
Robotic radical cystectomy with intracorporeal urinary diversion: beyond the initial experience
Introduction
Robotic assisted radical cystectomy (RARC) is an established and safe procedure within minimally invasive urologic oncology (1). Multiple studies, including prospective randomized clinical trials, have demonstrated similar oncologic outcomes and complication rates when comparing RARC to conventional open radical cystectomy (ORC) (2-6). More recently, the RAZOR trial, a randomized, open label phase-3 non inferiority study, demonstrated that RARC was non inferior to ORC with regards to 2-year progression free survival (7). Of note, all of the patients in these trials had an extracorporeal urinary diversion (ECUD). Completely intracorporeal urinary diversion (ICUD) was first described in 2003 (8). Though ICUD was initially performed in only 9% of cases in 2005, the most recent update by the International Robotic Cystectomy Consortium (IRCC) reports an increase to 97% in 2015 among their group (9). While a prospective randomized controlled trial comparing RARC with ICUD to ORC is enrolling, long-term outcomes following ICUD appear similar to historic open cohorts (4,10-12).
Reported benefits of ICUD have included decreased evaporative fluid losses and blood loss with faster return of bowel function and shorter hospital stays (13-15). However, ICUD poses its own challenges within the context of an already demanding, extirpative procedure. Herein, we describe the learning curve, technical points, and unique complications associated with ICUD.
Learning curve
Each surgical procedure has its own learning curve, and much of the trepidation in adoption of ICUD is derived from concerns regarding technical proficiency and perioperative morbidity; this is particularly true with intracorporeal orthotopic continent diversion (9,16). In 2010, the IRCC sought to define technical parameters for RARC, at which time the majority were performed with ECUD (17). Hayn and colleagues reported that 21 cases were needed to obtain operative times less than 6.5 hours and 30 cases were needed to obtain a lymph node yield of 20. Most importantly, they demonstrated that 30 cases were required to have an overall positive surgical margin rate of 5% or lower (17).
The most recent assessment by the IRCC revealed that in 2016, 81% of all urinary diversions in RARC are intracorporeal ileal conduits (IIC). Intracorporeal neobladder (IN) represented 17% of all diversions (9). In their report of transition from EC—ileal conduit to IIC (68 vs. 59 patients, respectively), Tan et al. noted shorter total operative times, blood loss and 30-day overall complication rate in the ICUD cohort (15). Moreover, they had noted a shorter total operative (300 vs. 360 min) in their last 29 ICUD cases compared to their first 30. Similar findings with respect to operative times and complication rate were reported by Porreca et al. in a retrospective analysis of the first 100 ICUD performed after completion of a strict, modular training program; ICUD included ileal conduit, orthotopic neobladder, and cutaneous ureterostomy (18).
Collins et al. prospectively evaluated the learning curve on the first 67 RARC with IN at their institution amongst two surgeons (14). In having to navigate the learning curve individually without the assistance of a more experienced surgeon, the “mentor” surgeon had significant improvements in operative time and overall complication rates amongst 47 patients. The “mentee” surgeon did not have statistically significant improvements in these parameters, though operative times did trend downwards. There were no differences in outcomes between the two surgeons apart from an average lower blood loss in the “mentee” (613 vs. 462 mL), which may be explained by higher blood loss in earlier cases performed by the “mentor.” This suggests that the learning curve of intracorporeal diversion can be abbreviated by the “mentee” working closely with a surgeon who has already overcome it and achieved mastery. In addition, the familiarity of the operative team with the procedure and ability to troubleshoot and bedside assist improves with each case. This mentorship practice model may facilitate bringing the reported advantages of ICUD to a larger population of patients.
At our institution, the mentor surgeon proctors up to the initial 50 cases depending on the mentee’s prior robotics experience. In addition, RARC with ICUD are often performed on the same day by the mentor and mentee surgeons in adjacent operating rooms to facilitate communication and operative assistance. The mentor surgeon is also notified and available for particularly challenging cases, such as in the post-radiation setting. There is a group scrub of nurses and technicians that participate almost exclusively in robotic cases. Laparoscopic assistance for the procedure is provided by a rotating cohort of junior residents and urologic oncology and minimally invasive surgery fellows.
