return home on the day of the procedure following routine post-operative observation.
Complications
Almost all patients will experience minor, self-limiting side effects from the operation. These can include perineal pain or discomfort, bruising, haematuria (14.5%) and haematospermia (37.5%) (2,4). Temporary erectile dysfunction might be experienced by some patients (4). Sepsis occurs in less than 1% of patients (6). Voiding difficulties are common, especially in patients with pre-existing lower urinary tract symptoms, and acute urinary retention can occur. In a large series of 3000 patients, the morbidity of TPP biopsy positively correlated with the number of cores taken (14).
Additional considerations
Preoperative MRI
Preoperative mpMRI can identify the location of significant prostatic lesions, allowing for targeted sampling. The PROMIS trial demonstrated that targeted biopsy diagnosed 18% more significant PCa lesions than those receiving random TRUS-biopsy, which in turn reduced the need for repeat biopsy and over-treatment of clinically insignificant disease (3). Additionally, a targeted biopsy requires fewer cores and, where present, contains longer lengths of cancer per core. This further assists in reducing perioperative morbidity and improving specificity (19). Given the funding of mpMRI by Medicare to Australian patients who meet the criteria and the current recommendation of the EAU Guidelines (2), mpMRI is now strongly suggested for all patients before undergoing prostate biopsy (2,20).
Targeted biopsy
Targeted biopsies are performed utilising imaging results from a preoperative mpMRI. MRI targeted biopsy can be performed with cognition fusion, real-time ultrasound fusion software, or performed in-bore with real-time MRI guidance (see Table 1). Using mpMRI to target suspicious lesions does not significantly improve the overall detection rate of PCa, however it does increase the detection of clinically significant PCa and lowers the detection of clinically insignificant PCa (see Table 2) (15). Hansen et al. (2018) demonstrated that detection of clinically significant prostate cancer, defined as a Gleason score 7–10, was significantly higher when using combined template guided plus targeted biopsy (36). This combined technique showed 71% specificity, compared to 59% for template and 61% for targeted alone (P<0.001). Therefore initial biopsy should be a combined technique (15,36).
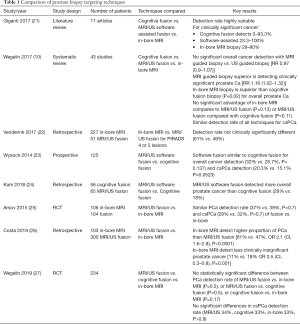
Full table
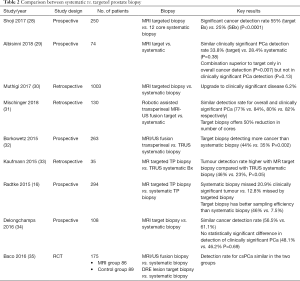
Full table
Cognitive fusion requires the clinician to fuse preoperative mpMRI results with real-time US imaging using their anatomical knowledge and clinical skill alone. A brachytherapy grid can be used and corresponding grid hole coordinates can be seen on US, allowing the clinician to perform systematic and targeted biopsy. In experienced hands this has been shown to be acceptably precise (7). However, this technique requires that clinicians be adequately trained to produce consistent results and can involve a steep learning curve (37).
Multiparametric MRI-US fusion software fuses preoperative mpMRI results with real-time TRUS imaging. Examples of fusion software currently available include BiopSee (Pi Medical), iSR’obotic Mona Lisa (Biobot Surgical) and BioJet (DK technologies). Imaging can be either elastic, which means the software compensates for differences between the preoperative imaging and in situ ultrasound images, whereas rigid software does not. There has been no significant difference noted in outcomes between the two types (38). Cores are taken through brachytherapy grid holes to ensure sampling of the designated areas (7,10). Like the cognitive fusion technique, mpMRI-US fusion software also requires additional clinician training to ensure consistency and reproducibility of results (37). Despite this, mpMRI-US fusion appears to be more reliable than cognitive fusion for less experienced clinicians (7,37).
