
Review of the evidence for robotic-assisted robotic cystectomy and intra-corporeal urinary diversion in bladder cancer
Introduction
In 2018 the GLOBOCAN cancer database estimates there were almost 550,000 new bladder cancer cases recorded worldwide with 199,000-bladder cancer related deaths (1), making bladder cancer the 11th most common cancer worldwide. Approximately 25% of new bladder cancer cases present with muscle-invasive disease (2). Radical cystectomy (RC), pelvic lymph node dissection (PLND) and urinary diversion (UD) is the “Gold standard” surgical treatment for muscle-invasive bladder cancer (MIBC) and certain high risk or treatment refractory non-muscle invasive bladder cancers. Whilst open radical cystectomy (ORC) dates back to the 1800’s, creation of an ileal conduit is a more recent development first popularised by Bricker in the 1950’s (3). Today there are a variety of surgical techniques including ORC, laparoscopic radical cystectomy (LRC), robotic-assisted radical cystectomy (RARC), intra-corporeal urinary diversion (ICUD), extra-corporeal urinary diversion (ECUD) and bladder substitution with orthotopic neobladder.
ORC is a complex surgical procedure associated with high morbidity; complication rates range from 30–70% (4). LRC was introduced to mitigate against the morbidity associated with open surgery, however due to the prolonged learning curve and technically challenging procedure it failed to reach wide-spread adoption. RARC with its superior optics, increased dexterity and appealing ergonomics has largely superseded LRC as the principal minimally invasive surgical option. The adoption of RARC over the last 15–20 years has been impressive with a 25-fold increase in the US from 0.7% in 2002 to 18.5% in 2012 (5). Comparative studies and meta-analysis have shown equivalent oncological outcomes (6) with improvements in perioperative markers of surgical quality such as transfusion rates and length of stay (7). To date the majority of randomised control studies (RCTs) assessing RARC have only included RARC with ECUD. This hybrid approach may diminish some of benefits of the purely minimally invasive surgery seen in ICUD.
This review focuses on the available evidence for RARC providing comparison on oncological, perioperative, functional, surgeon-specific and cost outcomes. A section is dedicated to the evidence for ICUD and its role within robotic cystectomy.
Oncological outcomes
The recently reported RAZOR trial (8) is the only RCT with a primary outcome comparing oncological outcomes of ORC vs. RARC. This multi-institute phase-3 non-inferiority trial found no inferiority of RARC compared to ORC in 2-year progression-free survival rates (RARC: 71.6% and ORC: 72.3%, Pnon-inferiority =0.001). Furthermore, similar 2-year survival outcomes were reported in several retrospective analyses (9,10). Admittedly, the 2-year follow-up period appears short in the RAZOR trial, however several studies reveal that 2-year follow-up is adequate to identify up to 81% of recurrences (11,12). Bochner et al.’s RCT (13) provides longer-term oncological outcomes with 5-year follow-up and found no statistical difference in recurrence-free, cancer-specific and overall survival when comparing ORC with RARC, albeit this was a small (n=118) single-institute RCT. Three other RCTs have used lymph node yields and positive surgical margin status as markers of oncological outcomes and found no statistical difference between ORC and RARC (14-16). Table 1 provides a description and comparison of the five RCTs comparing RARC with ORC.
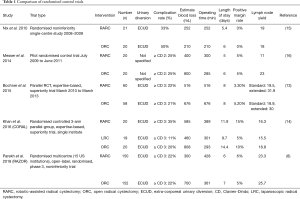
Full table
The International Robotic Cystectomy Consortium (IRCC) multi-institute prospective database on RARC reports the longest follow-up period for oncological outcomes of RARC. Their recently published 2019 study provides 10-year follow-up and notes 10-year recurrence-free, disease specific and overall survival rates of 59%, 65% and 35% respectively, comparable to similar open series (17).
The significance of patterns of recurrence when comparing ORC and RARC is somewhat controversial. Initially there were concerns that RARC leads to tumour spillage, inadequate extirpation and dissemination of tumour cells. Nguyen et al.’s (18) retrospective analysis found no difference in the rates of local and distant recurrence rates between ORC and RARC but noted a difference in the pattern of distant recurrences. A higher rate of peritoneal carcinomatosis was seen in the RARC group (RARC 21% vs. ORC 8%) and a higher rate of recurrence in the extraperitoneal lymph nodes (ORC 15% vs. RARC 23%). It is implied that higher rates of recurrence within the extra-pelvic lymph nodes may be due to less comprehensive lymph node dissection during the robotic procedure, despite the lymph node yields being the similar between the surgical modalities (median ORC node yield: 20, median node RARC yield: 21). It is postulated the higher peritoneal carcinomatosis seen in the robotic procedure may be due to tumour dispersion from the pneumoperitoneum. It is important to note that no statistical analysis was applied to these patterns of recurrence in Nguyen’s study leading to recent criticism (19). Secondary analysis of Bochner et al.’s 2015 RCT (13) found a similar propensity to local/regional first recurrence in the RARC group. In contrast the RAZOR trial (8) did not find any difference in rates of peritoneal carcinomatosis when comparing RARC with ORC. Furthermore, Tan et al.’s (20) retrospective analysis of ORC versus RARC with ICUD found no statistical difference in the rate or pattern of recurrence between the modalities. Corroborated by Collins et al.’s (21) multi-institute series, noting no difference in recurrence patterns and leading the author to assert that tumour biology had a greater impact on recurrence rates and patterns than surgical modality.