The learning curve, in reality, likely extends beyond the initial estimates of approximately 30 cases. The IRCC has reported outcomes of 1094 ICUD over a 10-year period (9). They found that high grade complications in ICUD decreased significantly from 25% in 2005 to 6% in 2015, while a similar decrease in complications was not identified (13% in 2006 to 14% in 2015) in the ECUD cohort. The consortium also demonstrated shorter operative times with ICUD compared to ECUD, as well as lower blood loss.
A dedicated care pathway can also improve convalescence. Tan et al. evaluated the role of an enhanced recovery after surgery (ERAS) pathway in their transition from ORC, to RARC-ICUD without an ERAS protocol, and subsequently RARC-ICUD with an ERAS protocol (19). Despite having a higher American Society of Anesthesiologists score, the ERAS cohort had a significantly shorter median length of stay compared to the RARC non-ERAS group and the ORC group (7 vs. 11 vs. 17 days, respectively). The ERAS group also had significantly lower 90-day readmission rates.
Clearly, a committed effort to performing ICUD will allow the learning curve to be overcome, as has been demonstrated with robotic prostatectomy and robotic partial nephrectomy (20). Given the potential advantages when compared to ECUD or traditional ORC, initial trepidation regarding proficiency should not preclude its uptake.
Technical points
Port placement
The success of ICUD is predicated on adhering to the principles of open surgery, particularly for orthotopic ICUD; several authors have described their techniques (16,21-23). When compared to robotic prostatectomy, the ports in RARC are placed more cephalad to allow for extended pelvic lymph node dissection (LND), and manipulation of the afferent/proximal bowel limb for diversion (1,24). At our institution, we favor placing ports in a “W” configuration. This consists of a midline 12 mm camera port placed 5 cm cranial to the umbilicus, three 8mm robotic ports, and a 12 mm assistant port, usually as the right most port (24). Per surgeon preference, an additional 12 mm suprapubic assistant port can be placed to facilitate bowel reconstitution during ICUD. Of note, an emphasis on cephalad and medial placement of the right robotic port is critical to facilitate ICUD. The Karolinska group similarly places the camera port, though the remaining ports are positioned at the level of the umbilicus (1,25). The City of Hope group places ports 20 or 23 cm from the pubic symphysis, with an additional subcostal assistant port contralateral to the 4th robotic arm (1,21). In addition, robotic port configuration has been described for both the DaVinci Si and Xi robotic platforms (Intuitive Surgical, Sunnyvale, CA) by Pathak et al. and the Wake Forest group (26). Of note, they report that placing an additional 12 mm assistant port close to the midline facilitates bladder pedicle takedown during the extirpative component of the cystectomy. The group also describes supine side-docking of the Xi robot as opposed to positioning between the legs in Trendelenburg, which can eliminate risks of ophthalmic, pulmonary and positional nerve injury. Moreover, the use of the TruSystemTM OR Table (Trumpf Medical, Saalfeld, Germany) allows for position changes without the need to re-dock if a hybrid approach is used.
IIC
Our group is able to perform IIC with a standard 5 port configuration. After the extirpative component of the procedure, the left ureter is brought under the sigmoid colon mesentery. Stay stitches are placed on the proximal and distal segments of a 15 cm segment of ileum 15cm proximal to the ileocecal valve. An avascular segment of the mesentery is taken down at the proximal and distal aspect, and a 60 mm load of the Endo-GIA stapler (Covidien, Dublin, Ireland) is inserted through the 12 mm right most assistant port to divide the ileal loop. We then reconstitute the bowel prior to the uretero-ileal anastomosis (UIA). After ensuring proper orientation with the use of stay stiches, two 60 mm Endo-GIA staple loads are used to complete the side-to-side anastomosis. One staple load is used for the transverse component. The UIA are performed in Bricker fashion with two running 4-0 Vicryl sutures (Ethicon, Summerville, USA) beginning at the apex of the spatulated ureter. 8 Fr × 30 cm double J ureteral stents are placed directly into the ureter half-way through each anastomosis. Once they have reached the upper tract, the distal coil is then positioned within the conduit, and the anastomosis is completed. The stents are not externalized. The stoma is then matured after specimen extraction.
