Genetic evaluation of male infertility
Introduction
Men with azoospermia or severe oligozoospermia (<5 million sperm/mL ejaculate fluid) are predisposed to genetic abnormalities. Genetic testing, including karyotype/cytogenetic testing, Y chromosome microdeletion (YCMD) testing, congenital hypogonadotropic hypogonadism (HH) mutation screening, and cystic fibrosis transmembrane conductance regulator (CFTR) gene screening, may reveal the etiology and likelihood of successful paternity, and potential risks to offspring (1). Informed discussion with the patient about the role of genetic testing, and the prognostic and psychological effects of genetic findings, should be completed prior to testing. Many genetic mutations and polymorphisms have been identified that have putative direct or indirect effects on spermatogenesis or yet unknown exact phenotypic consequences. Further the cause of infertility can be demonstrated in approximately 20-30% of men with severe oligozoospermia or azoospermia, while up to 80% remain with an unknown genetic cause requiring additional research to identify candidate gene(s) (2).
Diagnosis
Differentiation between obstructive (physical blockage due to absence of parts of the male excurrent ductal system) and non-obstructive (spermatogenic failure without sperm in pellet of a centrifuged ejaculate) causes is of paramount importance in selection of appropriate genetic testing. History and physical examination including scrotal evaluation and serum lab tests are critical to this delineation to quantitate testis volume, signs of hypogonadism, and abnormal laboratory values especially serum follicle-stimulating hormone (FSH). In up to 70% of men, such preliminary workup will identify a cause for infertility (3).
Genetic testing: who should have it?
All genetic testing options should not be completed for every azoospermic patient. Testing should be considered given the clinical context for each patient (Figure 1). For men with normal testis volume, palpable vasa on physical examination, and strong suspicion for obstruction with normal serum FSH and normal semen volume, no genetic testing is indicated. Genetic testing is also not recommended for patients with known prior history of fertility or those patients with previously documented sperm concentration >5 million/mL of ejaculate fluid. Patients with known prior toxic exposure such as cytotoxic chemotherapy or radiation also do not require genetic testing (4). Patients who should be tested include those with suspected congenital obstruction (normal testis volume and FSH), primary testicular failure (low testis volume and high FSH), “incomplete” testicular failure with either low testis volume or increased FSH, or HH (low testicular volume, low or low-normal LH, and low testosterone) (Figure 1).
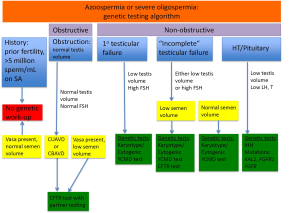
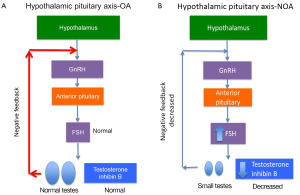
Men with primary testicular failure and severe oligospermia or non-obstructive azoospermia (NOA) with impaired endocrine and exocrine function and sperm count lower than five million per mL of ejaculate fluid will classically have small, atrophic, soft testes, normal semen volume and seminal pH, and elevated FSH. These characteristics reflect poor spermatogenesis or reduction in seminiferous tubules. Decreased testicular endocrine and exocrine processes result in decreased testosterone and inhibin B negative feedback on the hypothalamus and pituitary, resulting in increased FSH (and sometimes LH if testosterone is significantly decreased) (Figure 2). Inhibin B, a glycoprotein hormone produced by the testis, is inversely related to FSH and strongly correlated with testicular volume and sperm count. There are some cases, such as late maturation arrest, that may demonstrate normal FSH and inhibin B (5). Although FSH and inhibin B are most sensitive when obtained together, inhibin B does not provide valuable independent diagnostic information and is therefore not routinely measured in clinical practice at this time. Men with classic primary testicular failure should undergo karyotype and YCMD analysis (1,6). Patients with severe oligospermia or azoospermia along with testicular volume and serum FSH that have an incomplete primary testicular failure clinical picture (i.e., normal testis volume with elevated FSH) also require karyotyping and YCMD testing prior to any possible treatment (7). Men with normal FSH, normal testicular volume without evidence of obstruction on physical exam could have testicular maturation arrest, commonly associated with abnormalities of genes including MutL homolog 1 (MLH1) gene, MutS homolog 2 (MSH2), and excision repair cross-complementation group 1 (ERCC1) (8-11). Additionally, CFTR testing should be completed in the setting of low semen volume due to association with seminal vesicle atresia (discussed further in cystic fibrosis section below).