Major/minor complications
RC is a complex procedure performed in elderly, often highly co-morbid, patients resulting in significant morbidity. The Clavien-Dindo (CD) system (22) is used for reporting complications. CD 3–5 grades are considered major complications and CD 1–2 grades minor complications. The 5 RCTs comparing ORC with RARC all report on complications. Nix et al. (15) did not differentiate between major and minor complications but found no significant difference in absolute number of complications; 7 (33%) in the RARC group and 10 (50%) in the ORC group (P=0.2789) or in the mean CD grade, 2.3 and 2.6, respectively (P=0.5622). Messer et al.’s (16) pilot prospective RCT did not report minor CD 1 complications and reported transfusion as a separate complication but found no statistical difference between RARC and ORC for CD 3–5 complications, albeit this was a small study (n=40) with primary outcome measuring health-related quality of life (HRQol). The CORAL study (14), a single-centre 3-arm RCT comparing 30- and 90- day complications rates for ORC, LRC and RARC hypothesised that due to “the greater precision offered by robotic technology” RARC would reduce complication rates compared to the other two modalities. This hypothesis proved to be incorrect as the study found no significant difference in 30-day major (CD 3–5) complication rates between RARC and either ORC or LRC and, similarly, no significant difference in 90-day all grade complication rates between modalities. Bochner et al. (13) compared 90-day CD grade 2–5 complications and found no statistical difference between ORC and RARC, 66% and 62% respectively (P=0.7), meeting the trials futility criteria and facilitating early closure of the trial. More recently the RAZOR (8) trial found no significant different in 90 days all grade complication rates between ORC and RARC.
Meta-analysis and observational studies have revealed a mixed picture in regards to complication rates; Leow et al. (23) conducted a retrospective study of 279 hospitals including an impressive 34,672 patients undergoing ORC and 2,101 RARC patients. RARC was associated with 46% decreased odd of CD 1–2 complications (P=0.03) and no difference in CD 3–5 complications. Several early meta-analyses assessed complication rates; Tang et al. (24), Li et al. (25) and Fonseka et al. (26) all favoured RARC with significantly less perioperative complications when comparing RARC to ORC. Ishii et al. (27) found no difference in overall or CD 1–2 complications but found significantly more major complications in ORC. More recently, Shen et al. (28) and Tan et al. (29) found no statistical difference in CD 2–4 complications between the surgical techniques. Early meta-analyses were largely based on retrospective observational studies perhaps explaining the heterogeneity in published results.
Perioperative outcomes
Perioperative outcomes associated with quality of surgery including estimated blood loss, transfusion requirement, lengths of stay (LOS) and operating time have all been widely reported in RARC. Blood loss and transfusion requirement, in particular, have become the focus of renewed interest because several recent trials have noted an association between transfusion and worse survival outcomes. A 2016 meta-analysis of eight studies by Cata et al. (30) found blood transfusion was associated with a reduction of 27%, 29% and 12% in overall survival, cancer specific survival and recurrence free survival, respectively, in patients undergoing RC. Furthermore, Wang et al.’s (31) meta-analysis of 7,080 patients undergoing RC reported similar findings. Thus, the importance of blood loss and transfusion requirement may have been previously overlooked and highlights an area of possible advantage for RARC over ORC. Rai et al.’s (32) excellent Cochrane review, including the five aforementioned RARC vs. ORC RCTs, comprehensively applied the validated GRADE tool (33) to assess the quality of evidence and concluded with moderate certainty that RARC leads to substantially fewer blood transfusions (193 fewer transfusions per 1,000 RARC participants (95% CI: 262 fewer to 92 fewer) based on 460 transfusion per 1,000 participants for ORC). Sathianathen et al.’s (34) meta-analysis found a similar reduction in transfusion rates of 42% in the RARC group compared to ORC. The decrease in blood loss and transfusion requirement may largely be due to the tamponade effect seen with CO2 insufflation and the benefit has been reproduced in meta-analysis comparing robotic prostatectomy versus open prostatectomy (35).