In-bore MRI-guided biopsy is performed with the patient in the MRI machine, using MRI to guide needle placement into MRI-visible prostatic lesions. This technique has been found to have higher accuracy of needle placement and therefore requires fewer cores than other biopsy techniques (7,37). However, this technique is expensive, time consuming and requires the use of MRI-compatible equipment (7,39).
Current evidence does not suggest a difference in clinically significant prostate cancer detection between cognitive fusion and US-fusion software techniques, however inexperienced clinicians may benefit from using software fusion to locate suspicious targets (7,10). At the time of writing, there are no head-to-head studies comparing different mpMRI-US fusion software techniques. In a review by Wegelin et al., in-bore MRI guided biopsies were shown to have a higher detection rate of clinically significant prostate cancer detection than cognitive targeted biopsy (10). The same review stated that there was no significant difference in the cancer detection rates of in-bore MRI guided biopsies compared to US-fusion software techniques. However, in-bore MRI guided biopsies were demonstrated to have a higher detection rate of clinically significant prostate cancer, and a lower detection rate of clinically insignificant prostate cancer compared to systematic TRUS-biopsy (10).
Despite the improvement in sampling that mpMRI offers, pre-operative imaging does carry some risks. First, not all significant lesions can be seen on mpMRI. Studies have shown that the number of clinically significant cancers missed on mpMRI is up to 10% (19). Second, patient re-positioning for TPP-biopsy can mean that the patient’s anatomy may not correlate perfectly to pre-operative imaging, meaning that significant lesions may be missed on biopsy (19,40).
Free hand vs. brachytherapy grid vs. robotic sampling
Free-hand TPP-biopsy involves the clinician sampling the prostate using knowledge of surface anatomy and TRUS imaging, without the use of a brachytherapy grid. This technique requires a high degree of skill and is associated with a steep learning curve. It is typically performed in lithotomy with local anaesthetic and sedation. The biopsy can be taken from one or two puncture sites through the perineum. The puncture site is used as a pivot point through which samples are taken by redirecting the needle. A single midline puncture site is possible however it places the urethra at increased risk of penetration. Alternatively, two puncture sites, one for each lobe, may be used, lowering the risk of urethral penetration (41). A meta-analysis of available data demonstrated that there is no difference between the cancer detection rate of free-hand TPP-biopsy and TRUS-biopsy (42).
Template-guided TPP-biopsy is the most common technique used by clinicians. A brachytherapy grid is placed over the perineum via the stepper. This can be used to correlate with needle placement on real time US imaging. The grid allows the needle to be guided into pre-planned areas of the prostate, whether performing template or targeted biopsy (7,41). The use of a brachytherapy grid and associated equipment is more time consuming and expensive than free-hand TPP-biopsy. Also, each sample is taken through a new puncture in the perineum, which can be associated with increased pain and subcutaneous bruising. However, it does allow for standardized sampling and is easier for less-experienced clinicians to use (41).
Robot-guided TPP-biopsy uses robotic guidance for the US probe and needle placement for prostate biopsy. One example would be Mona Lisa by Biobot®. The technique uses pre-operative mpMRI-US fusion software for real-time sampling, and can target pre-planned areas of suspicion within the prostate (7,43). The needle placement is calculated by the robot’s software, and accounts for depth and angle of patient positioning. As with free-hand TPP-biopsy, multiple samples can be taken through only one or two skin punctures. Robot-guided TPP-biopsy has been demonstrated to have greater accuracy of needle placement compared to template-guided TPP-biopsy, and greater detection of clinically significant PCa with fewer cores taken (7,31). However, access to this equipment comes at some expense and is not readily available to many urologists.
