Urinary biomarkers in pelvic-ureteric junction obstruction: a systematic review
Introduction
One of the most common causes of renal failure in infants and children is obstructive nephropathy due to congenital hydronephrosis secondary to pelvic-ureteric junction obstruction (PUJO) (1,2). Despite extensive clinical and experimental studies have been undergone over the past decades, fundamental issues regarding the evaluation and management of children with upper urinary tract obstruction remain unsolved.
It is known that the majority of the children with antenatally diagnosed hydronephrosis will have a spontaneous resolution of the dilatation. However, there is a significant amount of cases that will worsen with the risk of a progressive and permanent loss of renal function. The main goal of paediatric urologists is the preservation of kidney function through early selection of patients who will require surgical intervention as opposed to those who have chances to improve spontaneously.
The classic diagnostic tools for investigation of children with upper tract renal obstruction include ultrasound scans, nuclear medicine assessments [(dimercaptosuccinic acid) scan and technetium-99m (Tc-99m DMSA) mercaptoacetyltriglycine (MAG3) renal studies], contrast studies such as micturating cystourethrogram (MCUG) and conventional markers of renal function (such as serum creatinine).
However, these tests are not always adequate predictors for disease progression, particularly for border-line cases. Commonly indication for surgery is currently based on: (I) half-time of the elimination phase (T1/2) of diuretic renogram >15–20 minutes, (II) differential renal function (DRF) less than 40%, (III) deteriorating renal function (more than 5% in successive radionuclide scans), (IV) progressive thinning of the renal cortex with or without compensatory hypertrophy of the other kidney (V) frequent pyelonephritis (VI) significant grade hydronephrosis, usually greater than 20 mm (VII) other symptoms such as hypertension, kidney stones, flank pain (3).
However, there is an increasing concern among experts that the aforementioned criteria can’t timely identify patients who need surgery from those who can be managed conservatively. The use of urinary biomarkers has been advocated, which could discriminate patients whose renal function will deteriorate from those who will spontaneously improve at an early stage, preventing at the same time unnecessary surgeries. Recently, proteomic has become a very important diagnostic and prognostic tool in the armamentarium of clinicians. Evaluating proteins using proteomic technologies has the ability to increase the understanding and elucidate the cellular and the molecular basis of a particular disorder. In addition, the proteins analysed could be used to identify and quantify a disease at an early stage of occurrence. The etiopathogenesis of renal injury and progression of renal disease in obstructive nephropathy has been well described. It consists of a sequence of cellular and molecular events which includes renal hemodynamic responses, macrophage infiltration of the interstitium, tubular dilatation and apoptosis, accumulation of interstitial fibroblasts through proliferation of resident fibroblasts and epithelial-mesenchymal transformation of renal tubular cells. This process is under the influence of multiple enzymes, cytokines, chemokines, growth factors, signaling molecules and genes (4). Using proteomic analysis, in the last years several attempts have been made to evaluate molecules that could be utilized as potential biomarkers in PUJO in children, most of them concluding to promising results. We have performed an up-to-date systematic review of the literature on biomarkers of renal injury and dysfunction, potential targets for diagnosis, prognosis and treatment of children with PUJO.
Methods
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Metanalysis Statement (http://www.prisma-statement.org/). In order to identify eligible studies, a broad search was conducted in the electronic database MEDLINE from inception through March 2019 using various combinations of keywords such as pelvic-ureteric [All Fields] AND junction [All Fields] AND obstruction [All Fields] AND “biomarkers” [MeSH Terms] OR “biomarkers” [All Fields] OR “biomarker” [All Fields]. To broaden the research, additional records were identified through hand-searching review of the references reported in each article previously selected.
Selection was limited to original articles in English language presenting promising protein biomarkers of PUJO in children. Two independent reviewers (Irene Paraboschi and Massimo Garriboli) performed data extraction and quality assessment. Any disagreement was resolved by consensus or by arbitration of a third author (Guglielmo Mantica) not involved in the initial procedure.
An electronic schedule (Microsoft Excel 2007, Redmond, WA, USA) was pre-established listing authors, article title, journal, year of publication, study period, study design, sample size, average age, hydronephrosis definition used, surgical indication, duration and pattern of follow-up, details on biomarker studied, diagnostic test characteristics, area under the curve (AUC) on receiver operating characteristic (ROC) analysis with best cut-off (BCO) values, sensitivity, specificity and outcomes.