Karyotype analysis of all numeric (gains/losses) and structural abnormalities (most frequently Robertsonian, but also reciprocal translocations, inversions) of entire chromosomes is associated with approximately 6% of all male infertility. Four percent of men receiving ICSI for male subfertility have chromosomal abnormalities, the majority of which involve sex chromsomes (12). Karyotypic abnormalities are identified in 3% to 5% of severely oligozoospermic (often translocations) and 14% to 19% of men with NOA (most frequently nonmosaic Klinefelter syndrome, 47,XXY) and are eight times more common in infertile than fertile men (13-15). Klinefelter syndrome (47,XXY) is identified in one in 600 males among the general population. Patients with Klinefelter syndrome present with a spectrum of phenotypes (dependent on testosterone level) but all have atrophic testes with characteristic abnormal exocrine and endocrine function (elevated LH, FSH, low testosterone) and azoospermia. Reduced Leydig cell size along with germ cell loss, Sertoli cell loss, and tubular hyalinization contribute to testicular dysfunction. Deficient androgen production and excess estradiol from overexpressed CYP19 aromatase is manifested as delayed progression of puberty with decreased facial/body hair, decreased muscle development, bone density loss, and gynecomastia. This altered hormonal milieu may predispose to learning disabilities and social maldevelopment. Risk of sperm and subsequent embryo aneuploidy may be slightly increased in some studies (16), while others show no increased risk of aneuploidy (17). 46,XX is another possible but rare karyotype (1:20,000 live births) identified in azoospermic men, often resulting from translocation of the distal tip of Y chromosome short arm (containing SRY gene) to the distal tip of the X chromosome short arm. The remaining Y chromosome is not present, including AZF regions, and therefore spermatogenesis is absent making TESE not possible (18). Yq loss also occurs in isodicentric Y chromosome which may be unstable and also lack AZF regions. Additionally, abnormalities of X chromosome linked genes (i.e., androgen receptor Xq11.2-12) may exhibit a spectrum of androgen insensitivity based on specific mutation and CAG repeat length and can result in 46,XY azoospermic males (19,20).
Increasing deterioration of spermatogenesis (decreased sperm count) is associated with increased prevalence of karyotype abnormalities (for example increasing number of X chromosomes associated with increased clinical severity). Karyotype should be offered to all men with severe oligospermia or NOA prior to treatment. Following culture of leukocytes from the patient’s blood, treatment with protease and characteristic staining (G banding after treatment with trypsin and Giemsa staining) is completed, and additional cytogenetic analysis including extended karyotyping or fluorescence in situ hybridization (FISH) is sometimes necessitated. Genetic counseling should outline risk of paternal transmission of chromosomal abnormalities (reciprocal more than Robertsonian translocations, inversions) and consideration should be given to preimplantaion genetic diagnosis (PGD) since karyotypic abnormalities can translate to pregnancy loss and offspring risks (21).
YCMD, located on the long arm of the Y chromosome (Yq), involve three azoospermic factor (AZF) regions associated with spermatogenesis. YCMD are not typically identified on standard karyotypic analysis due to small size, but instead are detected by PCR amplification (using DNA extracted from peripheral blood) in 5-10% and up to 10-20% of men with severe oligospermia and NOA, respectively. There is, however, variation in YCMD according to ethnic/geographic background (22-24). YCMD testing detects regions AZFa (USP9Y and DDX3Y), AZFb (including EIF1AY, HSFY, SMCY, RPS4Y2, PRY), and AZFc (including BPY2, CDY, DAZ) using sequence-tagged sites (STS). STS are pre-chosen unique DNA sequences sets which reflect presence or absence of the different Y chromosomal regions. YCMD testing has prognostic value and guides choice of microdissection TESE/ICSI vs. donor sperm/adoption. Sixty percent of YCMDs do not result in retrievable sperm with TESE, and these include all men with AZFa (Sertoli cell only syndrome), AZFb (maturation arrest, P5-proximal P1) and AZFb+c (maturation arrest, P5-distal P1) deletions, none of which have had sperm identified to date with TESE in the few available studies (25,26). Among infertile men, AZFc is the most frequent de novo microdeletion of the Y chromosome (1:4,000 men) presenting with severe oligospermia in two out of three cases, with remaining men being azoospermic (27). Men with AZFc microdeletions, which encompass seven groups of transcription units, have retrievable sperm 70% to 80% of the time and IVF/ICSI results are comparable to controls (28). However, it is important to note that AZFc microdeletions are transmitted from affected fathers to all sons resulting in compromised fertility potential (29,30). Additionally, partial AZFb and AZFc deletions (gr/gr, b1/b3, b2/b3) may exist that may be compatible with normal sperm count or reduced sperm count amenable to successful sperm retrieval (31,32).
