Robot-assisted laparoscopic retroperitoneal lymph node dissection: a minimally invasive surgical approach for testicular cancer
Introduction
Testicular cancer is typically a disease of young men (1) with multiple available treatment options. Therapeutic pathways depend on disease stage and pathologic features and typically result in high survival rates. Treatments include active surveillance, retroperitoneal lymph node dissection (RPLND), primary chemotherapy, and radiotherapy (seminoma only) (2) and choice of therapy is often based on the patient’s perception of treatment toxicities. Surgery has typically been utilized as primary treatment for clinical stage (CS) I non-seminomatous germ cell tumor (NSGCT) as well as for post-chemotherapy residual masses.
When considering a surgical approach, open RPLND (O-RPLND) has been long considered the standard of care and the basis for comparison of alternative surgical techniques. Access for O-RPLND, however, historically has involved a xiphoid to pubis abdominal wall incision to allow access to the entire retroperitoneum and can be associated with significant morbidity. Given the pain and morbidity associated with this incision many young men choose chemotherapy as primary treatment for CS I NSGCT despite its significant toxicities (3,4).
Laparoscopic RPLND (L-RPLND) was introduced in 1992 as an alternative to O-RPLND (5) and was utilized in patients with CS I and low volume CS II disease. Compared to O-RPLND, L-RPLND resulted in significantly decreased morbidity, however, given that the majority of patients undergoing L-RPLND had lower lymph node yields (6), and those found to have positive retroperitoneal lymph nodes received adjuvant chemotherapy, it was mostly considered a staging procedure (7). Additionally, L-RPLND is a challenging operation with difficulty accessing the lymph nodes posterior to the great vessels and controlling vascular bleeding. It was thus limited only to a few centers of excellence with expert laparoscopic surgeons.
Robotic surgery is an alternative minimally invasive surgical technique resulting in the benefit of decreased surgical morbidity associated with laparoscopic surgery while providing improved visualization, instrument dexterity and ergonomics. R-RPLND was first performed in 2006 (8), and since then, multiple investigators have demonstrated it to be a viable and safe surgical approach (9-11). With R-RPLND, there is improved capability to dissect behind the great vessels and to more easily control major bleeding. As experience with R-RPLND has improved, it has transformed a minimally invasive approach to RPLND from a staging operation to a therapeutic one.
Indications and staging for R-RPLND
Patients with suspected testicular cancer should routinely have tumor markers drawn prior to orchiectomy, including alpha fetal protein (AFP), beta-human chorionic gonadotropin (B-HCG), and lactate dehydrogenase (LDH). Axial imaging of the chest, abdomen, and pelvis is performed with computed tomography (CT) scan. Based on orchiectomy pathology results, tumor markers, and imaging findings, the appropriate clinical stage can be determined.
R-RPLND can be employed as primary treatment for patients with tumor marker negative, high-risk, CS I NSGCT. Those with pathology specimens demonstrating lymphovascular invasion or greater than 50% embryonal carcinoma in the orchiectomy specimen are considered high risk. Patients with low risk CS I NSGCT are best managed with active surveillance. Some investigators are also exploring the use of RPLND as primary treatment for low-stage seminoma, however, the results of this are yet to be determined (12). Patients with tumor marker negative CS IIA NSGCT and certain cases of CS IIB NSGCT may be eligible for primary treatment with R-RPLND (2). Additionally, NSGCT patients with post-chemotherapy masses larger than 1 cm and negative tumor markers are candidates for R-RPLND (2). Patients with post chemotherapy masses obstructing the inferior vena cava which may require vascular replacement are best managed with an open approach.