The Roswell Park group has previously described the “Marionette” technique for IIC (23,27). Key differences in their approach are as follows. Initially, a silk stay stitch is passed through the abdominal wall, inferior to the site of the future stoma, through the distal end of the conduit and back through the abdominal wall. This stitch is not tied, rather it is manipulated by the instrument arm like a “marionette” throughout the remainder of the procedure. Ileal loop isolation is performed similar to our technique, though bowel reconstitution is performed after the UIA. A Bricker UIA is performed. Stent placement is facilitated through a suction tip, which is passed via the assistant port through a distal enterotomy. It is maneuvered through conduit to the site of the UIA. Single-J ureteral stents are then placed via the suction tube, sutured into position, and the suction tip is subsequently removed. Bowel continuity is then re-established though the placement of an additional suprapubic port. The stoma is then matured.
Intracorporeal continent cutaneous diversion—Indiana pouch
Desai et al. have reported on a series of 10 patients undergoing continent cutaneous (Indiana Pouch) ICUD and discuss several components unique to this form of diversion (28). Mainly, working on the right colon requires the addition of three ports and upsizing another, along with undocking and redocking the robot. If utilizing the DaVinci Si console (Sunnyvale, USA), the patient must also be repositioned to the right side up position, which can be avoided with the DaVinci Xi. Their oncologic outcomes and complication rates were similar to that reported in other RARC literature, but they do recommend other ICUD experience prior to undertaking Indiana Pouch formation. Moreover, they suggested that surmounting the learning curve may be more challenging than other ICUD given that Indiana Pouch formation is a less common type of diversion.
IN
A variety of techniques have been used to describe IN (1,16,25,29). Using the Idea, Development, Exploration, Assessment, Long-term follow-up (IDEAL) Collaboration guidelines, the University of Florence group reported on a series of 18 patients with an emphasis on reconstruction of a “neo-trigone” with “orthotopic” UIA (29). After isolation of a 50 cm ileal loop, the urethroileal anastomosis is performed, leaving 20 cm of bowel proximal and 30cm distal to the anastomosis resulting in an asymmetric U. The ileum is then detubularized and the posterior plate is reconfigured as an “L”, effectively creating an orthotopic neo-trigone. The bladder neck is reconfigured anteriorly and the posterior plate is then folded anteriorly to close the defect. The ureters are then spatulated and re-anastomosed at the neo-trigone in Bricker fashion. Of note, this neobladder does not contain an afferent limb.
The Karolinska group also favors performing the urethroileal anastomosis prior to bowel isolation and reconstitution (1,25). The dependent portion of 50 cm of ileum is isolated, of which the distal 40 cm is detubularized. They also perform rotation and double folding to obtain a more spherical reservoir, and do leave a 10 cm aperistaltic afferent limb upon which the ureters are anastomosed in Wallace fashion.
The USC group first isolates approximately 60 cm loop of ileum, of which the proximal 15 cm becomes the afferent limb (16,21). The distal ileum is then detubularized. The posterior plate is reconstructed and then rotated 90 degrees counter clockwise prior to performing the urethroileal anastomosis at the midpoint of the distal, rotated edge. The anterior closure is then performed by cross-folding, and the ureteroileal anastomosis is performed in Bricker fashion.