Although men with NOA most often have primary testicular failure, it is also possible that there is secondary testicular failure (congenital or acquired) due to hypothalamic-pituitary axis deficiency resulting in decreased gonadotropin production and thereby decreased testosterone production. HH of congenital etiology is characterized by consistent hypogonadism throughout life that is, by definition, not acquired. This hypogonadism results from deficient gonadotropin production (LH, FSH) from the hypothalamus or anterior pituitary. The most common cause of congenital HH gonadotropin deficiency is Kallmann Syndrome, a developmental genetic disorder, affecting up to 1:10,000 men. Kallmann Syndrome includes a range of phenotypes including isolated congenital HH without other abnormalities or classic Kallmann’s syndrome with olfactory abnormalities of anosmia or severe hyposmia. Anosmia or hyposmia are due to aberrant migration of GnRH-secreting neurons and olfactory axons during embryogenesis and may occur simultaneously with other phenotypic abnormalities. Multiple genetic etiologies have been described including various KAL1 mutations responsible for X-linked Kallmann syndrome (33,34). Autosomal genes FGFR1, FGF8, PROKR2 and others have been linked to HH without olfactory abnormalities (35,36). Treatment includes hormone replacement therapy based on deficiency (36,37). Genetic tests are not yet readily available, however data about inheritance patterns (X-linked, autosomal dominant, autosomal recessive) may be useful for counseling patients regarding risks to offspring and need for PGD. More exhaustive search for a genetic source may be considered in familial cases.
Obstructive azoospermia (OA) occurs with normal sperm production but blocked flow in the male excurrent dutal system. With obstruction, testicular function, testicular volume (~20 mL), and FSH (1-8 IU/mL) are normal. FSH is normal due to Leydig and Sertoli cells’ negative feedback (via testosterone, inhibin B respectively) on the hypothalamus and pituitary. In the case of congenital unilateral absence of the vas deferens (CUAVD) or congenital bilateral absence of the vas deferens (CBAVD), physical examination reveals unilateral or bilateral absence of the vas deferens and/or indurated, irregular epididymal segments or even partial absence of epididymal segments. The caput of the epididymis is always present due to different embryologic origin than the cauda or corpus. If CUAVD or CBAVD is likely, then genetic testing for CFTR gene mutation (chromosome 7q31.2) is performed with simultaneous renal ultrasound to rule out renal agenesis (38). Men with CBAVD exhibit two affected CFTR alleles (up to 80% of patients) with up to a 15% chance of additional abnormalities. Of the two CFTR alleles, there is most frequently one severe allele and one mild allele in 85% of patients (39). If renal agenesis occurs concomitantly with CBAVD, however, this is secondary to abnormal mesonephric duct formation unrelated to CFTR mutation genetics (40).
Cystic fibrosis is the most common autosomal recessive condition occurring clinically in 1:1,600 patients of Northern European descent/non-Hispanic white population with a progressive course adversely affecting lung, pancreas, and other parts of the gastrointestinal system (41). Cystic fibrosis results from abnormalities in the CFTR gene, which typically regulates exocrine epithelial cell tubal secretion consistency and increases sweat sodium chloride concentration via cyclic AMP pathways. The vast majority of males with CF demonstrate CBAVD (more than 95%) with resultant OA. Greater than 1,700 mutations and abnormalities have been demonstrated, with severity resulting from decreased or absent CFTR protein based on maternal and paternal allele contributions. Phenotypes range from isolated CBAVD, vas without a true lumen, atrophic or absent epididymal corpus/cauda, to classic cystic fibrosis (42). The majority of CF screening panels include between 30 and 50 mutations (including the most common abnormality in CBAVD, F508del as well as 5T, 7T, and 9T variants) which are identified most commonly in patients with clinical symptoms of CF (43,44). Even with complete CFTR gene screening, mutations may still be undetectable in 25% of CBAVD patients. Comprehensive analysis of the CFTR gene with DNA sequencing is reserved for patients with CF history or CBAVD (45). In CBAVD, which is classified as surgically unreconstractable OA, microsurgical epididymal sperm aspiration (MESA) is the optimal treatment with subsequent assisted reproductive technology (ART) (46,47).