The eligibility for the systematic review were studies of any design that reported interleukin 6 (IL6), neutrophil gelatinase-associated lipocalin (NGAL), monocyte chemotactic peptide-1 (MCP1), transforming growth factor β1 (TGFβ1), carbohydrate antigen 19.9 (CA19.9), kidney injury molecule 1 (KIM1), glutathione S-transferases (GSTs), antimicrobial peptides (AMPs), such as B defense 1 (BD1), cathelicidin (LL37), hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein (HIP/PAP), and human a defensin 5 (HD5), caspase 3 enzyme, tumor necrosis factor α (TNFα), thioredoxin (Trx), proximal tubule metallopeptidases (such as CD10, CD13, CD26), intercellular adhesion molecule 1 (ICAM1), lysosomal exoglycosidases (such as HEX A, HEX B, FUC, GAL, MAN, GLU), epidermal growth factor (EGF), emmprin, matrix metalloproteinase 9 (MMP9), tissue inhibitor of metalloproteinase 1 (TIMP1), procollagen III aminoterminal propeptide (PIIINP), regulated on activation, normal T cell expressed and secreted (RANTES), sFas/Apo-1 (CD95), angiotensinogen (AGT), macrophage inflammatory protein 1a (MIP1a), interferon-γ-inducible protein-10 (IP10), osteopontin (OPN), cystatin C (CyC), β2microglobulin (β2M), heme oxygenase 1 (HO1), N-acetyl-β-D-glucosaminidase (NAG), alkaline phosphatase (ALP), γ-glutamyl transferase (GGT), endothelin 1 (ET1), semaphorin-3A (SEMA3A), netrin-1, liver-type fatty acid-binding proteins (L-FABP) in urine samples of children (until 18 years old) with unilateral or bilateral congenital PUJO compared to healthy controls or to children with dilated but not obstructed kidneys.
Histopathological studies were excluded from the systematic reviews as well as large scale urinary proteome analyses carried out through liquid chromatography coupled to tandem mass spectrometry (HPLC-MS/MS) or by mean high throughput techniques as matrice assisted laser desorption ionization or surface enhanced laser desorption ionization mass spectrometry (MALDI/SELDI-MS). Studies with no control group, studies with participants suffering from concomitant urological diseases and studies published in language other than English were also excluded.
Due to the heterogeneity of studies and especially the very small number of studies referring to the same biomarker a meta-analysis of the available data was deemed unfeasible.
Results
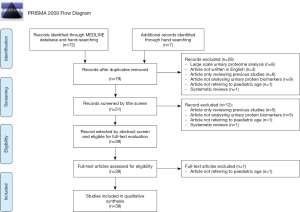
Out of 79 studies that were initially identified from the main search and through hand-searching review 40 were excluded: 28 were excluded on a title basis, 12 were excluded on abstract basis. Thirty-nine studies were evaluated on a full-text basis. Out of them, 1 had to be excluded due to inappropriate study population age, leaving in the end 38 studies that have been included in this review.
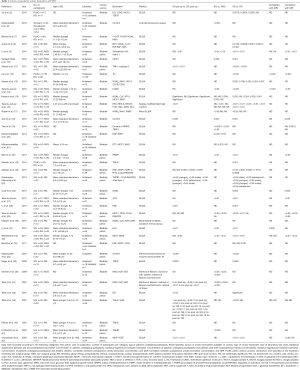
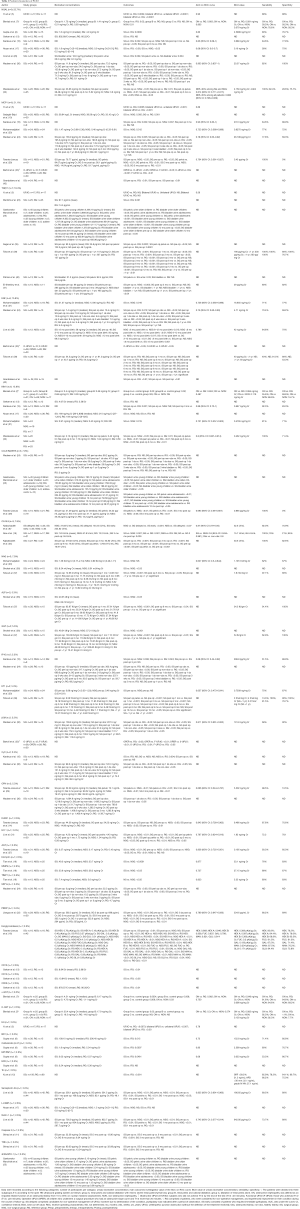
As shown in Prisma flow diagram (Figure 1), 8 articles were excluded because based on large scale urinary proteome analysis, 3 articles because they were written in language other than in English, 11 articles because they only reviewed previous studies, 14 articles because they did not analyse urinary protein biomarkers, 3 articles because they did not refer to paediatric age and 2 articles because they were systematic reviews. Table 1 summarized data of the 38 studies published between November 1997 and October 2018. Out of the 38 studies included in this systematic review, all (100%) searched for urine biomarkers and 5 (13.2%) (7,12,18,22,34), included also serum samples. Thirty-two (84.2%) studies considered only patients with unilateral PUJO while 6 (15.8%) included also patients with bilateral obstruction (5,6,20,21,33,35). The quantitative urine protein analysis was performed using