Obtaining a tension free urethroileal anastomosis can be among the most challenging components of IN. We have described several maneuvers to facilitate completion of this step, apart from careful ileal loop selection (24). This includes, in a step-wise approach, the following: maximizing urethral length at the prostatic apex, removing the sigmoid colon out of the pelvis after the extirpative portion of the procedure, reduction of pneumoperitoneum and decreasing steep Trendelenburg, application of perineal pressure, utilizing barbed suture and detubularizing the ileal loop closer to the mesentery to lengthen the posterior plate. As a last resort, incising the mesentery and releasing the mesenteric fat can produce additional length. Of note, we previously performed our urethroileal anastomosis prior to loop detubularization, but now favor anchoring the posterior bowel/neobladder wall to the posterior urethral plate as seen in Figure 1. This modification was made as we found that it was easier to tension the initial urethroileal anastomotic sutures. Figure 2 demonstrates the Bricker ureteroileal anastomosis. Figure 3 displays anchoring of the suprapubic catheter after completion of the urethral anastomosis, closure of the anterior neobladder wall and ureteroileal anastomosis completion.
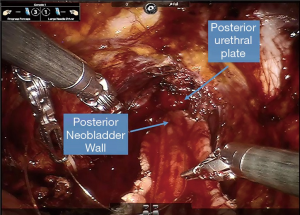
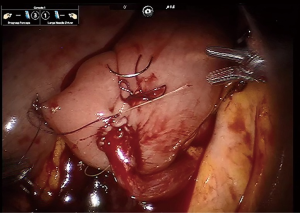
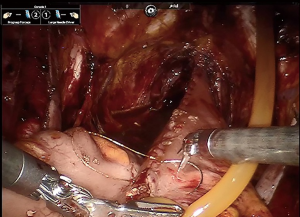
Image guided surgery
The use of indocyanine green can facilitate may components of RARC and ICUD including tumor identification, sentinel lymph node drainage, mesenteric angiography during bowel transection and reconstitution, aid left ureteral tunneling under the sigmoid mesentery, identifying ischemic ureteric segments during anastomosis and verifying non-ischemic enteroenteric anastomosis (30,31). Manny and Hemal reported on a series of 10 patients undergoing fluorescence-enhanced RARC, of which 8 underwent IIC. Mesenteric angiography with identification of bowel arcades prior to bowel stapling was successful in all 8 of the patients undergoing IIC, with vascular identification occurring at a median time of less than 1 min (30). No short term complications relating to anastomotic stricture or stomal stenosis were noted in short term follow up.
Surgery related complications
Radical cystectomy remains among the more morbid procedures in urology due to the risk factors for bladder cancer and the resulting patient comorbidities, as well as the extent of surgery with a urinary diversion (32).
Novara et al. have performed a systematic review and meta-analyses of RARC with ICUD and ECUD (33). In the subset analysis of ICUD, the overall 30 day complication rate was 67% (range, 42–86%) for ileal conduit and 46% (range, 43–62%) for neobladder with high grade complication rates of 24% (range, 0–54%) and 28% (15–33%), respectively (33). Mortality rates ranged from 0–3% across ICUD.
LND can confer a survival benefit in patients with bladder cancer, and while the optimum extent of dissection is still debated, its diagnostic and prognostic value is not (34,35). LND, however, is not without morbidity. In their analysis of ICUD, Schumacher et al. detected a 12% 30 day complication incidence related to LND (36). Of the 5 patients with LND related complications, 3 developed a lymphocele, 1 developed iliac vessel bleeding, and another had deep venous thrombus formation.
A theoretical advantage of ICUD is a decreased risk of distal ureteral ischemia and subsequent ureteral leak or stricture given the shorter length of ureter required when compared to ECUD or ORC. The reported benign anastomotic stricture rate in large ORC series is between 3–10% (37,38). Anderson et al. (39) compared ORC to RARC-ECUD and noted a stricture rate of 8.5 vs. 12.6%, respectively (P=0.2). It seems appropriate that the stricture rate would be similar, given that the ureteral length required for the diversion is similar in both arms. In comparison, review of series of ICUD with a minimum of 100 patients demonstrate a UIA stricture or leak rate ranging from 2–3.8% (16,23,32). Schumacher et al. reported 2 UIA strictures (4.4%) successfully managed with balloon dilation in their cohort of 45 patients undergoing ICUD (36). The use of ICG, particularly antegrade via percutaneous nephrostomy tube if in place, can identify ischemic areas of the distal ureter prior to anastomosis due to lack of fluorescence (31).