Patients with CUAVD/CBAVD classically have low semen volume, with low pH (<7) due to absent alkaline seminal vesicle secretions and fructose due to seminal vesicle atresia which is common in these patients (48). If vasa are palpable, but the patient has low semen volume (<1.5 mL), CFTR mutation testing is indicated since seminal vesicle atresia/obstruction could reflect partial absence of the vas with normal scrotal vas and missing retroperitoneal vas. In addition, CUAVD patients frequently have contralateral obstruction of the seminal vesicle and manifest CFTR mutations. If idiopathic epididymal obstruction is considered, then some groups recommend CFTR mutation testing since up to 50% of men may demonstrate mutations.
In rare cases, open testicular biopsy (optimally with microsurgical approach) may be indicated to differentiate OA from NOA in azoospermic men with normal volume testes, palpable vasa, normal FSH levels, and a negative serum anti-sperm antibody test (49). In men with CBAVD, testicular biopsy commonly shows active spermatogenesis and is not needed before definitive aspiration for IVF/ICSI. Female partner testing and genetic counseling is required prior to ART since up to 5% of partners may be heterozygotes among Caucasians. If the female partner is identified to be CF heterozygote, PGD selection of unaffected embryos may reduce the risk of having offspring with infertility or clinical CF.
Management and additional considerations
For all aforementioned genetic tests, genetic counseling should be provided for patients describing limits and benefits of genetic diagnosis prior to actual genetic testing (6). Management of oligospermia and azoospermia will be discussed in further detail in this issue, but includes IVF/ICSI (50) in conjunction with microdissection TESE for men with NOA. Such treatment yields successful sperm retrieval in approximately 60% of patients (51). For men with severe oligospermia or OA, treatment with IVF/ICSI and with MESA/testicular tissue extraction with IVF/ICSI, respectively, results in very high success rates (52-55).
Genetic testing for male infertility may reveal more than only fertility risk. Men with CUAVD or CBAVD with CFTR abnormalities obviously must be monitored for sequelae associated with the spectrum of possible disorders. Men with NOA and oligospermia have been recognized in some studies to have defective DNA repair, with 2.9 fold and 1.4 fold respective increase in cancer risk (56) including testis, prostate, and hereditary nonpolyposis colorectal cancer (Lynch Syndrome) (57,58). Impaired systemic homologous recombination, DNA repair, microsatellite stability, and apoptotic response likely underlie this predisposition and necessitate consideration of an increased cancer risk on clinical examination.
Additional study, of sperm microRNA (miRNA) and small noncoding RNA which regulate transcriptional and post-transcriptional gene expression, has identified expression differences according to testicular histology which could enhance diagnosis and therapy in the future (59). Specifically, a number of miRNAs are produced by male germ cells throughout spermatogenesis and have been identified in testis tissue and semen (60,61). Differential miRNA expression has been noted using miRNA microarray assays with quanititative real-time PCR (qRT-PCR) confirmation. Two miRNA families, miR-34 and miR-449, have been identified to be significantly different between testis tissue of azoospermic men with SCO, MA (all with significant down-regulation of miRNA) when compared with normal spermatogenesis tissue (59). Additionally, differences in seminal miRNA expression profiles have been noted between oligoasthenozoospermic (including miR-34b, miR-34b*, miR-15b, miR-34c-5p) and asthenozoospermic (miR-34b, miR-122 and miR-1973) men and men with normal spermatogenesis (62,63). Additional miRNAs (miR-34c-5p, miR-122, miR-146b-5p, miR-181a, miR-374b, miR-509-5p and miR-513a-5p) have been shown to be markedly decreased in azoospermia while increased in asthenozoospermia (63). The predictive value of the identified miRNA to differentiate control versus azoospermic cases and control versus asthenozoospermia cases had an AUC in ROC analysis ranging from 0.733-0.836 and 0.822-0.921, respectively (63). Late meiotic cells and haploid germ cells are the main contributing source of miRNA during spermatogenesis, and miRNA aberrations may serve as modifiers early post-fertilization and persist in blastocysts (64,65).