commercially available human sandwich enzyme-linked immunosorbent assay (ELISA) kits in 32 (84.2%) studies. Fourteen (36.8%) studies compared patients undergoing pyeloplasty [surgical group (SG)] with patients with mild non-obstructive dilatation not requiring surgery [non-surgical group (NSG)] and healthy children [reference group (RG)] (9,10,14-18,20,22,27,29,31-33). Seven (18.4%) articles analysed urine biomarker concentrations in patients undergoing surgery (SG) and related them with patients conservatively treated (NSG) (6,19,21,28,36,37,41). Thirteen (34.2%) studies reported the comparison between surgical patients (SG) and healthy subjects (RG) (8,11,13,24-26,30,34,35,38-40,42). Four (10.5%) articles compared patients with PUJO with healthy children (RG) without differentiating in the former group those who required surgery (5,7,12,23). Twenty-two (57.9%) studies evaluated the effect of pyeloplasty on voided urine biomarker concentrations, comparing their values before and after surgery (9,11,14-16,18,22,24,25,27-32,34,35,37-39,41,42). Twelve (30.8%) studies investigated the correlation between preoperative biomarker concentration with the anterior posterior renal pelvis diameter (DAP) (9,12,14,16,18,22,23,25,27,30-32) while 20 (52.6%) studies investigated the correlation between preoperative biomarker concentration with the split renal function (SRF) evaluated on nuclear medicine assessments (8,9,12-14,16,18,19,22,25-32,39,41,42). ROC curves were used to investigate the performance of urinary biomarkers in the total patient data set in 21 (55.3%) studies (5-9,14-22,24,27-32). Table 2 summarized the results of the 38 articles classified them according to the type of urinary biomarker analysed.
Full table
Full table
NGAL
Preoperative urinary NGAL levels in surgical PUJO patients significantly differed from healthy controls in nine studies (5,7,8,13,15,17,26,30,32), and from dilated non-obstructed patients in two studies (17,32).
TGFβ1
Preoperative TGFβ1 levels in surgical PUJO patients were significantly higher than in healthy control group in 3 studies (23,35,39) while Palmer et al. (40) did not identify any significant difference. Preoperative TGFβ1 levels in surgical PUJO patients were significantly higher than in patients with mild non-obstructive hydronephrosis in the study of El-Sherbiny et al. (41).
A minority of studies performed a ROC analysis to define the diagnostic profile of TGFβ1. In the series reported by El-Sherbiny et al. (41) mean bladder TGFβ1 value 3 months after surgery showed a trend towards a decrease, albeit still insignificant. Conversely postoperative mean TGFβ1 concentration was significantly lower than the preoperative value according to Sager et al. (35) and Taha et al. (39).
MCP1
Several studies found that MCP1 levels were significantly increased in preoperative samples compared to healthy controls (10,15,24,29,42). Some authors (10,15,20,29) exhibited that MCP1 concentrations were greater in the surgical PUJO group compared to patients with mild non-obstructive hydronephrosis. Additionally, in the SG, the difference between the pre- and post-operative concentrations became significant lower between 2 and 4 months after surgery according to Grandaliano et al. (42).
EGF
Preoperative concentrations of urinary EGF were significantly increased compared to healthy controls according to Madsen et al. (24) and compared to children with dilated non-obstructed kidneys according to Mohammadjafari et al. (21). Conversely, data reported from Grandaliano et al. (42) showed significantly lower urinary EGF concentrations in the SG compared to healthy children and they were also significantly lower compared to the observational group according to Li et al. (28).