Operative time and length of stay vary significantly based on individual surgeon’s experience and particular institutions post-operative practices. Thus, reported data from single centre/surgeon trials may be of limited use. Protracted operative time is associated with poor outcomes such as venous thromboembolism and increased anaesthetic risk (36). Sathianathen et al. (34) found that RARC had significantly greater operative time compared to ORC with a mean difference of 68.51 min (95% CI: 30.55–105.48 min) and this increased operative time is consistently reported across the early literature. Increased operative time is perhaps offset by decreased LOS; all five RCTs provided data for LOS and cumulative analysis notes a modest mean reduction of 0.67 days for RARC over ORC (32). The introduction of the enhanced recovery protocol is likely to improve LOS irrespective of surgical modality and evidence has shown its benefit in other surgeries (37).
HRQoL
A diagnosis of MIBC leading to RC has a serious deleterious effect on the HRQoL of patients; first, a large proportion of patients undergo neo-adjuvant/adjuvant systemic therapy with associated debilitating side-effects, second, the surgery is highly morbid and third, patients face the psychological impact of managing life-long UD/bladder substitution. A variety of HRQoL tools have been employed in the RC literature to date including the bladder cancer index (BCI), Functional Assessment of Cancer Therapy-Vanderbilt Cystectomy Index (FACT-VCI) and the European Organisation for the Research and Treatment of Cancer QOL core 30 (QLQ-C30) questionnaires. Four of the described RCTs provided data on QOL; Bochner et al. (13) utilised the non-bladder cancer specific QLQ-C30 questionnaire at baseline, 3- and 6-month intervals post-operatively and found no statistical difference in questionnaire scores between the modalities. More recently, the RAZOR trial (8) assessed HRQoL using the FACT-VCI questionnaire at baseline, 3- and 6-month and finding no statistical difference in survey scores between ORC and RARC.
A recent meta-analysis (38) assessing the effect of UD type on HRQoL found significant improvement in emotional functioning (85 vs. 79, P=0.023), cognitive functioning (93 vs. 85, P<0.001), constipation (16 vs. 31, P<0.001) and abdominal bloating flatulence scores (12 vs. 25, P<0.001) for ileal orthoptic neobladder compared to ileal conduit but the opposite for domains relating to sexual and urinary function. There are several criticisms of HRQoL research in RC, in part due to the heterogeneity of assessment tools making meaningful meta-analysis impossible. Furthermore, the majority of trials report the first post-operative assessment at 3 months negating the potential early benefit of RARC over ORC in terms of expedient return to normal activities. Analogous research (39) in colorectal surgery reveals statistically higher QoL scores at 2-week follow-up after minimally invasive bowel resection compared to the open approach perhaps highlighting the benefit of earlier post-operative recovery. Similarly, there is a lack of long-term HRQoL data for RARC and whilst we see follow-up periods approaching 10 years in robotic prostatectomy HRQoL studies (40), no such evidence exists in RARC.
Surgeon-specific factors
Almost all research on RC focuses on patient-specific outcomes and there is scant research on surgeon-specific outcomes besides learning curves. RC is a complex prolonged procedure, which requires great mental aptitude from the surgeon and thus surgeon-specific outcomes related to fatigue and efficiency are important considerations. Moore et al. (41) found that robotic systems allowed a shorter time to reach proficiency in surgical tasks and greater transferability of skills compared to laparoscopy. Multiple studies have shown surgeon stress has a deleterious effect on surgical performance (42). Novel work assessing cardiovascular parameters during stressful surgical tasks noted more favourable surgeon outcomes during robotic tasks compared to laparoscopic tasks (43). Abdelrahman et al.’s meta-analysis (44) noted indicators of workload, heart rate and reporting of musculoskeletal symptoms to be significantly less in robotic surgery compared to laparoscopic. However, there may be negative consequences of robotic surgery due to the increased demands on surgical assistants and poor communication due to the re-location of the primary surgeon away from the operating table to the surgical console (45). To the authors knowledge there are no comparative studies comparing ORC with RARC with respect to markers of surgeon stress or fatigue.
A variety of definitions for the learning curve exist but broadly speaking it is the time taken for a surgeon to achieve competence in a new procedure. The metrics used to gauge competency vary and importantly it pertains to the whole surgical team not just the primary surgeon. The Pasadena consensus panel (46) described the following parameters for surgical competence in RARC; estimated blood loss, operative time and positive surgical margin status. Hayn et al.’s (47) prospective study including 496 RARCs from 14 different institutes noted that operative time plateaued after just 21 procedures and acceptable lymph node yields and positive surgical margin status were achieved after 30 cases. Collins et al. (48) assessed the learning curve for their first 67 patients undergoing RARC with intracorporeal bladder substitute. The group noted a significant decrease in complications with increasing experience but no change in lymph node yield, LOS or estimated blood loss. Similarly, Richards et al. (49) noted significantly decreasing operating times and rates of complications over the duration of their 60-patient case series. For comparison, studies have shown a comparable learning curve for robotic partial nephrectomy with competency reached at 25–30 cases and plateauing at 70–75 cases (50). It is important to note that the majority of surgeons undertaking RARC have experience in robotic surgery through performing robotic prostatectomy and thus the learning curve might be somewhat abbreviated.