Operative technique and perioperative management
After full pre-operative assessment and appropriate patient counseling has been completed, informed consent is obtained and if the patient is under 18 years of age, parental consent is necessary. Potential risks of the procedure that are discussed include major vascular injury requiring repair or vascular surgery consultation, bowel injury, nephrectomy, deep venous thrombosis with pulmonary embolus, lymphatic fluid leakage including chylous ascites, and ejaculatory dysfunction. Patients are routinely counseled to back sperm prior to R-RPLND. Patients undergo a modified bowel preparation with a clear liquid diet and magnesium citrate starting 24 hours prior to surgery. Induction of general anesthesia is performed with deep paralysis to allow for full pneumoperitoneum. Mechanical and chemical deep vein thrombosis prophylaxis is given with placement of sequential compression devices and subcutaneous injection of unfractionated or low molecular weight heparin. Perioperative antibiotics, typically a cephalosporin, are administered within 30 minutes of incision time. An orogastric tube is placed to decompress the stomach and a urethral catheter is inserted to decompress the bladder and keep track of urine output.
Room set-up and patient positioning
Our technique for R-RPLND is via a supine approach. We find that this allows for a complete, bilateral dissection if needed. Both the da Vinci (Intuitive Surgical; Sunnyvale, CA) Si and Xi platforms can be utilized, although, the Xi is our preferred platform as it allows multi-quadrant access without the need to redock the robot.
The patient is placed supine on a full torso gel pad which prevents patient movement when placed in the Trendelenburg position. Arms are padded and tucked and legs are supported. A Veress needle is used to gain entry at Palmer’s point (subcostal margin left upper quadrant) and establish pneumoperitoneum. If the da Vinci Si model is being used, a 12-mm trocar is placed 3–4 cm below the umbilicus in the midline. A 0-degree camera is used to place the remaining ports under direct vision. An 8-mm right robotic port and 4th arm port is placed on the patient’s left side and an 8 mm left robotic port is placed on the patient’s right side. A 12-mm assistant port is placed between the camera port and the left robotic port (Figure 1). As demonstrated in the figure, all arms are placed caudad to the level of the umbilicus in an off-set fashion, thus facilitating cephalad dissection. There should be at least 7 cm of space between ports to prevent external arm collisions. For the Xi model, a linear port configuration is utilized using four robot 8 mm ports and a 12 mm assistant port (Figure 2). These ports are placed at least 6–7 cm apart. A 0-degree lens is utilized initially to gain access to the retroperitoneum but later switch to a 30-degree down lens during lymphatic dissection. We routinely use the Air Seal (Conmed; Utica, New York, USA) insufflation device is used to maintain pneumoperitoneum at 12 mmHg. The patient is placed in 20–30 degrees Trendelenburg position to allow the small intestine to fall cephalad and the robot is docked.
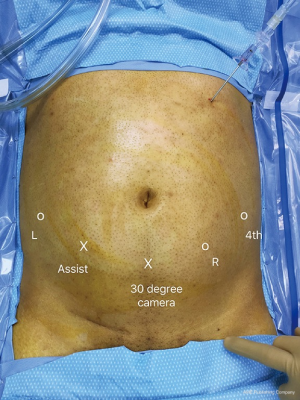
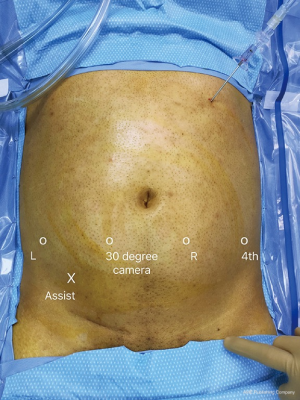
The assistant is usually placed on the patient’s right side and scrub nurse will be on the patient’s left side. A Mayo stand will be positioned between the patient’s legs and will hold laparoscopic and robotic instruments commonly used throughout the case. If utilizing the da Vinci Si model, the robot is brought from the head of the bed over the left shoulder. The entire retroperitoneal dissection can be performed from this docking location except for the removal of the spermatic cord within the inguinal canal. Removal of the spermatic cord requires re-docking of the da Vinci Si at the end of the case alongside the patient’s ipsilateral leg with the ports redirected towards the internal inguinal ring. Docking can be from any direction with the da Vinci Xi due to the rotating boom, but our routine is to dock on the side opposite the bedside assistant. It does not need to be redocked to excise the spermatic cord as it is designed to allow for extended reach of its arms thus providing multi-quadrant access.
