Endoscopic management of upper tract urothelial carcinoma—tips and tricks
Introduction
Upper tract urothelial carcinoma (UTUC) is a relatively rare malignancy accounting for 5–10% of urothelial tumors, with a slight increase in the past few decades (1,2). Approximately 60% of cases are invasive at the time of diagnosis (3). Radical resection of the ipsilateral kidney and ureter with bladder cuff excision is considered the gold standard treatment of UTUC (4). However, studies of nephron-sparing surgery for renal cell carcinoma have shown that radical nephrectomy is associated with chronic kidney disease (5), increased risk for cardiovascular disease and mortality compared to partial nephrectomy (6). In addition, there is a 2–6% risk of UTUC recurrence in the solitary contralateral kidney after nephroureterectomy (7). These untoward sequelae motivated the search for renal-preserving treatments for UTUC. The main current nephron-sparing approaches include segmental ureterectomy, distal ureterectomy with ureteral re-implantation, and endoscopic treatments. Several characteristics of UTUC have been identified in order to aid in selecting the most appropriate treatment approach. Among them are tumor grade, hydronephrosis, tumor focality, and tumor size (8,9). The updated 2019 European Association of Urology guidelines for UTUC state that endoscopic treatments can be offered when the disease is of “low risk”: low pathological and cytological grade, unifocal, no evidence of invasion on cross-sectional imaging, and is less than 2 cm in size. Similarly, National Comprehensive Cancer Network (NCCN) guidelines limit endoscopic treatment for UTUC to unifocal, low-grade papillary tumors less than 1.5 cm in size (10).
Ureteroscopy was first described in 1912, when a pediatric cystoscope was accidently inserted into a child's ureter (11). With the introduction of fiberoptics, it was possible to develop flexible miniature devices, which are able to reach the upper collecting system. These devices were eventually able to contain a working channel and enable introduction of devices, such as forceps, baskets, and laser fibers. In 1994, Grasso and Bagley described the use of a new device, a 7.5 F flexible ureteronephroscope with a 3.6 F working channel (12). With advances in laser technology and the production of safer, high-output laser generators, the indications for ureteroscopic treatment for stone disease have broadened, and wide experience with ureteroscopic devices was gained. This experience has been implemented into the diagnosis and treatment of UTUC as well. Having been first introduced in the mid-1980s (13), ureteroscopy is now a part of common guidelines for diagnosis, staging, and treatment of UTUC (4,10).
At our institution, we treat low-grade tumors endoscopically, when feasible, regardless of tumor size or focality. The objective of this review is to describe the surgical technique, and provide tips and tricks which we use in our practice of endoscopic retrograde treatment of UTUC.
Patient positioning and anesthesia
Patients are positioned in the dorsal lithotomy position. Although regional or local anesthesia are optional (14), we prefer general anesthesia for two reasons: first, treating upper tumors can be a lengthy procedure in cases of hard-to-reach tumors or a high tumor burden, and second, treating upper tract tumors is a delicate and a precise procedure. Control over kidney movement through respiratory rate and tidal volume is crucial during the procedure (15). In contrast to stone lithotripsy, laser energy is delivered very close to the collecting system wall, and it can easily cause damage if delivered to an inappropriate location. This is even more important when using the neodymium laser, which penetrates deeper into the tissue.
Preliminary cystoscopy
All endoscopic procedures should start with a formal cystoscopy in which a thorough investigation of the urethra and bladder wall is conducted. Any tumor or suspicious-looking mucosa is documented. Transurethral resection of bladder tumor (TURBT) is delayed until the end of the ureteroscopic procedure, unless the ureteral orifice (UO) is covered by a bladder tumor and cannot be identified. At the end of the ureteroscopic procedure, a zebra guidewire (™Boston Scientific) is left in the ureter, and the TURBT can be safely completed. This wire does not conduct electricity, making it safe for use during TURBT and for avoiding ureteral damage if it comes into contact with the resectoscope. At the conclusion of the procedure, bladder irrigation is performed prior to ureteral stenting in order to flush out malignant cells and avoid seeding into the upper tract (16).