Cost outcomes
The comparison of cost between surgical modalities is fraught with difficulty due to a number of confounders; first of all there is much heterogeneity in the payment and public insurance systems between countries; second, the methods by which hospitals collect and report cost data varies widely; third, the assessment of indirect costs through re-admission and complications is often not included in cost analysis and maybe substantial given the associated morbidity of RC and finally, the acquisition cost and maintenance contracts for running a robotic program are often ignored and maybe significant.
Bochner et al.’s (13) RCT was the only RCT to provide cost analysis. The group found a significant cost advantage in terms of inpatient and operating room costs for ORC over RARC. RARC with ileal conduit cost an extra $1,740 (P<0.05) and RARC with bladder substitution an extra $3,920 (P<0.0001). The increased cost in the robotic group pertained to the greater physician and operating room costs. Lee et al.’s (51) prospective study comparing ORC with RARC also noted a cost advantage for ORC when assessing direct costs ($13–1,085: all types of UD). However, when indirect costs relating to complications were included RARC with ileal conduit outperformed ORC by $4,846. If a bladder substitution was performed the robotic procedure was more expensive by $1,966. Furthermore, Joice et al. (52) also concluded that UD type had an effect on overall cost and continent diversion lead to greater 90-day costs due to increased hospital costs and greater number of re-admissions. Most recently, Hu et al. (53) study included 439 RARC patients and 7,308 ORC in their comparison of perioperative outcomes and hospital costs. The study noted non-significant difference in in-patient costs (RARC: $24,051 vs. ORC: $21,637, P=0.08). but greater RARC costs at 30 days ($31,009 vs. $27,947) and 90 days ($36,121 vs. $32,521). Leow et al. (23) performed the largest comparative cost analysis study including an impressive 34,672 ORC patients and 2,101 RARC patients. They noted significantly higher supply costs, almost double, for RARC vs. ORC ($6,041 vs. $3,638; P<0.0001). Similarly, at 90-day RARC was $4,326 more expensive. Interestingly, sub-group analysis of highest volume surgeons, defined as >8 cases per year, or high-volume institutions, >19 cases per year, found no statistical difference in costs. This may be explained by reduced LOS, complications and streamlined post-operative care in those high-volume institutions with surgeons further along the learning curve and maybe an argument for centralisation of cancer care.
ICUD
The adoption of ICUD has been rapid in the RARC community over the last 10 years; Hussein’s retrospective analysis (54) of 2,125 patients from the IRCC database noted 51% of the cohort underwent ICUD. With increased mentoring and refinement in technique a trend towards IUCD was noted with 9% performed in 2005 rising to 97% in 2016, the majority of these were ileal conduits. However, the study included high volume institutions only and thus the data may not be representative of the cystectomy community as a whole. It is postulated that completely minimally invasive RC with IUCD may be advantageous due to smaller incisions, less post-operative pain, decreased bowel handling, decreased ureteric dissection leading to fewer stenosis and faster post-operative recovery (55).
Despite the potential benefits and increasing adoption of ICUD there is no level-1 evidence for its use; 4 of the 5 RCTs (8,13-15) assessing RARC only included patients with the hybrid ECUD approach, and the remaining RCT (16) did not comment on the UD approach taken. Hussein et al. (54) aforementioned study represents the largest retrospective study and noted less blood loss, fewer blood transfusions and shorter operative time (357 vs. 400 min) (P<0.001) in the IUCD group compared to the EUCD group. However, a greater tendency for complications in ICUD was noted, 57% vs. 43% (P<0.001). This may partly be explained by the surgeon’s learning curve because over time the rate of high grade complication in ICUD decreased; 25% in 2005 to 6% in 2015 (P<0.001), whilst the complicate rate for ECUD remained stable at 13–14% over the same time period representing prior surgeon experience with EUCD and plateauing of outcomes. Furthermore, a decreased operative time was seen in the ICUD group over the ECUD, 357 min (297–420 min) and 400 min (338–480 min) respectively (P>0.001). In contrast Bertolo et al. (56) conducted a single-institute prospective 2-arm comparative trial between RARC with ICUD vs. RARC with ECUD. They noted increased operative time in ICUD by 1 hour (P=0.0004) but comparable overall complication rates; ICUD: 26.7% vs. ECUD: 34.8%, (P=0.3).