A 0-degree camera is used initially to gain retroperitoneal exposure, but is later changed to a 30 degree down lens for node dissection. Our instruments of choice include a monopolar scissors, a fenestrated bipolar, and a Progasp grasper for the 4th arm. If vascular injury is encountered, we use robotic needle drivers. Two additional instruments that are very helpful for R-RPLND include a robotic clip applier, which allows control of lymphatics not able to be reached by the assistant, and a robotic vessel sealer, which is ideal for controlling lumbar vessels.
The bedside assistant will have access to a suction-irrigation device, laparoscopic clip appliers (for both titanium and polymer clips), graspers, and laparoscopic needle drivers to aid in passing stitches to the surgeon. “Rescue stitches” are created beforehand in case urgent vascular control is required. We favor a 4-0 polypropylene suture cut to 12 cm with a small absorbable clip at the end. An endocatch bag is utilized to remove the lymphatic specimens as they are freed.
Dissection technique
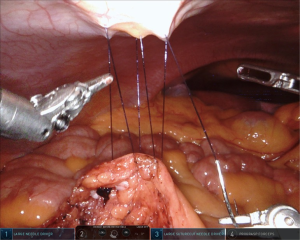
Access is gained to the retroperitoneum by incising the posterior peritoneum just medial to the cecum extending to the ligament of Treitz. We typically create a hammock-like barrier by suturing both cut ends of the parietal peritoneum to the anterior abdominal wall. We typically use a 3-0 monofilament suture and secure it with polymer clips (Figure 3). This, along with the patient’s Trendelenburg position, acts to retract the small bowel away from the field, allowing visualization of the distal lower retroperitoneum. The duodenum is identified and carefully mobilized, if needed, and then gently retracted cephalad utilizing the Prograsp. This creates the upper retroperitoneal exposure needed to allow for a full dissection.
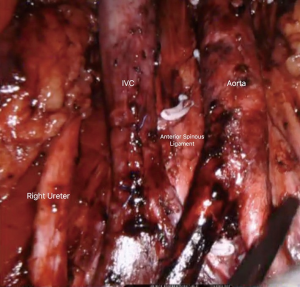
For both right-sided and left-sided template dissections, the ipsilateral renal vessels represent the upper limit of dissection (Figure 4). The inferior mesenteric artery (typically spared) and the ipsilateral ureteral crossing over the common iliac vessels represent, respectively, the inferior medial and inferior lateral limits of dissection. A right-sided template dissection entails removal of paracaval, precaval, retrocaval, interaortocaval, and paraaortic nodal packets. A left-sided template dissection includes removal of the paraaortic, preaortic, retroaortic, and interaortocaval nodal packets. A full, bilateral template dissection combines both right-sided and left-sided templates.
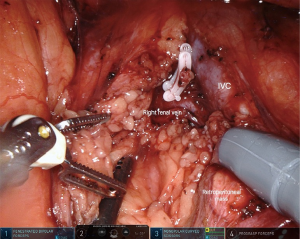
A split-and-roll technique is utilized to remove all lymph nodes and allows good access to retrocaval and retroaortic nodal tissue. Polymer clips are placed on lymphatic channels for node removal and can be placed by the bedside assistant or the surgeon via a robotic clip applier. We always ensure precise control of the lymphatics crossing over the left renal vein as well as the cisterna chyli between the inferior vena cava and aorta in order to prevent chylous ascites (Figure 5). The inferior mesenteric artery is typically preserved but can be sacrificed if needed. We routinely perform a nerve-sparing operation to prevent retrograde ejaculation especially in post chemotherapy patients requiring a bilateral dissection. Sympathetic post-ganglionic nerve fibers can be identified at their origin at the sympathetic chain. These are traced to the hypogastric plexus overlying the distal aorta. Care is taken not to use electrocautery along the nerves and nerve fibers to prevent inadvertent thermal injury.