The need for intraoperative or perioperative blood transfusion in patients undergoing radical cystectomy has been previously identified as an independent risk factor for overall mortality and high grade complications in the ORC and RARC literature (32,40-42). ICUD has been shown to be associated with less blood loss compared to ECUD. Interestingly, the IRCC noted a significantly decreased rate of blood transfusion in the ICUD cohort compared to ECUD (4% vs. 19%), though there was a small but statistically significant increase in incidence of high grade complications in the ICUD group (13 vs. 10%, P=0.02) (9). This is likely attributed to high grade complications occurring more frequently early in the learning curve, as high grade complication rate decreased with time in the ICUD cohort but not in the ECUD group. Of note, ICUD was not an independent predictive factor of high grade complications in their assessment.
Conclusions
RARC with ICUD is a minimally invasive alternative to conventional ORC. ICUD is technically demanding though the learning curve can be surmounted with consistent exposure to the procedure. Variations in technique exist, though non-continent, continent cutaneous and orthotopic continent diversions have all been reported with acceptable oncologic and functional outcomes. Overall complication rates are similar to ECUD and ORC. Potential advantages of ICUD include decreased rates of intraoperative blood transfusion and distal ureteral ischemia along with faster convalescence. An ongoing prospective, randomized clinical trial comparing RARC with ICUD to ORC will help clarify these benefits.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Ashok K. Hemal) for the series “Robotic-assisted Urologic Surgery” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: The series “Robotic-assisted Urologic Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chan KG, Guru K, Wiklund P, et al. Robot-assisted Radical Cystectomy and Urinary Diversion: Technical Recommendations from the Pasadena Consensus Panel. Eur Urol 2015;67:423-31. [Crossref] [PubMed]
- Bochner BH, Dalbagni G, Marzouk KH, et al. Randomized Trial Comparing Open Radical Cystectomy and Robot-assisted Laparoscopic Radical Cystectomy: Oncologic Outcomes. Eur Urol 2018;74:465-71. [Crossref] [PubMed]
- Khan MS, Gan C, Ahmed K, et al. A Single-centre Early Phase Randomised Controlled Three-arm Trial of Open, Robotic, and Laparoscopic Radical Cystectomy (CORAL). Eur Urol 2016;69:613-21. [Crossref] [PubMed]
- Tan WS, Sridhar A, Ellis G, et al. Analysis of open and intracorporeal robotic assisted radical cystectomy shows no significant difference in recurrence patterns and oncological outcomes. Urol Oncol 2016;34:257.e1-257.e9. [Crossref] [PubMed]
- Raza SJ, Wilson T, Peabody JO, et al. Long-term oncologic outcomes following robot-assisted radical cystectomy: results from the International Robotic Cystectomy Consortium. Eur Urol 2015;68:721-8. [Crossref] [PubMed]
- Menon M, Hemal AK, Tewari A, et al. Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion. BJU Int 2003;92:232-6. [Crossref] [PubMed]
- Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet 2018;391:2525-36. [Crossref] [PubMed]
- Beecken WD, Wolfram M, Engl T, et al. Robotic-assisted laparoscopic radical cystectomy and intra-abdominal formation of an orthotopic ileal neobladder. Eur Urol 2003;44:337-9. [Crossref] [PubMed]