Access to upper urinary tract
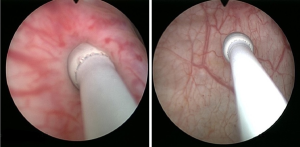
Identification of the UO location and configuration is done during rigid cystoscopy. Retrograde ureteropyelography with an 8FR cone-tipped catheter (™BARD) is performed at this point (Figure 1). The cone-tipped catheter is composed of an 8F body and a distal cone-shaped tip. This cone is inserted into the UO, occluding the lumen and preventing backflow of contrast material to the bladder (Figure 2). This approach provides high-quality images and aids in identification of filling defects. Next, the semi-rigid ureteroscope is introduced into the UO and up the ureter as proximally as possible by applying the “no-touch” technique as described by Tawfiek et al. (17). No safety guide is used in this technique since the wire might chip off small tumors and thereby prevent their detection or, alternatively, injure larger tumors, cause bleeding and obscure visualization. If a ureteral tumor is encountered, several biopsies are obtained, and the tumor is ablated with a holmium:YAG (Ho:YAG) laser. The Ho:YAG laser is safe to use on ureteral tumors since it does not penetrate more than 0.5 mm into the tissue and does not cause subsequent stricture. A neodymium:YAG (Nd:YAG) laser should be used with caution while treating ureteral tumors. While the tumor’s intraluminal portion can be treated safely with Nd:YAG, this laser should not be used when treating the ureteral wall due to a high rate of stricture formation (18). At this point, a guide-wire is inserted through the semi-rigid ureteroscope up to the most proximal level that had been reached. The semi-rigid ureteroscope is withdrawn, and a flexible ureteroscope is advanced over the wire up to the level last visualized by the semirigid ureteroscope. The rest of the ureter, renal pelvis, and calices are then surveyed. If a large tumor is encountered, a ureteral access sheath (UAS) is inserted up to the proximal ureter. The wire we use is a 0.038-inch hydrophilic-coated wire (™Radifocus) with a floppy tip and a stiff body. Although the hydrophilic properties of the wire make it slippery to handle and might cause it to easily slip out of the ureter, this is rarely the case in experienced hands. Nonetheless, hydrophilic wires are atraumatic and, therefore, the most appropriate for UTUC treatment.
The UAS is an important tool in the treatment of UTUC, since it allows efficient drainage of bleeding from the resected area. It also enables the use of high-flow irrigation without elevating the intrapelvic pressure (19), hence improving visibility. These properties facilitate the treatment of large-volume tumors.
The default UAS size we use is 12/14 F. Although some reports claim that UAS use may cause ureteral damage, our group and others recently demonstrated that UAS does not cause clinically significant ureteral strictures, even in unstented ureters (20,21).
Irrigation
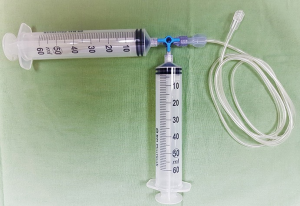
As mentioned above, high-flow irrigation is essential for UTUC treatment, especially for large-volume tumors. Irrigation through the ureteroscope’s working channel is initially used with normal saline and a pressure-regulating pump set at 200 mmHg. Visibility is occasionally hampered by bleeding from tumoral tissue, and manual syringe-based power irrigation with distilled water operated by an assistant is used for these cases (Figure 3). The assistant can control the irrigation rate according to the bleeding rate. Since distilled water is hypotonic, water enters red blood cells by diffusion and then blasts them. The use of distilled water during ureteronephroscopy was shown to improve visibility compared to normal saline (22). The rate of water absorption into the circulation is about 1–2 mL/min. Therefore, prolonged procedures are safe and do not cause hyponatremia (23). In our experience, distilled water delivered through a manual syringe system provides the best visibility and enables the treatment of high-volume disease in one session. The manual syringe system is a simple solution that generates the high-pressure flow necessary for this procedure (24).
Biopsy
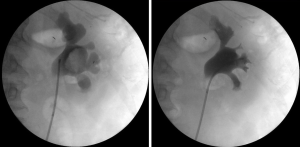
Biopsy from a suspicious-looking lesion must be obtained in every procedure, including follow-up ureteroscopies. Biopsy results in the first procedure will determine tumor grade and whether the patient is suitable for endoscopic treatment. Biopsies are also important to rule out grade progression in cases of recurrence. A 3 F cup biopsy forceps should be used for small or solid lesions. Multiple specimens should be obtained since the amount of tissue that can be captured by these forceps is low and taking several biopsies was shown to increase diagnostic accuracy (25). The preferred device for larger tumors is the 2.4 F stainless steel flat-wire basket. This basket is composed of ribbon-like strands made out of stainless steel, which imparts its unique characteristics of cutting the tumor like a guillotine, performing debulking of the tumor, as well as obtaining a large-volume high-quality biopsy (Figure 4). The flat-wire basket was shown to provide a more accurate diagnosis compared to the 3F cup biopsy forceps, and should be used when possible (26). Moreover, although the BIGopsy forceps (™Cook Medical) was shown to provide a more accurate diagnosis compared to the 3FR forceps (27), difficult manipulation and decreased visualization make it less attractive for use in this setting.