Transperineal biopsy under local anaesthesia
TPP biopsy has a lower sepsis rate and allows better sampling of the anterior prostate compared to traditional transrectal prostate biopsy. However, the uptake of the technique has been slow due to the perceived need for general or spinal anaesthesia. In the US for example, the expense of performing GA means that outpatient prostate biopsies are favoured. This obstacle is likely to change in the near future with newly emerged evidence showing TPPB is safe and feasible under local anaesthesia. A recent large series by Stefanova analysed 1,287 patients undergoing TPPB under local anaesthesia using a free-hand technique. The anaesthesia was performed with infiltration of the skin followed by peri-prostatic infiltration. Their results suggest that the tolerability of TPPB under LA is similar to TRUS biopsy. The post-operative complications remain low comparing to conventional TPPB under GA (44). Similar results are demonstrated in smaller published series. Kum et al. published a series of 176 patients undergoing TPPB with LA in either day surgery unit (60%) or clinic setting (40%) (45). The tolerability of TPPB under LA was similar. Interestingly, patients who underwent the procedure in the clinic setting had lower VAS (Visual Analogue Scale) comparing to those in the day surgery unit. The authors hypothesised that the differences may have been due to the anxiety that might have been created due to the longer check-in process (45).
Conclusions
The diagnosis of prostate cancer by prostate biopsy demands a trade between acceptable specificity and sensitivity and patient morbidity. TPP-biopsy offers a safe and effective way of obtaining tissue for diagnosis. Emerging technologies and techniques are available with comparable results. As targeting techniques continue to improve, the detection of clinically significant prostate cancer will improve whilst decreasing the detection of insignificant disease and patient morbidity.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Andrology and Urology for the series “Surgery for Urologic Cancers”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/doi: 10.21037/tau.2019.12.40). The series “Muscle-Invasive Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. SS served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Translational Andrology and Urology from Jul 2018 to Jun 2020. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Mottet N, Bellmunt J, Briers, et al. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer. Arnhem, The Netherlands: EAU Guidelines Office; 2017.
- Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 2017;389:815-22. [Crossref] [PubMed]
- Borghesi M, Ahmed H, Nam R, et al. Complications After Systematic, Random, and Image-guided Prostate Biopsy. Eur Urol 2017;71:353-65. [Crossref] [PubMed]
- Ong WL, Weerakoon M, Huang S, et al. Transperineal biopsy prostate cancer detection in first biopsy and repeat biopsy after negative transrectal ultrasound-guided biopsy: the Victorian Transperineal Biopsy Collaboration experience. BJU Int 2015;116:568-76. [Crossref] [PubMed]
- Grummet JP, Weerakoon M, Huang S, et al. Sepsis and 'superbugs': should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int 2014;114:384-8. [Crossref] [PubMed]
- Grummet J, Pepdjonovic L, Huang S, et al. Transperineal vs. transrectal biopsy in MRI targeting. Transl Androl Urol 2017;6:368-75. [Crossref] [PubMed]
- Radtke JP, Boxler S, Kuru TH, et al. Improved detection of anterior fibromuscular stroma and transition zone prostate cancer using biparametric and multiparametric MRI with MRI-targeted biopsy and MRI-US fusion guidance. Prostate Cancer Prostatic Dis 2015;18:288-96. [Crossref] [PubMed]
- Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. [Crossref] [PubMed]
- Wegelin O, van Melick HHE, Hooft L, et al. Comparing Three Different Techniques for Magnetic Resonance Imaging-targeted Prostate Biopsies: A Systematic Review of In-bore versus Magnetic Resonance Imaging-transrectal Ultrasound fusion versus Cognitive Registration. Is There a Preferred Technique? Eur Urol 2017;71:517-31. [Crossref] [PubMed]
- Shariat SF, Roehrborn CG. Using biopsy to detect prostate cancer. Rev Urol 2008;10:262-80. [PubMed]
- Toner L, Bolton DM, Lawrentschuk N. Prevention of sepsis prior to prostate biopsy. Investig Clin Urol 2016;57:94-9. [Crossref] [PubMed]
- Therapeutic Guidelines Limited. Surgical prophylaxis for urological surgery eTG Complete. 2019 [cited 2019 Apr 8].
- Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology 2013;81:1142-6. [Crossref] [PubMed]
- Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic resonance imaging-targeted biopsy may enhance the diagnostic accuracy of significant prostate cancer detection compared to standard transrectal ultrasound-guided biopsy: a systematic review and meta-analysis. Eur Urol 2015;68:438-50. [Crossref] [PubMed]
- Radtke JP, Kuru TH, Boxler S, et al. Comparative Analysis of Transperineal Template Saturation Prostate Biopsy Versus Magnetic Resonance Imaging Targeted Biopsy with Magnetic Resonance Imaging-Ultrasound Fusion Guidance. J Urol 2015;193:87-94. [Crossref] [PubMed]
- Barzell WE, Melamed MR. Appropriate patient selection in the focal treatment of prostate cancer: the role of transperineal 3-dimensional pathologic mapping of the prostate--a 4-year experience. Urology 2007;70:27-35. [Crossref] [PubMed]
- Kuru TH, Wadhwa K, Chang RTM, et al. Definitions of terms, Processes and a minimum dataset for transperineal prostate biopsies: A standardization approach of the Ginsburg Study Group for enhanced prostate diagnostics. BJU Int 2013;112:568-77. [Crossref] [PubMed]
- Martini A, Cumarasamy S, Tewari AK. MRI-Targeted Biopsy for Prostate-Cancer Diagnosis. N Engl J Med 2018;379:589. [Crossref] [PubMed]
- Grummet J. How to Biopsy. Urol Clin North Am 2017;44:525-34. [Crossref] [PubMed]
- Giganti F, Moore CM. Moore, A critical comparison of techniques for MRI-targeted biopsy of the prostate. Transl Androl Urol 2017;6:432-43. [Crossref] [PubMed]
- Venderink W, van der Leest M, van Luijtelaar A, et al. Retrospective comparison of direct in-bore magnetic resonance imaging (MRI)-guided biopsy and fusion-guided biopsy in patients with MRI lesions which are likely or highly likely to be clinically significant prostate cancer. World J Urol 2017;35:1849-55. [Crossref] [PubMed]
- Wysock JS, Rosenkrantz A, Huang W, et al. A prospective, blinded comparison of magnetic resonance (MR) imaging-ultrasound fusion and visual estimation in the performance of MR-targeted prostate biopsy: the PROFUS trial. Eur Urol 2014;66:343-51. [Crossref] [PubMed]
- Kam J, Yuminagga Y, Kim R, et al. Does magnetic resonance imaging-guided biopsy improve prostate cancer detection? A comparison of systematic, cognitive fusion and ultrasound fusion prostate biopsy. Prostate Int 2018;6:88-93. [Crossref] [PubMed]
- Arsov C, Rabenalt R, Blondin D, et al. Prospective randomized trial comparing magnetic resonance imaging (MRI)-guided in-bore biopsy to MRI-ultrasound fusion and transrectal ultrasound-guided prostate biopsy in patients with prior negative biopsies. Eur Urol 2015;68:713-20. [Crossref] [PubMed]
- Costa DN, Goldberg K, Leon AD, et al. Magnetic Resonance Imaging-guided In-bore and Magnetic Resonance Imaging-transrectal Ultrasound Fusion Targeted Prostate Biopsies: An Adjusted Comparison of Clinically Significant Prostate Cancer Detection Rate. Eur Urol Oncol 2019;2:397-404. [Crossref] [PubMed]
- Wegelin O, Exterkate L, van der Leest M, et al. The FUTURE Trial: A Multicenter Randomised Controlled Trial on Target Biopsy Techniques Based on Magnetic Resonance Imaging in the Diagnosis of Prostate Cancer in Patients with Prior Negative Biopsies. Eur Urol 2019;75:582-90. [Crossref] [PubMed]
- Shoji S, Hiraiwa S, Ogawa T, et al. Accuracy of real-time magnetic resonance imaging-transrectal ultrasound fusion image-guided transperineal target biopsy with needle tracking with a mechanical position-encoded stepper in detecting significant prostate cancer in biopsy-naïve men. Int J Urol 2017;24:288-94. [Crossref] [PubMed]