- Hussein AA, May PR, Jing Z, et al. Outcomes of Intracorporeal Urinary Diversion after Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. J Urol 2018;199:1302-11. [Crossref] [PubMed]
- Brassetti A, Cacciamani G, Anceschi U, et al. Long-term oncologic outcomes of robot-assisted radical cystectomy (RARC) with totally intracorporeal urinary diversion (ICUD): a multi-center study. World J Urol 2019. [Epub ahead of print]. [PubMed]
- Catto JWF, Khetrapal P, Ambler G, et al. Robot-assisted radical cystectomy with intracorporeal urinary diversion versus open radical cystectomy (iROC): protocol for a randomised controlled trial with internal feasibility study. BMJ Open 2018;8:e020500. [PubMed]
- Sandberg JM, Hemal AK. Robot-assisted laparoscopic radical cystectomy with complete intracorporeal urinary diversion. Asian J Urol 2016;3:156-66. [Crossref] [PubMed]
- Wilson TG, Guru K, Rosen RC, et al. Best Practices in Robot-assisted Radical Cystectomy and Urinary Reconstruction: Recommendations of the Pasadena Consensus Panel. Eur Urol 2015;67:363-75. [Crossref] [PubMed]
- Collins JW, Tyritzis S, Nyberg T, et al. Robot-assisted radical cystectomy (RARC) with intracorporeal neobladder – what is the effect of the learning curve on outcomes? BJU Int 2014;113:100-7. [Crossref] [PubMed]
- Tan WS, Kelly JD. Is experience with extracorporeal urinary diversion following robotic assisted radical cystectomy necessary before transitioning to intracorporeal urinary diversion? Transl Androl Urol 2018;7:S735-7. [Crossref] [PubMed]
- Desai MM, Gill IS, de Castro Abreu AL, et al. Robotic Intracorporeal Orthotopic Neobladder during Radical Cystectomy in 132 Patients. J Urol 2014;192:1734-40. [Crossref] [PubMed]
- Hayn MH, Hussain A, Mansour AM, et al. The Learning Curve of Robot-Assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. Eur Urol 2010;58:197-202. [Crossref] [PubMed]
- Porreca A, Mineo Bianchi F, Romagnoli D, et al. Robot-assisted radical cystectomy with totally intracorporeal urinary diversion: surgical and early functional outcomes through the learning curve in a single high-volume center. J Robot Surg 2019. [Epub ahead of print]. [PubMed]
- Tan WS, Tan MY, Lamb BW, et al. Intracorporeal robot-assisted radical cystectomy, together with an enhanced recovery programme, improves postoperative outcomes by aggregating marginal gains. BJU Int 2018;121:632-9. [Crossref] [PubMed]
- Abboudi H, Khan MS, Guru KA, et al. Learning curves for urological procedures: a systematic review. BJU Int 2014;114:617-29. [Crossref] [PubMed]
- Goh AC, Gill IS, Lee DJ, et al. Robotic Intracorporeal Orthotopic Ileal Neobladder: Replicating Open Surgical Principles. Eur Urol 2012;62:891-901. [Crossref] [PubMed]
- Tan WS, Lamb BW, Kelly JD. Evolution of the neobladder: A critical review of open and intracorporeal neobladder reconstruction techniques. Scand J Urol 2016;50:95-103. [Crossref] [PubMed]
- Azzouni FS, Din R, Rehman S, et al. The First 100 Consecutive, Robot-assisted, Intracorporeal Ileal Conduits: Evolution of Technique and 90-day Outcomes. Eur Urol 2013;63:637-43. [Crossref] [PubMed]
- Almassi N, Zargar H, Ganesan V, et al. Management of Challenging Urethro-ileal Anastomosis During Robotic Assisted Radical Cystectomy with Intracorporeal Neobladder Formation. Eur Urol 2016;69:704-9. [Crossref] [PubMed]
- Jonsson MN, Adding LC, Hosseini A, et al. Robot-Assisted Radical Cystectomy with Intracorporeal Urinary Diversion in Patients with Transitional Cell Carcinoma of the Bladder. Eur Urol 2011;60:1066-73. [Crossref] [PubMed]