Epigenetic studies have demonstrated differences in testicular DNA methylation (addition of methyl group to C5 on cytosine which may alter DNA expression) in azoospermic men using, for example, the Illumina Infinium™ Human Methylation27 BeadChip DNA methylation arrays. In one study of 94 azoospermic men, NOA and OA men were demonstrated to have significantly different DNA methylation profiles (assessed by CpG sites). Additionally, abnormal DNA methylation of imprinted genes in spermatozoa of oligozoospermic men have been identified (66). Histone/protamine modification profiles may also prove useful in identifying further genetic causes of oligospermia and azoospermia (67-69). Precursor (pre-mRNA) splicing regulators (studied in mice so far) (70), copy number variations (CNVs), and seminal mRNA transcript analyses may also prove helpful (71,72). Finally differences in sperm mitochondrial genome deletions may be an additional area for exploration (70). Lastly, work has proven promising in generating primordial germ cell-like cells for spermatogenesis from embryonic and induced pluripotent stem cells (from mice) (73) and human primodial germ cell reprogramming (74). With translation of such work to humans, profound characterization of the genetics of germ cells may provide additional treatment avenues for men with defects in spermatogenesis and future multi-gene predictive assays as have been formulated in prostate cancer (75) bladder cancer (76), and breast cancer prognosis (77).
Conclusions
Genetic testing is necessitated in all severely oligospermic and non-obstructive azoospermic men. Such men demonstrate small-to-atrophic, soft testes and increased FSH. Karyotype chromosome structural and numeric abnormalities, YCMD, and other genetic mutations have been implicated in male subfertility. Klinefelter syndrome (47,XXY) is the most common karyotypic abnormality, while AZFc microdeletion is the most common Y microdeletion. HH is diagnosed and may result from multiple genetic abnormalities with differing modes of inheritance. OA caused by CUAVD/CBAVD requires cystic fibrosis mutation testing (including less common variants identified specifically in CBAVD population) of the patient (who otherwise demonstrates normal volume testes and normal FSH) and his partner. The above testing for severely oligospermic and azoospermic men will provide prognostic information and guide medical management, sperm retrieval options, or in some cases alert the patient and clinician to a lack of any spermatogenesis in the case of 46,XX, and AZFa, AZFb, or AZFb+c microdeletions. Future study of sperm genetic expression profiles, stem cells, miRNA, and epigenetic modifications will optimize our genetic testing for male infertility and provide potential therapeutic targets.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Practice Committee of American Society for Reproductive Medicine in collaboration with Society for Male Reproduction and Urology. The management of infertility due to obstructive azoospermia. Fertil Steril 2008;90:S121-4. [PubMed]
- Dohle GR, Halley DJ, Van Hemel JO, et al. Genetic risk factors in infertile men with severe oligozoospermia and azoospermia. Hum Reprod 2002;17:13-6. [PubMed]
- Bhasin S, de Kretser DM, Baker HW. Clinical review 64: Pathophysiology and natural history of male infertility. J Clin Endocrinol Metab 1994;79:1525-9. [PubMed]
- Meistrich ML. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil Steril 2013;100:1180-6. [PubMed]
- Foresta C, Bettella A, Petraglia F, et al. Inhibin B levels in azoospermic subjects with cytologically characterized testicular pathology. Clin Endocrinol (Oxf) 1999;50:695-701. [PubMed]
- Male Infertility Best Practice Policy Committee of the American Urological Association. Report on evaluation of the azoospermic male. Fertil Steril 2006;86:S210-5. [PubMed]
- Vogt PH, Edelmann A, Kirsch S, et al. Human Y chromosome azoospermia factors (AZF) mapped to different subregions in Yq11. Hum Mol Genet 1996;5:933-43. [PubMed]