Tumor ablation
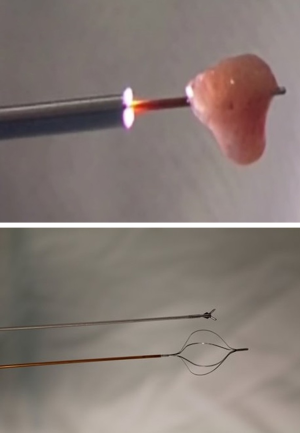
We treat UTUC endoscopically using a dual laser generator (Figure 5) which contains two different lasers, a neodymium laser (Nd:YAG) and a holmium laser (Ho:YAG). The Nd:YAG is a continuous laser with a 1064-micron wavelength and a 5–10 mm tissue penetration depth, while the Ho:YAG is a pulsed laser with a 2,140 micron wavelength and a 0.5–1 mm tissue penetration depth. The two lasers are operated alternatively through the same laser fiber by using a dual foot pedal (Figure 5). The treatment starts by coagulating the tumoral tissue surface with the Nd:YAG which creates a layer of necrotic tissue while maintaining excellent hemostasis (Figure 6). Next, the necrotic tissue is resected with the Ho:YAG laser. This process is repeated until the entire intraluminal tumoral tissue has been treated. The combination of the two lasers enables the surgeon to operate in a “bloodless” field and thereby treat relatively large tumors (28). In addition, coagulating the tumor prior to resecting it results in non-viable cancer cells in the urine, which may potentially reduce seeding and decrease tumor recurrence (29). An additional advantage of the Nd;YAG laser is that it is hemostatic and provides excellent hemostasis when delivered against an open vessel. For this reason, this type of laser is used in the treatment of hemangiomas in the urinary bladder as well as in other organs (30,31).
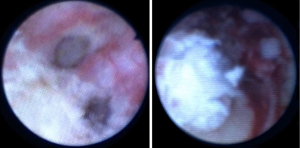
The laser setting we use for Nd:YAG is 30 J in continuous mode, which may be brought up to 60 J (with a 365-micron laser fiber) according to tissue effect, tumor size, and distance from the collecting system’s wall. For Ho:YAG, we use 0.6 J and 10 Hz, which resects the tumor efficiently with good control over the tumoral surface. Our preferred laser fiber diameter is 365-micron, which delivers higher energy and treat the tumor effectively. The 272-micron laser fiber occupies less space in the ureteroscope's working channel, which results in higher flow rate. We use this fiber in cases where better visualization is required. The 272-micron laser fiber is also the fiber of choice in cases of lower-pole tumors, which require down-deflection of the flexible ureteroscope in order to reach it. The 365-micron fibers significantly decrease that deflection compared to the 272 fiber (32). We use a 2 F Bugbee diathermy electrode connected to a standard electrosurgical generator at 20 W in extreme cases of lower-pole tumors where the 272-micron fiber limits the flexible ureteroscope’s deflection and prevents the scope from reaching the tumor. This electrode is extremely delicate and does not limit the deflection to any degree (Figure 7).

Conclusions
Technological advances and greater surgical experience have led to the wide acceptance of endoscopic management of UTUC in well-selected patients. Refined techniques in diagnosis and treatment, and the availability of precision surgical devices have led to improved surgical outcomes. We believe that endoscopy in the setting of UTUC will continue to evolve and become applicable to a wider selection of patients.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (John J. Knoedler and Jay D. Raman) for the series “Upper-Tract Urothelial Carcinoma: Current State and Future Directions” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.01.07). The series “Upper-Tract Urothelial Carcinoma: Current State and Future Directions” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical statement: the authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Raman JD, Messer J, Sielatycki JA, et al. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int 2011;107:1059-64. [Crossref] [PubMed]
- Margulis V, Shariat SF, Matin SF, et al. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer 2009;115:1224-33. [Crossref] [PubMed]
- Rouprêt M, Babjuk M, Burger M, et al. EAU Guidelines on Urothelial Carcinomas of the Upper Urinary Tract. 2019.
- Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol 2006;7:735-40. [Crossref] [PubMed]
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317-23. [Crossref] [PubMed]
- Novara G, De Marco V, Dalpiaz O, et al. Independent predictors of contralateral metachronous upper urinary tract transitional cell carcinoma after nephroureterectomy: multi-institutional dataset from three European centers. Int J Urol 2009;16:187-91. [Crossref] [PubMed]
- Shibing Y, Liangren L, Qiang W, et al. Impact of tumour size on prognosis of upper urinary tract urothelial carcinoma after radical nephroureterectomy: a multi-institutional analysis of 795 cases. BJU Int 2016;118:902-10. [Crossref] [PubMed]
- Cho KS, Hong SJ, Cho NH, et al. Grade of hydronephrosis and tumor diameter as preoperative prognostic factors in ureteral transitional cell carcinoma. Urology 2007;70:662-6. [Crossref] [PubMed]
- Flaig TW, Spiess PE, Agarwal N, et al. NCCN Guidelines version 1.2020 Upper GU Tract Tumors.National Comprehensive Cancer Network.