- Pathak RA, Patel M, Hemal AK. Comprehensive Approach to Port Placement Templates for Robot-Assisted Laparoscopic Urologic Surgeries. J Endourol 2017;31:1269-76. [Crossref] [PubMed]
- Guru K, Seixas-Mikelus SA, Hussain A, et al. Robot-assisted Intracorporeal Ileal Conduit: Marionette Technique and Initial Experience at Roswell Park Cancer Institute. Urology 2010;76:866-71. [Crossref] [PubMed]
- Desai MM, Simone G, de Castro Abreu AL, et al. Robotic Intracorporeal Continent Cutaneous Diversion. J Urol 2017;198:436-44. [Crossref] [PubMed]
- Minervini A, Vanacore D, Vittori G, et al. Florence robotic intracorporeal neobladder (FloRIN): a new reconfiguration strategy developed following the IDEAL guidelines. BJU Int 2018;121:313-7. [Crossref] [PubMed]
- Manny TB, Hemal AK. Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography, tumor marking, and mesenteric angiography: the initial clinical experience. Urology 2014;83:824-9. [Crossref] [PubMed]
- Pathak RA, Hemal AK. Intraoperative ICG-fluorescence imaging for robotic-assisted urologic surgery: current status and review of literature. Int Urol Nephrol 2019;51:765-71. [Crossref] [PubMed]
- Tan WS, Lamb BW, Tan MY, et al. In-depth Critical Analysis of Complications Following Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion. Eur Urol Focus 2017;3:273-9. [Crossref] [PubMed]
- Novara G, Ficarra V, Rosen RC, et al. Systematic Review and Meta-analysis of Perioperative Outcomes and Complications After Robot-assisted Radical Prostatectomy. Eur Urol 2012;62:431-52. [Crossref] [PubMed]
- Skinner DG. Management of Invasive Bladder Cancer: A Meticulous Pelvic Node Dissection Can Make a Difference. J Urol 1982;128:34-6. [Crossref] [PubMed]
- Gschwend JE, Heck MM, Lehmann J, et al. Extended Versus Limited Lymph Node Dissection in Bladder Cancer Patients Undergoing Radical Cystectomy: Survival Results from a Prospective, Randomized Trial. Eur Urol 2019;75:604-11. [Crossref] [PubMed]
- Schumacher MC, Jonsson MN, Hosseini A, et al. Surgery-related Complications of Robot-assisted Radical Cystectomy With Intracorporeal Urinary Diversion. Urology 2011;77:871-6. [Crossref] [PubMed]
- Shimko MS, Tollefson MK, Umbreit EC, et al. Long-Term Complications of Conduit Urinary Diversion. J Urol 2011;185:562-7. [Crossref] [PubMed]
- Katherine AA, Emily AV, Gillian S, et al. Predictors of Benign Uretero-enteric Anastomotic Strictures After Radical Cystectomy and Urinary Diversion. Urology. 2018. [Crossref] [PubMed]
- Anderson CB, Morgan TM, Kappa S, et al. Ureteroenteric anastomotic strictures after radical cystectomy-does operative approach matter?. J Urol 2013;189:541-7. [Crossref] [PubMed]
- Morgan TM, Barocas DA, Chang SS, et al. The relationship between perioperative blood transfusion and overall mortality in patients undergoing radical cystectomy for bladder cancer. Urol Oncol 2013;31:871-7. [Crossref] [PubMed]
- Moschini M. Effect of Allogeneic Intraoperative Blood Transfusion on Survival in Patients Treated With Radical Cystectomy for Nonmetastatic Bladder Cancer: Results From a Single High-Volume Institution. Clin Genitourin Cancer 2015;13:562-7. [Crossref] [PubMed]
- Ahmed K, Khan SA, Hayn MH, et al. Analysis of Intracorporeal Compared with Extracorporeal Urinary Diversion After Robot-assisted Radical Cystectomy: Results from the International Robotic Cystectomy Consortium. Eur Urol 2014;65:340-7. [Crossref] [PubMed]