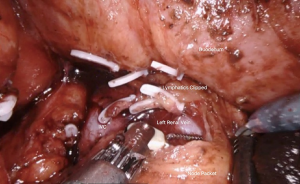
If R-RPLND is being performed for CS I NSGCT, typically a unilateral template dissection is performed. Lymph nodes are sent for frozen analysis and, if positive, a full bilateral template dissection is completed. If R-RPLND is being performed for a post-chemotherapy residual mass, a full bilateral template dissection is always undertaken (Figure 6). All specimens should be placed in endoscopic retrieval bags, which are removed at the end of the case.
Spermatic cord excision is lastly performed with dissection carried out caudally. If the da Vinci Si is used, re-docking is needed along the patient’s ipsilateral leg. There is no re-docking needed for the da Vinci Xi. Dissection is performed out of the internal inguinal ring until sutures from the prior radical orchiectomy are seen. These, along with the entire cord stump, are removed.
Post-operative management
Post-operatively, patients are placed on a clear liquid diet, which is advanced as tolerated to a low-fat diet (<20 grams of fat). This low-fat diet is continued for two weeks after the operation to minimize the risk of chylous ascites. Chylous ascites, should it develop, is typically managed with bowel rest and total parenteral nutrition. Should this not resolve, then procedural or operative intervention should be considered. Patients should also bank sperm prior to surgery in the event of retrograde ejaculation.
Discussion
As experience with R-RPLND has increased, it has been utilized as primary treatment for low-stage NSGCT (9,10) and for post-chemotherapy masses (13). Pearce et al. (14) had the largest study examining outcomes of primary R-RPLND in patients with CS I–IIA NSGCT. Six surgeons at four different institutions, over a 5-year period, performed R-RPLND on 47 patients, of which 42 had CS I disease. The median operative time was 235 minutes and the median estimated blood loss (EBL) was 50 mL. Risk factors for the retroperitoneal disease included lymphovascular invasion and involvement of embryonal carcinoma in greater than 40% of the orchiectomy specimen. The median lymph node count was 26 nodes and the median length of stay (LOS) was one day. Intraoperative complications included a vascular injury requiring open repair and a pancreatic injury that resolved with drain placement and non-operative management. Complications within the first 30 days included two Clavien grade I complications and two Clavien grade III complications, which included a large body-wall hematoma requiring transfusion and chylous ascites requiring paracentesis. Eight of the 42 patients ultimately had node positive disease, with seven or these patients with pN1 disease and the remaining patient with pN2 disease. Five of these patients received adjuvant chemotherapy with the remaining three managed with surveillance. The 2-year recurrence-free survival rate was 97% overall (100% in patients not treated with adjuvant chemotherapy), however, the median follow-up was only 6 months, constituting a major limitation of this study (14).
The largest single institution study was previously published by our group in 2016 and examined R-RPLND utilizing both the lateral and supine approaches (11). The study included 19 patients that underwent 20 procedures, 16 of which were primary R-RPLND with the remaining four post-chemotherapy R-RPLND. Median operative time was 293 minutes and median EBL was 50 mL. The median lymph node count was 19.5 and the median LOS was one day with the vast majority of patients discharged within 24 hours of surgery. There was one intraoperative complication involving a recognized ureteral transection that was primarily repaired without long-term sequelae. No patients requiring blood transfusion. There were no major post-operative complications although two of the patients who underwent post-chemotherapy full template R-RPLND did experience retrograde ejaculation. Eight of the 19 patients ultimately were found to have pathologic stage II disease with three of these patients having teratoma. Only two patients received adjuvant chemotherapy, including one patient with pathologic stage IIC disease and another that developed a lung recurrence four months after RPLND. The remaining pathologic stage II patients were kept under surveillance with no evidence of recurrence at follow-ups of 46, 47, and 91 months, respectively (11).