- Ishikawa T, Fujioka H, Fujisawa M. Clinical and hormonal findings in testicular maturation arrest. BJU Int 2004;94:1314-6. [PubMed]
- Gonsalves J, Sun F, Schlegel PN, et al. Defective recombination in infertile men. Hum Mol Genet 2004;13:2875-83. [PubMed]
- Reitmair AH, Schmits R, Ewel A, et al. MSH2 deficient mice are viable and susceptible to lymphoid tumours. Nat Genet 1995;11:64-70. [PubMed]
- Maduro MR, Casella R, Kim E, et al. Microsatellite instability and defects in mismatch repair proteins: a new aetiology for Sertoli cell-only syndrome. Mol Hum Reprod 2003;9:61-8. [PubMed]
- Lee JY, Dada R, Sabanegh E, et al. Role of genetics in azoospermia. Urology 2011;77:598-601. [PubMed]
- Van Assche E, Bonduelle M, Tournaye H, et al. Cytogenetics of infertile men. Hum Reprod 1996;11 Suppl 4:1-24. [PubMed]
- Wosnitzer MS, Paduch DA. Endocrinological issues and hormonal manipulation in children and men with Klinefelter syndrome. Am J Med Genet C Semin Med Genet 2013;163C:16-26. [PubMed]
- Chandley AC. Chromosome anomalies and Y chromosome microdeletions as causal factors in male infertility. Hum Reprod 1998;13 Suppl 1:45-50. [PubMed]
- Staessen C, Tournaye H, Van Assche E, et al. PGD in 47,XXY Klinefelter’s syndrome patients. Hum Reprod Update 2003;9:319-30. [PubMed]
- Denschlag D, Tempfer C, Kunze M, et al. Assisted reproductive techniques in patients with Klinefelter syndrome: a critical review. Fertil Steril 2004;82:775-9. [PubMed]
- Vorona E, Zitzmann M, Gromoll J, et al. Clinical, endocrinological, and epigenetic features of the 46,XX male syndrome, compared with 47,XXY Klinefelter patients. J Clin Endocrinol Metab 2007;92:3458-65. [PubMed]
- Arnedo N, Nogués C, Bosch M, et al. Mitotic and meiotic behaviour of a naturally transmitted ring Y chromosome: reproductive risk evaluation. Hum Reprod 2005;20:462-8. [PubMed]
- Nenonen HA, Giwercman A, Hallengren E, et al. Non-linear association between androgen receptor CAG repeat length and risk of male subfertility--a meta-analysis. Int J Androl 2011;34:327-32. [PubMed]
- Escudero T, Abdelhadi I, Sandalinas M, et al. Predictive value of sperm fluorescence in situ hybridization analysis on the outcome of preimplantation genetic diagnosis for translocations. Fertil Steril 2003;79 Suppl 3:1528-34. [PubMed]
- Reijo R, Alagappan RK, Patrizio P, et al. Severe oligozoospermia resulting from deletions of azoospermia factor gene on Y chromosome. Lancet 1996;347:1290-3. [PubMed]
- Simoni M, Tüttelmann F, Gromoll J, et al. Clinical consequences of microdeletions of the Y chromosome: the extended Münster experience. Reprod Biomed Online 2008;16:289-303. [PubMed]
- Foresta C, Moro E, Garolla A, et al. Y chromosome microdeletions in cryptorchidism and idiopathic infertility. J Clin Endocrinol Metab 1999;84:3660-5. [PubMed]
- Hopps CV, Mielnik A, Goldstein M, et al. Detection of sperm in men with Y chromosome microdeletions of the AZFa, AZFb and AZFc regions. Hum Reprod 2003;18:1660-5. [PubMed]
- Hurles ME, Willey D, Matthews L, et al. Origins of chromosomal rearrangement hotspots in the human genome: evidence from the AZFa deletion hotspots. Genome Biol 2004;5:R55. [PubMed]
- Reijo R, Lee TY, Salo P, et al. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet 1995;10:383-93. [PubMed]
- Choi JM, Chung P, Veeck L, et al. AZF microdeletions of the Y chromosome and in vitro fertilization outcome. Fertil Steril 2004;81:337-41. [PubMed]
- Oates RD, Silber S, Brown LG, et al. Clinical characterization of 42 oligospermic or azoospermic men with microdeletion of the AZFc region of the Y chromosome, and of 18 children conceived via ICSI. Hum Reprod 2002;17:2813-24. [PubMed]
- Simoni M, Bakker E, Krausz C. EAA/EMQN best practice guidelines for molecular diagnosis of y-chromosomal microdeletions. State of the art 2004. Int J Androl 2004;27:240-9. [PubMed]
- Kleiman SE, Yogev L, Lehavi O, et al. The likelihood of finding mature sperm cells in men with AZFb or AZFb-c deletions: six new cases and a review of the literature (1994-2010). Fertil Steril 2011;95:2005-12, 2012.e1-4.