However, in the series reported by Taha et al. (39) no difference was identified between surgical patients and healthy children. Madsen et al. (24), Taha et al. (39) and Grandaliano et al. (42) considered the evolution of EGF concentrations after pyeloplasty: postoperative values decreased significantly between 2 and 4 months after surgery according to Grandaliano et al. (42), while no difference was reported by Madsen et al. (24) and by Taha et al. (39) 1 year after surgery.
KIM1
Preoperative values of KIM1 were reported significantly augmented compared to healthy controls by Karakus et al. (15) by Wasilewska et al. (32) and compared to conservative cases by Mohammadjafari et al. (21) and by Wasilewska et al. (32). Preoperative values of KIM1 were reported not significantly different compared to controls by Noyan et al. (17) Gerber et al. (13) and compared to conservative cases by Karakus et al. (15) and Noyan et al. (17).
CCL5/RANTES
Three studies analysed CCL5/RANTES as a promising biomarker of PUJO. According to Madsen et al. (24) and to Taranta-Janusz et al. (29) there was a significant difference between the CCL/RANTES concentration obtained from the pelvic urine of surgical PUJO patients and those obtained from the bladder of healthy children. This difference became insignificant postoperatively according to Madsen et al. (24) but not to Taranta-Janusz et al. (29).
CA19.9
Three studies (6,18,34) reported that preoperative CA19.9 values were significantly higher in the SG compared to the healthy RG. Atar et al. (18) also demonstrated that surgical PUJO patients have significantly higher preoperative values compared to dilated non-obstructive patients. Three months after surgery, the voided urine CA19.9 levels decreased significantly according to Atar et al. (18) and Kajbafzadeh et al. (34).
NAG
Two studies (36,37) stated that preoperative levels of NAG were significantly increased compared to the conservative cases. In addition, Taha et al. (37) demonstrated that younger children had significantly higher activities of voided urinary NAG compared to older ones at diagnosis.
Other markers
The other biomarkers were evaluated in two studies or less with different results and degrees of predicting success (See Table 2).
Discussion
Obstructive nephropathy represents the major cause of renal insufficiency in children and, among them, PUJO constitutes the most frequent condition (1). Currently, according to the European Association of Urology Guidelines, symptomatic obstruction, impaired DRF (less than 40%), decrease of more than 10% of renal function in subsequent investigations, poor drainage function after the administration of diuretic, increasing anteroposterior pelvic diameter (APD) on US, and grade III and IV hydronephrosis represent indications for surgery (43). However, in many cases, patients present border-line results that make difficult to predict whether the affected kidney is a risk of damage and, in some, the indication for surgery comes only after several radiological investigations, often when the evidence that a renal damage has already established.
A biomarker has been defined as a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (44). Animal model systems have provided new insights into the cellular response of the developing kidney to urinary tract obstruction, helping in identify molecules involved in the pathogenic response to kidney damage, mediators of interstitial inflammation, tubular apoptosis and fibrosis. They became targets of proteomic studies aiming to identify early predictors of renal sufferance that could be used for clinical decision-making, assisting in earlier and more reliable determination of patients at risk to develop renal damage, guiding more timely therapeutic decisions and monitoring the treatment efficacy.
In addition to their diagnostic relevance, they could also prove to be extremely useful in the designing molecular therapies to prevent or reverse the renal structural and functional consequences of obstructive nephropathy. We aimed to perform a systematic review of the published literature of urinary biomarkers used as a tool for diagnosis and prognosis of children with PUJO.
Analysis of the most used markers
NGAL
NGAL is a non-organ specific protein of the lipocalin family which is secreted by different tissues and is reabsorbed via epithelia endocytosis in the proximal renal tubules. Its expression is also induced in the thick ascending limb of Henle’s loop and in the collecting ducts in response to renal tubular injury. Thus, the combination of increased gene expression in the distal nephron and altered reabsorption in the proximal tubule make it one of the first indicators of renal damage in case of obstruction. Case-control prospective studies in children with severe hydronephrosis secondary to PUJO suggested that urine NGAL/creatinine ratio from bladder was significantly higher in affected patients compared to normal subjects (5,7,8,15,17,26,32).