Tselos et al. performed a systematic review of 11 studies examining a total of 116 patients (15). The mean operative time was 263 minutes and the median lymph node count was 22.3. The complication rate was 8%, with half of these designated minor complications (Clavien grade I–II) and the other half major complications (Clavien grade III–IV). Retrograde ejaculation was seen in 4.5% of patients. The median length of stay was 1.3 days and the average follow-up at 21.2 months demonstrated no evidence of disease recurrence (15).
The length of stay for all R-RPLND studies detailed above was better than those reported for O-RPLND (ranging from 3 days, at very high-volume institutes, to 6.6 days) (6,16-18) and for L-RPLND (2.6 to 3.3 days) (6,19). Like L-RPLND (204 to 258 min) (6,19), R-RPLND has a longer operative time (235 to 293 min) compared to O-RPLND (132 min at very high volume institutions, otherwise reported 186 to 270 min) (6,16-18).
Intraoperative complication rates have been reported for O-RPLND to range from 5–7% (16,17) with a 6% transfusion rate (17). EBL is typically higher than R-RPLND, with reported rates of 184 to 450 mL (6,16-18). Post-operative complication rates have been reported as high as 24% (17) with some data suggesting an all-comer overall complication rate of 33% (6).
Laparoscopic complication rates have been reported as high as 15.6% with a conversion rate of 3.8% (6) with most conversion rates due to bleeding and inability to control it laparoscopically. Long term oncologic outcomes after laparoscopic surgery, as a primary therapeutic modality, have been confounded with the high percentage of patients who have chemotherapy after positive nodes are discovered after surgery (20). The quality of life in patients who had L-RPLND, however, has been shown to be significantly higher than those patients who underwent O-RPLND (21).
There have been studies primarily looking at the feasibility of R-RPLND for post-chemotherapy patients as well. The major challenge with post-chemotherapy RPLND stems from significant fibrosis of the retroperitoneal mass causing adhesion to the great vessels. This creates an environment for possible vascular injury that could require urgent control. Singh et al. published the largest single institution series for post-chemotherapy R-RPLND involving 13 patients. The vast majority of these patients had a unilateral modified template dissection with a lateral approach. The median operative time was 200 minutes and median EBL was 120 mL. The median lymph node count was 20 and median LOS was four days. The only intraoperative complication was an aortic injury that was repaired robotically. Post-operatively, four patients developed chylous ascites (two of whom required operative repair) and five patients developed an ileus. Pathology demonstrated that three patients had teratoma and the remaining eight had necrosis. There was no evidence of disease recurrence at a median follow-up of 23 months (22).
Overs et al. reviewed 11 post-chemotherapy patients who underwent unilateral modified template R-RPLND via a lateral approach. Median operative time was 150 minutes and median EBL was 120 mL. The median lymph node count was seven and the majority of patients were discharged on hospital day three. There were no intraoperative complications reported. Post-operatively, one patient developed chylous ascites that was managed non-operatively. Over 70% of men retained ejaculatory function. Long-term follow-up was only available for six patients and none had evidence of recurrence at 24 months (23). Both studies highlight the feasibility of performing post-chemotherapy RPLND robotically. Critics may argue that, per NCCN guidelines, a full bilateral template dissection should be undertaken in the setting of residual post-chemotherapy masses (2). A supine approach, as described earlier, would allow for better access for a full template dissection without the need for re-docking, and should be considered.