- Krausz C, Giachini C, Xue Y, et al. Phenotypic variation within European carriers of the Y-chromosomal gr/gr deletion is independent of Y-chromosomal background. J Med Genet 2009;46:21-31. [PubMed]
- Legouis R, Hardelin JP, Levilliers J, et al. The candidate gene for the X-linked Kallmann syndrome encodes a protein related to adhesion molecules. Cell 1991;67:423-35. [PubMed]
- Montenegro LR, Silveira LF, Tusset C, et al. Combined use of multiplex ligation-dependent probe amplification and automatic sequencing for identification of KAL1 defects in patients with Kallmann syndrome. Fertil Steril 2013;100:854-9. [PubMed]
- Silveira LF, Trarbach EB, Latronico AC. Genetics basis for GnRH-dependent pubertal disorders in humans. Mol Cell Endocrinol 2010;324:30-8. [PubMed]
- Tommiska J, Toppari J, Vaaralahti K, et al. PROKR2 mutations in autosomal recessive Kallmann syndrome. Fertil Steril 2013;99:815-8. [PubMed]
- Laitinen EM, Tommiska J, Sane T, et al. Reversible congenital hypogonadotropic hypogonadism in patients with CHD7, FGFR1 or GNRHR mutations. PLoS One 2012;7:e39450. [PubMed]
- Oates RD, Amos JA. The genetic basis of congenital bilateral absence of the vas deferens and cystic fibrosis. J Androl 1994;15:1-8. [PubMed]
- Claustres M, Guittard C, Bozon D, et al. Spectrum of CFTR mutations in cystic fibrosis and in congenital absence of the vas deferens in France. Hum Mutat 2000;16:143-56. [PubMed]
- McCallum T, Milunsky J, Munarriz R, et al. Unilateral renal agenesis associated with congenital bilateral absence of the vas deferens: phenotypic findings and genetic considerations. Hum Reprod 2001;16:282-8. [PubMed]
- Liou TG, Rubenstein RC. Carrier screening, incidence of cystic fibrosis, and difficult decisions. JAMA 2009;302:2595-6. [PubMed]
- Radpour R, Gourabi H, Dizaj AV, et al. Genetic investigations of CFTR mutations in congenital absence of vas deferens, uterus, and vagina as a cause of infertility. J Androl 2008;29:506-13. [PubMed]
- American College of Obstetricians and Gynecologists Committee on Genetics. ACOG Committee Opinion No. 486: Update on carrier screening for cystic fibrosis. Obstet Gynecol 2011;117:1028-31. [PubMed]
- Pratt VM, Caggana M, Bridges C, et al. Development of genomic reference materials for cystic fibrosis genetic testing. J Mol Diagn 2009;11:186-93. [PubMed]
- Bareil C, Guittard C, Altieri JP, et al. Comprehensive and rapid genotyping of mutations and haplotypes in congenital bilateral absence of the vas deferens and other cystic fibrosis transmembrane conductance regulator-related disorders. J Mol Diagn 2007;9:582-8. [PubMed]
- Schlegel PN, Cohen J, Goldstein M, et al. Cystic fibrosis gene mutations do not affect sperm function during in vitro fertilization with micromanipulation for men with bilateral congenital absence of vas deferens. Fertil Steril 1995;64:421-6. [PubMed]
- Anger JT, Wang GJ, Boorjian SA, et al. Sperm cryopreservation and in vitro fertilization/intracytoplasmic sperm injection in men with congenital bilateral absence of the vas deferens: a success story. Fertil Steril 2004;82:1452-4. [PubMed]
- Samli H, Samli MM, Yilmaz E, et al. Clinical, andrological and genetic characteristics of patients with congenital bilateral absence of vas deferens (CBAVD). Arch Androl 2006;52:471-7. [PubMed]
- Lee R, Goldstein M, Ullery BW, et al. Value of serum antisperm antibodies in diagnosing obstructive azoospermia. J Urol 2009;181:264-9. [PubMed]
- Palermo G, Joris H, Devroey P, et al. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992;340:17-8. [PubMed]
- Su LM, Palermo GD, Goldstein M, et al. Testicular sperm extraction with intracytoplasmic sperm injection for nonobstructive azoospermia: testicular histology can predict success of sperm retrieval. J Urol 1999;161:112-6. [PubMed]
- Mulhall JP, Reijo R, Alagappan R, et al. Azoospermic men with deletion of the DAZ gene cluster are capable of completing spermatogenesis: fertilization, normal embryonic development and pregnancy occur when retrieved testicular spermatozoa are used for intracytoplasmic sperm injection. Hum Reprod 1997;12:503-8. [PubMed]
- Schlegel PN, Li PS. Microdissection TESE: sperm retrieval in non-obstructive azoospermia. Hum Reprod Update 1998;4:439. [PubMed]
- Schiff JD, Palermo GD, Veeck LL, et al. Success of testicular sperm extraction [corrected] and intracytoplasmic sperm injection in men with Klinefelter syndrome. J Clin Endocrinol Metab 2005;90:6263-7. [PubMed]
- Schlegel PN. Testicular sperm extraction: microdissection improves sperm yield with minimal tissue excision. Hum Reprod 1999;14:131-5. [PubMed]
- Eisenberg ML, Betts P, Herder D, et al. Increased risk of cancer among azoospermic men. Fertil Steril 2013;100:681-5. [PubMed]
- Hotaling JM, Walsh TJ. Male infertility: a risk factor for testicular cancer. Nat Rev Urol 2009;6:550-6. [PubMed]
- Walsh TJ, Schembri M, Turek PJ, et al. Increased risk of high-grade prostate cancer among infertile men. Cancer 2010;116:2140-7. [PubMed]
- Abu-Halima M, Backes C, Leidinger P, et al. MicroRNA expression profiles in human testicular tissues of infertile men with different histopathologic patterns. Fertil Steril 2014;101:78-86.e2.