- Young HH, McKay RW. Congenital valvular obstruction of the prostatic urethra. Surg Gynecol Obstet 1929;48:509-35.
- Grasso M, Bagley D. A. 7.5/8.2 F actively deflectable, flexible ureteroscope: a new device for both diagnostic and therapeutic upper urinary tract endoscopy. Urology 1994;43:435-41. [Crossref] [PubMed]
- Zincke H, Neves RJ. Feasibility of conservative surgery for transitional cell cancer of the upper urinary tract. Urol Clin North Am 1984;11:717-24. [PubMed]
- Park HK, Paick SH, Oh SJ, Kim HH. Ureteroscopic lithotripsy under local anesthesia: analysis of the effectiveness and patient tolerability. Eur Urol 2004;45:670-3. [Crossref] [PubMed]
- Emiliani E, Talso M, Baghdadi M, et al. The Use of Apnea During Ureteroscopy. Urology 2016;97:266-8. [Crossref] [PubMed]
- Kiss B, Furrer MA, Wuethrich PY, et al. Stenting Prior to Cystectomy is an Independent Risk Factor for Upper Urinary Tract Recurrence. J Urol 2017;198:1263-8. [Crossref] [PubMed]
- Tawfiek E, Bibbo M, Bagley DH. Ureteroscopic biopsy: technique and specimen preparation. Urology 1997;50:117-9. [Crossref] [PubMed]
- Schmeller NT, Hofstetter AG. Laser treatment of ureteral tumors. J Urol 1989;141:840-3. [Crossref] [PubMed]
- Auge BK, Pietrow PK, Lallas CD, et al. Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J Endourol 2004;18:33-6. [Crossref] [PubMed]
- Shvero A, Herzberg H, Zilberman D, et al. Is it safe to use a ureteral access sheath in an unstented ureter? BMC Urol 2019;19:80-5. [Crossref] [PubMed]
- Stern KL, Loftus CJ, Doizi S, et al. A Prospective Study Analyzing the Association Between High-grade Ureteral Access Sheath Injuries and the Formation of Ureteral Strictures. Urology 2019;128:38-41. [Crossref] [PubMed]
- Gielchinsky I, Pode D, Duvdevani M, et al. The Transparency of Irrigation Fluids Used in Endoscopic Surgery . J Endourol 2017;31:701-4. [Crossref] [PubMed]
- Cybulski P, Honey RJ, Pace K. Fluid absorption during ureterorenoscopy. J Endourol 2004;18:739-42. [Crossref] [PubMed]
- Proietti S, Dragos L, Somani BK, et al. In Vitro Comparison of Maximum Pressure Developed by Irrigation Systems in a Kidney Model. J Endourol 2017;31:522-7. [Crossref] [PubMed]
- Tavora F, Fajardo DA, Lee TK, et al. Small endoscopic biopsies of the ureter and renal pelvis: pathologic pitfalls. Am J Surg Pathol 2009;33:1540-6. [Crossref] [PubMed]
- Kleinmann N, Healy KA, Hubosky SG, et al. Ureteroscopic biopsy of upper tract urothelial carcinoma: comparison of basket and forceps. J Endourol 2013;27:1450-4. [Crossref] [PubMed]
- Breda A, Territo A, Sanguedolce F, et al. Comparison of biopsy devices in upper tract urothelial carcinoma. World J Urol 2019;37:1899-905. [Crossref] [PubMed]
- Verges DP, Lallas CD, Hubosky SG, et al. Endoscopic Treatment of Upper Tract Urothelial Carcinoma. Curr Urol Rep 2017;18:31-42. [Crossref] [PubMed]
- Liu Y, Lu J, Hong K, et al. Independent prognostic factors for initial intravesical recurrence after laparoscopic nephroureterectomy for upper urinary tract urothelial carcinoma. Urol Oncol 2014;32:146-52. [Crossref] [PubMed]
- Kato M, Chiba Y, Sakai K, et al. Endoscopic neodymium:yttrium aluminium garnet (Nd:YAG) laser irradiation of a bladder hemangioma associated with Klippel-Weber syndrome. Int J Urol 2000;7:145-8. [Crossref] [PubMed]
- Yang HY, Zheng LW, Yang HJ, et al. Long-pulsed Nd: YAG laser treatment in vascular lesions of the oral cavity. J Craniofac Surg 2009;20:1214-7. [Crossref] [PubMed]
- Akar EC, Knudsen BE. Evaluation of 16 New Holmium:Yttrium-Aluminum-Garnet Laser Optical Fibers for Ureteroscopy. Urology 2015;86:230-5. [Crossref] [PubMed]