Tan et al. (20) looked specifically at the short-term oncological outcomes for ORC vs. RARC with ICUD, including 184 patients with a mean follow-up of 33 months. The study found no significant difference in recurrence free survival, cancer specific survival, overall survival or pattern of recurrences between the two treatment modalities at 24 months. More recently, longer-term data is provided by Brassetti et al.’s (57) multi-institute retrospective study noting, 5-year recurrence free survival, cancer specific survival and overall survival probabilities were 58%±5%, 61%±5% and 54%±5%, respectively, comparable to RARC with EUCD.
Due to the greater technical difficulty and increased operative time for intra-corporeal bladder substitution its adoption has been modest compared to ileal conduit; Hussein et al. (54) noticed an increased trend in intra-corporeal bladder substitution from 7% in 2005 to 17% in 2015. Several studies have published refinements on the initial technique first reported by Beecken and the Frankfurt group (58) in 2003. In 2010 Guru et al. (59) described the passing of a 1-0 silk “Keith” needle through the abdominal wall to suspend the ileal segment for creation of the intra-corporeal neobladder. Wiklund et al. (60) provides a detailed, illustrated step-by-step guide for intra-corporeal neobladder formation and Goh et al. (61) describes the innovative use of intra-venous indigo-cyanide with fluorescence enhanced imaging to identify mesenteric vessels when isolating the ileal segment.
Tostivint et al. (62) prospectively compared ORC and RARC with intra-corporeal bladder substitution noting decreased length of stay (median 12 vs. 17 days; P<0.05) and decreased blood transfusion (0 vs. 23.6%; P<0.05) in favour of RARC but increased operative time (median 360 vs. 300 min; P<0.001) and increased rate of uretero-ileal stenosis (25.5% vs. 23% P<0.05). Albeit, this was a small study (n=72) and the RARC arm only included 17 patients. Satkunasivam et al. (63) compared RC with intra-corporeal neobladder formation with the open approach, specifically assessing urodynamic and functional outcomes. The study included 107 patients and confirmed urodynamically that the creation of a low-pressure, high-volume neobladder with the robotic approach was feasible. Furthermore, HRQoL was measured using the BCI with no significant difference between the surgical approach, however the robotic approach was associated with worse day-time wetness and increased pad size albeit the follow-up period was shorter for the robotic group. Case series from Desai et al. (64) and Tyritzis et al. (11) further confirm the feasibility of intra-corporeal bladder substitution.
Robotic cutaneous continent bladder substitution is a nascent technique with literature largely pertaining to feasibility studies, small case series and description of technique; Goh et al. (65) first described the use of a modified “Indiana pouch” with a continent stoma created through a robotic port site. Desai et al. (66) provide a 10-patient case series for patients undergoing intra-corporeal robotic Indiana pouch continent cutaneous diversion with a mean operative time of 6 hours and major complication rate (CD >3) of 20% at 1 year. The use of a cutaneous continent mechanism is a fairly rare event even in ORC, however, the described case series are important because now the full gambit of open UD techniques have been replicated in the robotic setting.
Conclusion and future perspective
The feasibility of RARC is well established; the extirpative component largely has a bearing on oncological outcomes and, at the very least seems, comparable to ORC. Much of the morbidity in RC stems from the UD; ICUD may prove beneficial in reducing this morbidity and the technique is developing at a rapid pace. At present all types of open UD have been replicated robotically. It is hoped with continued technical refinements and improved surgical efficiency robotics will lead to the improved perioperative outcomes necessary to justify the increased cost, beyond merely the reduction in transfusion rates seen to date. Furthermore, with the anticipated expansion of choice in robotic systems, it is likely that competitive forces may decrease the cost or robotic surgery further broadening robotic surgery’s appeal. Current research is hampered by the expected limitations of conducting RCTs on surgical procedures. None of the described RCTs in this review assess the potential benefits of truly minimally invasive RARC with ICUD despite its widespread adoption. The ambitious iROC trial (NCT03049410), currently recruiting in the UK, aims to fill this research gap by reporting on oncological, perioperative, functional, surgical and cost outcomes for RARC with ICUD to hopefully provide the definitive answer on the optimal surgical technique for RC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ja Hyeon Ku, Ho Kyung Seo, Seok Ho Kang) for the series “Muscle-Invasive Bladder Cancer” published in Translational Andrology and Urology. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.12.19). The series “Muscle-Invasive Bladder Cancer” was commissioned by the editorial office without any funding or sponsorship. Dr. Decaestecker reports personal fees and non-financial support from Intuitive Surgical, personal fees and non financial support from Covidien, non-financial support from Conmed, personal fees and non-financial support from Ab Medica, personal fees and non-financial support from Cordamed Biomedical, personal fees and non- financial support from MSD, personal fees and non-financial support from Takeda, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Witjes JA, Bruins M, Cathomas R, et al. Muscle-invasive and metastatic bladder cancer. European Association of Urology Guidelines, 2018.