Although, Madsen et al. (30) didn’t find any significant differences between bladder urine NGAL levels in PUJO patients and in controls, renal pelvis NGAL levels in obstructed kidneys were significantly higher than those in the bladder and the levels measured in the voided urine of healthy children. These data were confirmed by Cost et al. (26). NGAL levels tend to decrease at 3 to 6 months after surgery (15,30,32).
Finally, ROC curve was used to determine the BCO point of urinary NGAL levels in predicting PUJO patients and AUC and the corresponding 95% CI were calculated. The best performance was reported by Madsen et al. (30) who found at a cut off value of 20.57 ng/mg Cr, an AUC of 0.923 [95% confidence interval (CI), 0.837–1.000] which yielded 82% sensitivity and 100% specificity.
TGFβ1
TGFβ1 is a proinflammatory, proapoptotic, profibrotic cytokine and fibroblast chemoattractant which plays a major role in renal fibrosis, also via epithelial-mesenchymal transition. Up-regulation of TGFβ1 synthesis in the kidney is associated with accumulation of collagen and scarring, leading to the development of advanced chronic renal disease.
Experimental models documented that kidney responds to obstruction inducing a cascade of molecular events and histological changes starting from the up-regulation of the renin-angiotensin system, which increase the expression of tissue TGFβ1. The deduction is that its level could be used as a non-invasive tool to evaluate the progression of renal disease and to monitor the efficacy of surgery. TGFβ1 levels are remarkably elevated in surgical PUJO patients in confront to healthy children (23,35,39). Moreover, postoperative mean TGFβ1 concentration was significantly lower than preoperative TGFβ1, according to Sager et al. (35) and Taha et al. (39).
MCP1
MCP1 is a chemokine that promotes monocyte chemotaxis. In case of obstruction, its expression at the tubular level seems to be strictly associated with the recruitment of these inflammatory cells within the interstitial space. Thus, MCP1 urine excretion could be related to the extent of monocyte infiltration and the consequent progression of interstitial renal fibrosis.
All studies agreed in considering MCP1 levels highly related to the extent of tubular atrophy and interstitial fibrosis and demonstrated that its urinary concentration could successfully discriminate not only between children who require an intervention and healthy controls but also within the hydronephrosis group. In fact, various authors found that MCP1 urinary levels are significantly increased in surgical patients compared to healthy controls (5,10,15,24,29,42) and compared to children managed conservatively (10,15,20,29).
MCP1 could be used for long term follow-up in the postoperative period since its levels decreased and became similar to the healthy group between 2 and 4 months after surgery (15,24,42). Analysing the ROC curves, MCP1 maintained also a good diagnostic profile and therefore may be used to distinguish surgical PUJO patients.
EGF
EGF is a polypeptide growth factor which plays a fundamental role in normal tubulogenesis. It is synthesized by the ascending portion of Henle’s loop and by the distal convoluted tubule and displays fundamental effects on intact glomeruli, proximal tubules and collecting ducts. It was demonstrated that it has a central role in modulating tubular cell growth and tissue response in kidneys with tubule interstitial injury. However, the evaluation of urinary EGF concentration in PUJO children is still not fully understood and the studies that have investigated its urinary expression described contradictory results. This could be due to the fact that different methods were used in the studies: two studies considered also patients with bilateral disease, two reported also the concentration of the biomarker collected from the pelvic urine and two studies compared the urinary level of children with surgical PUJO with children with dilated but non-obstructed kidneys. Particularly, EGF urinary levels in patients undergoing surgery were significantly higher compared to those treated conservatively in the study of Mohammadjafari et al. (21) but they were significantly lower in the study of Li et al. (28). Although Grandaliano et al. (42) claimed that the preoperative concentrations of urinary EGF were significantly reduced in PUJO patients in comparison to their healthy controls, Madsen et al. (24) found that they were more elevated. To further confuse the situation, Taha et al. (39) reported that there was no significant difference of EGF values between surgical patients and controls and moreover there was no significant difference between preoperative and postoperative values even 1 year after surgery. In conclusion, the role of EGF as a urinary marker of PUJO seems, at least, debatable.
KIM1
KIM1 is a member of the type I transmembrane glycoprotein. Since it is undetectable in healthy children but is strongly expressed and released by damaged proximal tubular epithelial cells until complete recovery, it could be utilised as an early and sensitive biomarker for kidney injury in the setting of obstructive nephropathy.