- He Z, Kokkinaki M, Pant D, et al. Small RNA molecules in the regulation of spermatogenesis. Reproduction 2009;137:901-11. [PubMed]
- Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, et al. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One 2008;3:e1738.
- Abu-Halima M, Hammadeh M, Schmitt J, et al. Altered microRNA expression profiles of human spermatozoa in patients with different spermatogenic impairments. Fertil Steril 2013;99:1249-1255.e16.
- Wang C, Yang C, Chen X, et al. Altered profile of seminal plasma microRNAs in the molecular diagnosis of male infertility. Clin Chem 2011;57:1722-31. [PubMed]
- McCallie B, Schoolcraft WB, Katz-Jaffe MG. Aberration of blastocyst microRNA expression is associated with human infertility. Fertil Steril 2010;93:2374-82. [PubMed]
- Krawetz SA, Kruger A, Lalancette C, et al. A survey of small RNAs in human sperm. Hum Reprod 2011;26:3401-12. [PubMed]
- Kobayashi H, Sato A, Otsu E, et al. Aberrant DNA methylation of imprinted loci in sperm from oligospermic patients. Hum Mol Genet 2007;16:2542-51. [PubMed]
- Ferfouri F, Boitrelle F, Ghout I, et al. A genome-wide DNA methylation study in azoospermia. Andrology 2013;1:815-21. [PubMed]
- Aston KI, Punj V, Liu L, et al. Genome-wide sperm deoxyribonucleic acid methylation is altered in some men with abnormal chromatin packaging or poor in vitro fertilization embryogenesis. Fertil Steril 2012;97:285-92. [PubMed]
- Hammoud SS, Nix DA, Hammoud AO, et al. Genome-wide analysis identifies changes in histone retention and epigenetic modifications at developmental and imprinted gene loci in the sperm of infertile men. Hum Reprod 2011;26:2558-69. [PubMed]
- O’Connell M, McClure N, Lewis SE. A comparison of mitochondrial and nuclear DNA status in testicular sperm from fertile men and those with obstructive azoospermia. Hum Reprod 2002;17:1571-7. [PubMed]
- Aslani F, Modarresi MH, Soltanghoraee H, et al. Seminal molecular markers as a non-invasive diagnostic tool for the evaluation of spermatogenesis in non-obstructive azoospermia. Syst Biol Reprod Med 2011;57:190-6. [PubMed]
- Frühmesser A, Vogt PH, Zimmer J, et al. Single nucleotide polymorphism array analysis in men with idiopathic azoospermia or oligoasthenozoospermia syndrome. Fertil Steril 2013;100:81-7. [PubMed]
- Hayashi K, Ohta H, Kurimoto K, et al. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011;146:519-32. [PubMed]
- Gkountela S, Li Z, Vincent JJ, et al. The ontogeny of cKIT+ human primordial germ cells proves to be a resource for human germ line reprogramming, imprint erasure and in vitro differentiation. Nat Cell Biol 2013;15:113-22. [PubMed]
- Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics 2013;14:690. [PubMed]
- Riester M, Taylor JM, Feifer A, et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clin Cancer Res 2012;18:1323-33. [PubMed]
- Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol 2010;11:55-65. [PubMed]