- Albisinni S, Aoun F, Noel A, et al. Are concurrent systematic cores needed at the time of targeted biopsy in patients with prior negative prostate biopsies? Prog Urol 2018;28:18-24. [Crossref] [PubMed]
- Muthigi A, George AK, Sidana A, et al. Missing the Mark: Prostate Cancer Upgrading by Systematic Biopsy over Magnetic Resonance Imaging/Transrectal Ultrasound Fusion Biopsy. J Urol 2017;197:327-34. [Crossref] [PubMed]
- Mischinger J, Kaufmann S, Russo GI, et al. Targeted vs systematic robot-assisted transperineal magnetic resonance imaging-transrectal ultrasonography fusion prostate biopsy. BJU Int 2018;121:791-8. [Crossref] [PubMed]
- Borkowetz A, Platzek I, Toma M, et al. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. BJU Int 2015;116:873-9. [Crossref] [PubMed]
- Kaufmann S, Kruck S, Kramer U, et al. Direct comparison of targeted MRI-guided biopsy with systematic transrectal ultrasound-guided biopsy in patients with previous negative prostate biopsies. Urol Int 2015;94:319-25. [Crossref] [PubMed]
- Delongchamps NB, Portalez D, Bruguière E, et al. Are Magnetic Resonance Imaging-Transrectal Ultrasound Guided Targeted Biopsies Noninferior to Transrectal Ultrasound Guided Systematic Biopsies for the Detection of Prostate Cancer? J Urol 2016;196:1069-75. [Crossref] [PubMed]
- Baco E, Rud E, Eri L, et al. A Randomized Controlled Trial To Assess and Compare the Outcomes of Two-core Prostate Biopsy Guided by Fused Magnetic Resonance and Transrectal Ultrasound Images and Traditional 12-core Systematic Biopsy. Eur Urol 2016;69:149-56. [Crossref] [PubMed]
- Hansen NL, Barrett T. Kesch Cet al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naive men with suspicion of prostate cancer. BJU Int 2018;122:40-9. [Crossref] [PubMed]
- Brown AM, Elbuluk O, Mertan F, et al. Recent advances in image-guided targeted prostate biopsy. Abdom Imaging 2015;40:1788-99. [Crossref] [PubMed]
- Venderink W, de Rooij M, Sedelaar JPM, et al. Elastic Versus Rigid Image Registration in Magnetic Resonance Imaging-transrectal Ultrasound Fusion Prostate Biopsy: A Systematic Review and Meta-analysis. Eur Urol Focus 2018;4:219-27. [Crossref] [PubMed]
- Cicione A, De Nunzio C, Manno S, et al. An update on prostate biopsy in the era of magnetic resonance imaging. Minerva Urol Nefrol 2018;70:264-74. [PubMed]
- Puech P, Sufana Iancu A, Renard B, et al. Detecting prostate cancer with MRI-why and how. Diagn Interv Imaging 2012;93:268-78. [Crossref] [PubMed]
- Dundee PE, Grummet JP, Murphy DG. Transperineal prostate biopsy: Template-guided or freehand? BJU Int 2015;115:681-3. [Crossref] [PubMed]
- Shen PF, Zhu YC, Wei WR, et al. The results of transperineal versus transrectal prostate biopsy: A systematic review and meta-analysis. Asian J Androl 2012;14:310-5. [Crossref] [PubMed]
- Kaye DR, Stoianovici D, Han M. Robotic Ultrasound and Needle Guidance for Prostate Cancer Management: Review of the Contemporary Literature. Curr Opin Urol 2014;24:75-80. [Crossref] [PubMed]
- Stefanova V, Buckley R, Flax S, et al. Transperineal Prostate Biopsies Using Local Anesthesia: Experience with 1,287 Patients. Prostate Cancer Detection Rate, Complications and Patient Tolerability. J Urol 2019;201:1121-6. [Crossref] [PubMed]
- Kum F, Elhage O, Maliyil J, et al. Initial outcomes of local anaesthetic freehand transperineal biopsies in the outpatient setting. BJU Int 2020;125:244-52. [Crossref] [PubMed]