In this scenario, several clinical studies have shown that urinary KIM1 is higher in patients with PUJO compared to control groups (15) and to children with mild hydronephrosis (21,32). However, other studies failed in identifying differences between PUJO patients and healthy controls (13,17) or hydronephrotic patients (15,17). KIM1 levels have a tendency to decrease and normalize by 3 months after surgery (15,32). ROC analyses were performed to define the diagnostic profile of KIM1 in identifying children with an obstructed kidney condition with quite good results. The best AUC (0.89) was reported by Karakus et al. (15) with a cut-off value of 0.687 ng/mg Cr, which gave a sensibility of 92.3% and a specificity of 83.3%. It is, furthermore, possible to conclude that KIM1 urinary levels are closely related to the severity of renal damage, since a negative correlation was found with DRF in radionuclide scans (32). However, Gerber et al. (13) didn’t identify any significant linear correlations. This could have been consequence of the small sample size of their study or the presence of outliers in their small datasets, making any potential correlations difficult to demonstrate. No significant correlation was found with initial anterior posterior (AP) diameter (length) of the pelvis.
Critical review
Recently much has been learned about the pathophysiology of obstructive nephropathy and many novel biomarkers have been investigated for diagnostic and prognostic purposes. A number of endogenous molecules have been identified with different degrees of success, often with contradictory results. This wide range of outcomes could be due to a variety of reasons. First of all, the populations considered in the different articles were quite heterogeneous. Secondly, the heterogeneity of the populations considered and the different criteria adopted to classify patients made virtually impossible to compare the results. In addition to this, also the study designs were extremely wide-ranging. Another important bias was that these studies were held utilizing a wide range of patient ages. Elder children could therefore have suffered from ureterovascular hydronephrosis due to aberrant renal vessels. In these cases, decrements in renal function may occur discontinuously, inevitably interfering only episodically with urinary biomarker concentrations, which decrease during the diuretic phase of the renal impairment. In addition, it is also possible that the progression of the histopathological tubulointerstitial changes is extremely different between the age groups. Finally, the duration of follow-up was quite short especially in the more recent studies. All these shortcomings could be overcome by large prospective clinical trials with appropriate and strictly design protocols.
Urinary biomarkers have been extensively used as a promising tool for non-invasive assessment of PUJO in children. They could be helpful not only in the diagnosis of congenital obstructive uropathies but also in the differentiation between dilated but non-obstructed kidneys that could be managed conservatively.
Some studies also demonstrated that the urinary biomarkers could be useful in the evaluation of the surgical treatment success. Nevertheless, the existing literature is still lacking of solid and definitive studies. Large multicentre and carefully designed prospective studies are needed to evaluate the clinical usefulness of urinary biomarkers in the diagnosis and follow-up of children with obstructive nephropathy.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2020.01.01). NRD and CT report other from SpOtOn Clinical Diagnostics Ltd, NRD and CT have a patent Stable isotope albumin peptide internal standard issued. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Harambat J, van Stralen KJ, Kim JJ, et al. Epidemiology of chronic kidney disease in children. Pediatr Nephrol 2012;27:363-73. [Crossref] [PubMed]
- Nguyen HT, Herndon CD, Cooper C, et al. The Society for Fetal Urology consensus statement on the evaluation and management of antenatal hydronephrosis. J Pediatr Urol 2010;6:212-31. [Crossref] [PubMed]
- Heinlen JE, Manatt CS, Bright BC, et al. Operative versus nonoperative management of ureteropelvic junction obstruction in children. Urology 2009;73:521-5; discussion 525. [Crossref] [PubMed]
- Chevalier RL. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol 2006;2:157-68. Review. [Crossref] [PubMed]
- Yu L, Zhou L, Li Q, et al. Elevated urinary lipocalin-2, interleukin-6 and monocyte chemoattractant protein-1 levels in children with congenital ureteropelvic junction obstruction. J Pediatr Urol 2019;15:44.e1-7. [Crossref] [PubMed]
- Nabavizadeh B, Khorramirouz R, Amini E, et al. Value of urinary carbohydrate antigen 19-9 to predict failure of conservative management in children with ureteropelvic junction obstruction. J Pediatr Surg 2019;54:1650-3. [Crossref] [PubMed]
- Bieniaś B, Sikora P. Potential novel biomarkers of obstructive nephropathy in children with hydronephrosis. Dis Markers 2018;2018:1015726.