Operative time for R-RPLND for the above-mentioned studies is less than for O-RPLND (226 to 305 min) (16,17,24) and is on-par, if not somewhat faster, than for L-RPLND (183 to 213 min) (24-26). One has to consider, however, that both for R-RPLND and L-RPLND, most of these cases involved a modified template dissection on not a full bilateral template dissection. EBL was higher in both O-RPLND (413 to 1,000 mL) (16,17) with transfusion rates ranging from 14.2% to 42% (17,24). Complication rates have been reported to range from 12% to 38% (16,17,24). L-RPLND had EBL ranging from 260 to 400 mL (25,26) with conversion rates reported as high as 11.5% (26), which are more than the above mentioned rates for R-RPLND. LOS for O-RPLND varied based on institution anywhere from 4.8 #to 11.5 days (16,17,24) and 5–6 days for L-RPLND (24,26). The complexity of cases varied based on institutional experience and operative approach, and thus these factors must always be considered when determining how to treat patients.
Conclusions
R-RPLND has developed into a safe and effective approach for primary management of low stage NSGCT. In experienced hands, and with judicious patient selection, it can also be utilized with good effect for management of post-chemotherapy residual NSGCT masses. However, for large, bulky residual disease, R-RPLND may prove a challenge and one must employ caution in approaching these types of cases robotically. As expertise grows, techniques will continue to evolve. Our experience has demonstrated that a supine approach allows for superior retroperitoneal access facilitating full, bilateral template dissection. It is the preferred approach for both primary and post-chemotherapy R-RPLND. Ultimately, more studies with longer-term follow-up directly comparing R-RPLND to O-RPLND are needed to effectively compare clinical efficacy relative to complications.
Acknowledgments
All figures have been used with permission from “New Technologies and Techniques in Minimally Invasive Urologic Surgery: an ESUT Collection (2019)”.
Footnote
Conflicts of Interest: JR Porter: Speaker for Intuitive Surgical, Consultant for Ceevra, C-SATS advisory board. HR Mittakanti has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Noone AM, Krapcho M, Miller D, et al. SEER Cancer Statistics Review, 1975-2015. Bethesda, MD: National Cancer Institute, 2018.
- Motzer RJ, Agarwal N, Beard C, et al. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: testicular Cancer. J Natl Compr Canc Netw 2009;7:672-93. [Crossref] [PubMed]
- Tandstad T, Stahl O, Hakansson U, et al. One course of adjuvant BEP in clinical stage I nonseminoma mature and expanded results from the SWENOTECA group. Ann Oncol 2014;25:2167-72. [Crossref] [PubMed]
- Haugnes HS, Bosl GJ, Boer H, et al. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J Clin Oncol 2012;30:3752-63. [Crossref] [PubMed]
- Rukstalis DB, Chodak GW. Laparoscopic retroperitoneal lymph node dissection in a patient with stage 1 testicular carcinoma. J Urol 1992;148:1907-9; discussion 1909-10.
- Rassweiler JJ, Scheitlin W, Heidenreich A, et al. Laparoscopic retroperitoneal lymph node dissection: does it still have a role in the management of clinical stage I nonseminomatous testis cancer? A European perspective. Eur Urol 2008;54:1004-15. [Crossref] [PubMed]
- Nielsen ME, Lima G, Schaeffer EM, et al. Oncologic efficacy of laparoscopic RPLND in treatment of clinical stage I nonseminomatous germ cell testicular cancer. Urology 2007;70:1168-72. [Crossref] [PubMed]
- Davol P, Sumfest J, Rukstalis D, et al. Robotic-assisted laparoscopic retroperitoneal lymph node dissection. Urology 2006;67:199. [Crossref] [PubMed]