- Bricker EM. Bladder substitution after pelvic evisceration. Surg Clin North Am 1950;30:1511-21. [Crossref] [PubMed]
- Lobo N, Thurairaja R, Nair R, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion - The new 'gold standard'? Evidence from a systematic review. Arab J Urol 2018;16:307-13. [Crossref] [PubMed]
- Tyritzis SI, Collins JW, Wiklund NP. The current status of robot-assisted cystectomy. Indian J Urol 2018;34:101-9. [Crossref] [PubMed]
- Nepple KG, Strope SA, Grubb RL 3rd, et al. Early oncologic outcomes of robotic versus open radical cystectomy for urothelial cancer. Urol Oncol 2013;31:894-8. [Crossref] [PubMed]
- Novara G, Catto JW, Wilson T, et al. Systematic review and cumulative analysis of perioperative outcomes and complications after robot-assisted radical cystectomy. Eur Urol 2015;67:376-401. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Niegisch G, Nini A, Michalski R, et al. Comparison of 2-Year Oncological Outcome and Early Recurrence Patterns in Patients with Urothelial Bladder Carcinoma Treated with Open or Robot-Assisted Radical Cystectomy with an Extracorporeal Urinary Diversion. Urol Int 2018;101:224-31. [Crossref] [PubMed]
- Kim TH, Sung HH, Jeon HG, et al. Oncological Outcomes in Patients Treated with Radical Cystectomy for Bladder Cancer: Comparison Between Open, Laparoscopic, and Robot-Assisted Approaches. J Endourol 2016;30:783-91. [Crossref] [PubMed]
- Tyritzis SI, Hosseini A, Collins J, et al. Oncologic, functional, and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion. Eur Urol 2013;64:734-41. [Crossref] [PubMed]
- Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. J Urol 2011;185:456-61. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Sjoberg DD, et al. Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: A Randomized Clinical Trial. Eur Urol 2015;67:1042-50. [Crossref] [PubMed]
- Khan MS, Gan C, Ahmed K, et al. A Single-centre Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur Urol 2016;69:613-21. [Crossref] [PubMed]
- Nix J, Smith A, Kurpad R, et al. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer: perioperative and pathologic results. Eur Urol 2010;57:196-201. [Crossref] [PubMed]
- Messer JC, Punnen S, Fitzgerald J, et al. Health-related quality of life from a prospective randomised clinical trial of robot-assisted laparoscopic vs open radical cystectomy. BJU Int 2014;114:896-902. [Crossref] [PubMed]
- Hussein AA, Elsayed AS, Aldhaam NA, et al. Ten-Year Oncologic Outcomes Following Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol 2019;202:927-35. [Crossref] [PubMed]
- Nguyen DP, Al Hussein Al Awamlh B, Wu X, et al. Recurrence patterns after open and robot-assisted radical cystectomy for bladder cancer. Eur Urol 2015;68:399-405. [Crossref] [PubMed]
- Pourmalek F, Abdi H, Black PC. Re: Daniel P. Nguyen, Bashir Al Hussein Al Awamlh, Xian Wu, et al. Recurrence Patterns After Open and Robot-assisted Radical Cystectomy for Bladder Cancer. Eur Urol 2015;68:399-405. Eur Urol 2016;69:e35. [Crossref] [PubMed]
- Tan WS, Sridhar A, Ellis G, et al. Analysis of open and intracorporeal robotic assisted radical cystectomy shows no significant difference in recurrence patterns and oncological outcomes. Urol Oncol 2016;34:257.e1-9. [Crossref] [PubMed]
- Collins JW, Hosseini A, Adding C, et al. Early Recurrence Patterns Following Totally Intracorporeal Robot-assisted Radical Cystectomy: Results from the EAU Robotic Urology Section (ERUS) Scientific Working Group. Eur Urol 2017;71:723-6. [Crossref] [PubMed]
- Dindo D, Clavien PA. What is a surgical complication? World J Surg 2008;32:939-41. [Crossref] [PubMed]
- Leow JJ, Reese SW, Jiang W, et al. Propensity-matched comparison of morbidity and costs of open and robot-assisted radical cystectomies: a contemporary population-based analysis in the United States. Eur Urol 2014;66:569-76. [Crossref] [PubMed]
- Tang K, Xia D, Li H, et al. Robotic vs. open radical cystectomy in bladder cancer: A systematic review and meta-analysis. Eur J Surg Oncol 2014;40:1399-411. [Crossref] [PubMed]