- Gupta S, Jackson AR, DaJusta DG, et al. Urinary antimicrobial peptides: potential novel biomarkers of obstructive uropathy. J Pediatr Urol 2018;14:238.e1-6. [Crossref] [PubMed]
- Li X, Liu X, Li J, et al. Semaphorin-3A and Netrin-1 predict the development of kidney injury in children with congenital hydronephrosis. Scand J Clin Lab Invest 2018;78:55-61. [Crossref] [PubMed]
- Sadeghi-Bojd S, Alijani E, Mahdavian MS. Mean urinary cytokine MCP-1 in children with urinary tract obstruction and healthy children. Nephro-Urol Mon 2018;10:e63607. [Crossref]
- Shirazi M, Eslahi A, Sharifi V, et al. Evaluation of caspase 3 enzyme and TNF-alpha as biomarkers in ureteropelvic junction obstruction in children- a preliminary report. Pak J Med Sci 2017;33:315-9. [PubMed]
- Xu ZM, Li MJ, Tao C. Serum and urinary thioredoxin concentrations are associated with severity of children hydronephrosis. Clin Chim Acta 2017;466:127-32. [Crossref] [PubMed]
- Gerber C, Harel M, Lynch ML, et al. Proximal tubule proteins are significantly elevated in bladder urine of patients with ureteropelvic junction obstruction and may represent novel biomarkers: a pilot study. J Pediatr Urol 2016;12:120.e1-7. [Crossref] [PubMed]
- Taranta-Janusz K, Wasilewska A, Roszkowska R, et al. Is urine intercellular adhesion molecule-1 a marker of renal disorder in children with ureteropelvic junction obstruction? Biomarkers 2016;21:123-8. [Crossref] [PubMed]
- Karakus S, Oktar T, Kucukgergin C, et al. Urinary IP-10, MCP-1, NGAL, cystatin-C, and KIM-1 levels in prenatally diagnosed unilateral hydronephrosis: the search for an ideal biomarker. Urology 2016;87:185-92. [Crossref] [PubMed]
- Taranta-Janusz K, Zalewska-Szajda B, Chojnowska S, et al. Urine exoglycosidases are potential markers of renal tubular injury in children with ureteropelvic junction obstruction. Acta Paediatr 2015;104:e518-23. [Crossref] [PubMed]
- Noyan A, Parmaksiz G, Dursun H, et al. Urinary NGAL, KIM-1 and L-FABP concentrations in antenatal hydronephrosis. J Pediatr Urol 2015;11:249.e1-6. [Crossref] [PubMed]
- Atar A, Oktar T, Kucukgergin C, et al. The roles of serum and urinary carbohydrate antigen 19-9 in the management of patients with antenatal hydronephrosis. J Pediatr Urol 2015;11:133.e1-5. [Crossref] [PubMed]
- Tian F, Gu C, Zhao Z, et al. Urinary Emmprin, matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 as potential biomarkers in children with ureteropelvic junction narrowing on conservative treatment. Nephrology (Carlton) 2015;20:194-200. [Crossref] [PubMed]
- Mohammadjafari H, Rafiei A, Mousavi SA, et al. Role of urinary levels of endothelin-1, monocyte chemotactic peptide-1, and N-acetyl glucosaminidase in predicting the severity of obstruction in hydronephrotic neonates. Korean J Urol 2014;55:670-6. [Crossref] [PubMed]
- Mohammadjafari H, Rafiei A, Kosaryan M, et al. Determination of the severity of ureteropelvic junction obstruction using urinary epidermal growth factor and kidney injury molecule 1 levels. Biomark Med 2014;8:1199-206. [Crossref] [PubMed]
- Jianguo W, Zhenzhen L, Xianghua L, et al. Serum and urinary procollagen III aminoterminal propeptide as a biomarker of obstructive nephropathy in children. Clin Chim Acta 2014;434:29-33. [Crossref] [PubMed]
- Merrikhi A, Bahraminia E. Association of urinary transforming growth factor-β1 with the ureteropelvic junction obstruction. Adv Biomed Res 2014;3:123. [Crossref] [PubMed]
- Madsen MG, Nørregaard R, Palmfeldt J, et al. Epidermal growth factor and monocyte chemotactic peptide-1: potential biomarkers of urinary tract obstruction in children with hydronephrosis. J Pediatr Urol 2013;9:838-45. [Crossref] [PubMed]