- Williams SB, Lau CS, Josephson DY. Initial series of robot-assisted laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular cancer. Eur Urol 2011;60:1299-302. [Crossref] [PubMed]
- Cheney SM, Andrews PE, Leibovich BC, et al. Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int 2015;115:114-20. [Crossref] [PubMed]
- Stepanian S, Patel M, Porter JR. Robot-assisted Laparoscopic Retroperitoneal Lymph Node Dissection for Testicular Cancer: Evolution of the Technique. Eur Urol 2016;70:661-7. [Crossref] [PubMed]
- Hu B, Daneshmand S. Retroperitoneal Lymph Node Dissection as Primary Treatment for Metastatic Seminoma. Adv Urol 2018;2018:7978958. [Crossref] [PubMed]
- Kamel MH, Littlejohn N, Cox M, et al. Post-Chemotherapy Robotic Retroperitoneal Lymph Node Dissection: Institutional Experience. J Endourol 2016;30:510-9. [Crossref] [PubMed]
- Pearce SM, Golan S, Gorin MA, et al. Safety and Early Oncologic Effectiveness of Primary Robotic Retroperitoneal Lymph Node Dissection for Nonseminomatous Germ Cell Testicular Cancer. Eur Urol 2017;71:476-82. [Crossref] [PubMed]
- Tselos A, Moris D, Tsilimigras DI, et al. Robot-Assisted Retroperitoneal Lymphadenectomy in Testicular Cancer Treatment: a Systematic Review. J Laparoendosc Adv Surg Tech A 2018;28:682-9. [Crossref] [PubMed]
- Williams SB, McDermott DW, Winston D, et al. Morbidity of open retroperitoneal lymph node dissection for testicular cancer: contemporary perioperative data. BJU Int 2010;105:918-21. [Crossref] [PubMed]
- Subramanian VS, Nguyen CT, Stephenson AJ, et al. Complications of open primary and post-chemotherapy retroperitoneal lymph node dissection for testicular cancer. Urol Oncol 2010;28:504-9. [Crossref] [PubMed]
- Beck SD, Peterson MD, Bihrle R, et al. Short-term morbidity of primary retroperitoneal lymph node dissection in a contemporary group of patients. J Urol 2007;178:504-6; discussion 506. [Crossref] [PubMed]
- Bhayani SB, Ong A, Oh WK, et al. Laparoscopic retroperitoneal lymph node dissection for clinical stage I nonseminomatous germ cell testicular cancer: a long-term update. Urology 2003;62:324-7. [Crossref] [PubMed]
- Neyer M, Peschel R, Akkad T, et al. Long-term results of laparoscopic retroperitoneal lymph-node dissection for clinical stage I nonseminomatous germ-cell testicular cancer. J Endourol 2007;21:180-3. [Crossref] [PubMed]
- Poulakis V, Skriapas K, de Vries R, et al. Quality of life after laparoscopic and open retroperitoneal lymph node dissection in clinical Stage I nonseminomatous germ cell tumor: a comparison study. Urology 2006;68:154-60. [Crossref] [PubMed]
- Singh A, Chatterjee S, Bansal P, et al. Robot-assisted retroperitoneal lymph node dissection: Feasibility and outcome in postchemotherapy residual mass in testicular cancer. Indian J Urol 2017;33:304-9. [Crossref] [PubMed]
- Overs C, Beauval JB, Mourey L, et al. Robot-assisted post-chemotherapy retroperitoneal lymph node dissection in germ cell tumor: is the single-docking with lateral approach relevant? World J Urol 2018;36:655-61. [Crossref] [PubMed]
- Busch J, Magheli A, Erber B, et al. Laparoscopic and open postchemotherapy retroperitoneal lymph node dissection in patients with advanced testicular cancer--a single center analysis. BMC Urol 2012;12:15. [Crossref] [PubMed]
- Faria EF, Neves HS, Dauster B, et al. Laparoscopic Retroperitoneal Lymph Node Dissection as a Safe Procedure for Postchemotherapy Residual Mass in Testicular Cancer. J Laparoendosc Adv Surg Tech A 2018;28:168-73. [Crossref] [PubMed]
- Calestroupat JP, Sanchez-Salas R, Cathelineau X, et al. Postchemotherapy laparoscopic retroperitoneal lymph node dissection in nonseminomatous germ-cell tumor. J Endourol 2009;23:645-50. [Crossref] [PubMed]