- Li K, Lin T, Fan X, et al. Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy. Cancer Treat Rev 2013;39:551-60. [Crossref] [PubMed]
- Fonseka T, Ahmed K, Froghi S, et al. Comparing robotic, laparoscopic and open cystectomy: a systematic review and meta-analysis. Arch Ital Urol Androl 2015;87:41-8. [Crossref] [PubMed]
- Ishii H, Rai BP, Stolzenburg JU, et al. Robotic or open radical cystectomy, which is safer? A systematic review and meta-analysis of comparative studies. J Endourol 2014;28:1215-23. [Crossref] [PubMed]
- Shen Z, Sun Z. Systematic review and meta-analysis of randomised trials of perioperative outcomes comparing robot assisted versus open radical cystectomy. BMC Urol 2016;16:59. [Crossref] [PubMed]
- Tan WS, Khetrapal P, Tan WP, et al. Robotic assisted radical cystectomy with extracorporeal urinary diversion does not show a benefit over open radical cystectomy: a systematic review and meta-analysis of randomised controlled trials. PLoS One 2016;11:e0166221. [Crossref] [PubMed]
- Cata JP, Lasala J, Pratt G, et al. Association between perioperative blood transfusions and clinical outcomes in patients undergoing bladder cancer surgery: a systematic review and meta-analysis study. J Blood Transfus 2016;2016:9876394. [Crossref] [PubMed]
- Wang YL, Jiang B, Yin FF, et al. Perioperative blood transfusion promotes worse outcomes of bladder cancer after radical cystectomy: a systematic review and meta-analysis. PLoS One 2015;10:e0130122. [Crossref] [PubMed]
- Rai BP, Bondad J, Vasdev N, et al. Robotic versus open radical cystectomy for bladder cancer in adults. Cochrane Database Syst Rev 2019;4:CD011903. [PubMed]
- Guyatt GH, Oxman AD, Kunz R, et al. What is "quality of evidence" and why is it important to clinicians? BMJ 2008;336:995-8. [Crossref] [PubMed]
- Sathianathen NJ, Kalapara A, Frydenberg M, et al. Robotic Assisted Radical Cystectomy vs Open Radical Cystectomy: Systematic Review and Meta-Analysis. J Urol 2019;201:715-20. [Crossref] [PubMed]
- Ilic D, Evans SM, Allan CA, et al. Laparoscopic and robotic-assisted versus open radical prostatectomy for the treatment of localised prostate cancer. Cochrane Database Syst Rev 2017;9:CD009625. [Crossref] [PubMed]
- Daley BJ, Cecil W, Clarke PC, et al. How slow is too slow? Correlation of operative time to complications: an analysis from the Tennessee Surgical Quality Collaborative. J Am Coll Surg 2015;220:550-8. [Crossref] [PubMed]
- Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate A study of 4,500 consecutive primary hip and knee replacements. Acta Orthopaedica 2011;82:577-81. [Crossref] [PubMed]
- Cerruto MA, D'Elia C, Siracusano S, et al. Health-related Quality of Life After Radical Cystectomy: A Cross-sectional Study With Matched-pair Analysis on Ileal Conduit vs Ileal Orthotopic Neobladder Diversion. Urology 2017;108:82-9. [Crossref] [PubMed]
- Weeks JC. Short-term Quality-of-Life Outcomes Following Laparoscopic-Assisted Colectomy vs Open Colectomy for Colon Cancer A Randomized Trial. JAMA 2002;287:321-8. [Crossref] [PubMed]
- Prabhu V, Lee T, McClintock TR, et al. Short-, Intermediate-, and Long-term Quality of Life Outcomes Following Radical Prostatectomy for Clinically Localized Prostate Cancer. Rev Urol 2013;15:161-77. [PubMed]
- Moore LJ, Wilson MR, Waine E, et al. Robotic technology results in faster and more robust surgical skill acquisition than traditional laparoscopy. J Robot Surg 2015;9:67-73. [Crossref] [PubMed]
- Arora S, Sevdalis N, Nestel D, et al. The impact of stress on surgical performance: a systematic review of the literature. Surgery 2010;147:318-30, 330.e1-6.
- Moore LJ, Wilson MR, Waine E, et al. Robotically assisted laparoscopy benefits surgical performance under stress. J Robot Surg 2015;9:277-84. [Crossref] [PubMed]
- Abdelrahman AM, Lowndes B, Rand C, et al. Impact of Robotic Surgery Versus Laparoscopic Surgery on Surgeon Musculoskeletal Symptoms and Workload: A Systematic Review and Meta-analysis. The SAGES 2017 Annual Meeting; Houston, TX; 2017:abstr 80750.