- Gawłowska-Marciniak A, Niedzielski JK. Evaluation of TGF-β1, CCL5/RANTES and sFas/Apo-1 urine concentration in children with ureteropelvic junction obstruction. Arch Med Sci 2013;9:888-94. [Crossref] [PubMed]
- Cost NG, Noh PH, Devarajan P, et al. Urinary NGAL levels correlate with differential renal function in patients with ureteropelvic junction obstruction undergoing pyeloplasty. J Urol 2013;190:1462-7. [Crossref] [PubMed]
- Taranta-Janusz K, Wasilewska A, Dębek W, et al. Urinary angiotensinogen as a novel marker of obstructive nephropathy in children. Acta Paediatr 2013;102:e429-33. [Crossref] [PubMed]
- Li Z, Zhao Z, Liu X, et al. Prediction of the outcome of antenatal hydronephrosis: significance of urinary EGF. Pediatr Nephrol 2012;27:2251-9. [Crossref] [PubMed]
- Taranta-Janusz K, Wasilewska A, Dębek W, et al. Urinary cytokine profiles in unilateral congenital hydronephrosis. Pediatr Nephrol 2012;27:2107-13. [Crossref] [PubMed]
- Madsen MG, Nørregaard R, Palmfeldt J, et al. Urinary NGAL, cystatin C, β2-microglobulin, and osteopontin significance in hydronephrotic children. Pediatr Nephrol 2012;27:2099-106. [Crossref] [PubMed]
- Li Z, Liu X, Liu S, et al. Urinary heme oxygenase-1 in children with congenital hydronephrosis due to ureteropelvic junction obstruction. Biomarkers 2012;17:471-6. [Crossref] [PubMed]
- Wasilewska A, Taranta-Janusz K, Dębek W, et al. KIM-1 and NGAL: new markers of obstructive nephropathy. Pediatr Nephrol 2011;26:579-86. [Crossref] [PubMed]
- Bartoli F, Penza R, Aceto G, et al. Urinary epidermal growth factor, monocyte chemotactic protein-1, and β2-microglobulin in children with ureteropelvic junction obstruction. J Pediatr Surg 2011;46:530-6. [Crossref] [PubMed]
- Kajbafzadeh AM, Elmi A, Talab SS, et al. Urinary and serum carbohydrate antigen 19-9 as a biomarker in ureteropelvic junction obstruction in children. J Urol 2010;183:2353-60. [Crossref] [PubMed]
- Sager C, Lopez JC, Duran V, et al. Transforming growth factor-beta1 in congenital ureteropelvic junction obstruction: diagnosis and follow-up. Int Braz J Urol 2009;35:315-23; discussion 323-5. [Crossref] [PubMed]
- Shokeir AA, Taha MA. Role of urinary tubular enzymes in evaluation of children with ureteropelvic junction narrowing under conservative management. Urology 2009;73:1016-20. [Crossref] [PubMed]
- Taha MA, Shokeir AA, Osman HG, et al. Obstructed versus dilated nonobstructed kidneys in children with congenital ureteropelvic junction narrowing: role of urinary tubular enzymes. J Urol 2007;178:640-6. [Crossref] [PubMed]
- Taha MA, Shokeir AA, Osman HG, et al. Diagnosis of ureteropelvic junction obstruction in children: role of endothelin-1 in voided urine. Urology 2007;69:560-4; discussion 564-5. [Crossref] [PubMed]
- Taha MA, Shokeir AA, Osman HG, et al. Pelvi-ureteric junction obstruction in children: the role of urinary transforming growth factor-beta and epidermal growth factor. BJU Int 2007;99:899-903. [Crossref] [PubMed]
- Palmer LS, Maizels M, Kaplan WE, et al. Urine levels of transforming growth factor-beta 1 in children with ureteropelvic junction obstruction. Urology 1997;50:769-73. [Crossref] [PubMed]
- El-Sherbiny MT, Mousa OM, Shokeir AA, et al. Role of urinary transforming growth factor-beta1 concentration in the diagnosis of upper urinary tract obstruction in children. J Urol 2002;168:1798-800. [Crossref] [PubMed]
- Grandaliano G, Gesualdo L, Bartoli F, et al. MCP-1 and EGF renal expression and urine excretion in human congenital obstructive nephropathy. Kidney Int 2000;58:182-92. [Crossref] [PubMed]
- Tekgül S, Dogan HS, Hoebeke P, et al. EAU guidelines on paediatric urology. European Association of Urology 2016:290-323.
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 2001;69:89-95. [Crossref] [PubMed]