- Catchpole K, Bisantz A, Hallbeck MS, et al. Human factors in robotic assisted surgery: Lessons from studies 'in the Wild'. Appl Ergon 2019;78:270-6. [Crossref] [PubMed]
- Wilson TG, Guru K, Rosen RC, et al. Best practices in robot-assisted radical cystectomy and urinary reconstruction: recommendations of the Pasadena Consensus Panel. Eur Urol 2015;67:363-75. [Crossref] [PubMed]
- Hayn MH, Hussain A, Mansour AM, et al. The learning curve for robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2010;58:197-202. [Crossref] [PubMed]
- Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder—what is the effect of the learning curve on outcomes? BJU Int 2014;113:100-7. [Crossref] [PubMed]
- Richards KA, Kader K, Pettus JA, et al. Does initial learning curve compromise outcomes for robot-assisted radical cystectomy? A critical evaluation of the first 60 cases while establishing a robotic program. J Endourol 2011;25:1553-8. [Crossref] [PubMed]
- Mazzon G, Sridhar A, Busuttil G, et al. Learning Curves for Robotic Surgery: a Review of the Recent Literature. Curr Urol Rep 2017;18:89. [Crossref] [PubMed]
- Lee R, Ng CK, Shariat SF, et al. The economics of robotic cystectomy: cost comparison of open versus robotic cystectomy. BJU Int 2011;108:1886-92. [Crossref] [PubMed]
- Joice GA, Chappidi MR, Patel HD, et al. Hospitalisation and readmission costs after radical cystectomy in a nationally representative sample: does urinary reconstruction matter? BJU Int 2018;122:1016-24. [Crossref] [PubMed]
- Hu JC, Chughtai B, O'Malley P, et al. Perioperative Outcomes, Health Care Costs, and Survival After Robotic-assisted Versus Open Radical Cystectomy: A National Comparative Effectiveness Study. Eur Urol 2016;70:195-202. [Crossref] [PubMed]
- Hussein AA, May PR, Jing Z, et al. Outcomes of Intracorporeal Urinary Diversion after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol 2018;199:1302-11. [Crossref] [PubMed]
- Koie T, Ohyama C, Makiyama K, et al. Utility of robot-assisted radical cystectomy with intracorporeal urinary diversion for muscle-invasive bladder cancer. Int J Urol 2019;26:334-40. [Crossref] [PubMed]
- Bertolo R, Agudelo J, Garisto J, et al. Perioperative Outcomes and Complications after Robotic Radical Cystectomy With Intracorporeal or Extracorporeal Ileal Conduit Urinary Diversion: Head-to-head Comparison From a Single-Institutional Prospective Study. Urology 2019;129:98-105. [Crossref] [PubMed]
- Brassetti A, Cacciamani G, Anceschi U, et al. Long-term oncologic outcomes of robot-assisted radical cystectomy (RARC) with totally intracorporeal urinary diversion (ICUD): a multi-center study. World J Urol 2020;38:837-43. [Crossref] [PubMed]
- Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol 2003;44:337-9. [Crossref] [PubMed]
- Guru K, Seixas-Mikelus SA, Hussain A, et al. Robot-assisted intracorporeal ileal conduit: Marionette technique and initial experience at Roswell Park Cancer Institute. Urology 2010;76:866-71. [Crossref] [PubMed]
- Wiklund NP, Poulakis V. Robotic neobladder. BJU Int 2011;107:1514-37. [Crossref] [PubMed]
- Goh AC, Gill IS, Lee DJ, et al. Robotic intracorporeal orthotopic ileal neobladder: replicating open surgical principles. Eur Urol 2012;62:891-901. [Crossref] [PubMed]
- Tostivint V, Roumiguié M, Cabarrou B, et al. Orthotopic neobladder reconstruction for bladder cancer: robotic-assisted versus open-radical cystectomy for perioperative outcomes, functional results and quality of life. Prog Urol 2019;29:440-8. [Crossref] [PubMed]
- Satkunasivam R, Santomauro M, Chopra S. Robotic Intracorporeal Orthotopic Neobladder: Urodynamic Outcomes, Urinary Function, and Health-related Quality of Life. Eur Urol 2016;69:247-53. [Crossref] [PubMed]
- Desai MM, Gill IS, de Castro Abreu AL, et al. Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients. J Urol 2014;192:1734-40. [Crossref] [PubMed]
- Goh AC, Aghazadeh MA, Krasnow RE, et al. Robotic intracorporeal continent cutaneous urinary diversion: primary description. J Endourol 2015;29:1217-20. [Crossref] [PubMed]
- Desai MM, Simone G, de Castro Abreu AL, et al. Robotic intracorporeal continent cutaneous diversion. J Urol 2017;198:436-44. [Crossref] [PubMed]


