Surgical strategies for postchemotherapy testis cancer
Introduction
The surgical resection of disseminated disease has been an important component in the care of patients with testis cancer since the early 1900s (1). The utilization of and indications for retroperitoneal lymph node dissection (RPLND) have changed dramatically since the introduction of cisplatin based chemotherapy. Over 90% of patients with metastases are now cured, but a third will have residual disease after chemotherapy, thus necessitating the continued use of surgery to provide long-term survival (2).
The current indications for postchemotherapy (PC) resection vary by histology. The goal of surgery in those with nonseminomatous germ cell tumors (NSGCT) is to completely resect viable tumor or teratoma. Indications include residual masses ≥1 cm, residual disease after salvage chemotherapy, chemoresistant disease, late relapse of disease and residual masses not responding to salvage chemotherapy that remain resectable (3). The indications for PC resection in seminoma differ as 80–90% of residual masses are found to contain necrosis or fibrosis and there is no concern for residual teratoma (4,5) An intense desmoplastic reaction or scarring in the retroperitoneum makes PC RPLND for seminoma a difficult procedure with high risk of morbidity and need for adjunctive procedures. The indications for PC RPLND in seminoma are therefore more limited and include resection of growing residual masses without marker elevation or cases with solitary, resectable tissue after salvage chemotherapy (6).
There is ample evidence to suggest that experience is extremely important in the management of testis cancer following chemotherapy (2). A recent query of patients treated for testis cancer in the National Cancer Database found that overall survival is almost 10% lower (81% vs. 90%) at low-volume compared to high-volume hospitals, despite more favorable disease characteristics at the lower volume hospitals (7). Complication rates after RPLND have historically been as high as 30% with high-grade complications of up to 20% (8,9). Moreover, risks of complication and prolonged hospital say are higher with PC RPLND as compared to primary RPLND (10,11). PC RPLND is a challenging operation that requires complete resection in order to provide the greatest chance of cure. This requires surgeons to have an intimate familiarity with abdominal and retroperitoneal anatomy. Surgeons must also be comfortable with vascular techniques and be prepared for adjunctive procedures as necessary. Herein, we review the imaging options that are essential for surgical planning and the various surgical techniques that are often necessary in the challenging PC testis cancer surgery.
Imaging
The indications for surgery in PC NSGCT and seminoma rely on the radiographic identification of residual disease. In NSGCT, there is a risk of teratoma in 40–45% and viable malignancy in 10–15% of residual masses larger than 1 cm (12). Teratoma is chemoresistant with potential for growth, local destruction and ultimately death (13). Unpredictable somatic transformation to malignant histology is another concern with unresected teratoma (14). Advanced imaging with positron emission tomography (PET) in addition to routine computed tomography (CT) has been utilized to try to differentiate teratoma, viable malignancy and fibrosis/necrosis after chemotherapy for NSGCT. Unfortunately, PET scans have false negative rates up to 40% for NSGCT and can not distinguish between teratoma and necrosis, which have equivalent enhancement values (15). Thus, a CT scan is sufficient in the preoperative assessment of patients with NSGCT after chemotherapy and PET should not be used, as negative scans will not obviate the need for resection.
However, there is some evidence for the role of PET/CT in seminoma after chemotherapy. Several studies, including results from the prospective SEMPET trial have suggested sensitivities and specificities ranging from 78–82% and 90–100%, respectively, for PET in detecting viability of seminoma after chemotherapy (16-20). In contrast, the sensitivity and specificity of CT alone have both been reported between 73–100% for detecting viable seminoma in residual masses greater than 3 cm (17,18). While the earliest study of PET in PC seminoma reported a positive predictive value (PPV) of 100% (17), a later systematic review and meta-analysis found the PPV to be 56% (19). The concern for false positives is usually attributed to inflammation if scans are done less than 6 weeks after completion of chemotherapy or non-specific uptake in normal abdominal or retroperitoneal locations (21). A recent study by Cathomas et al. has brought further question to the utility of PET in PC seminoma (22). They included 90 patients with metastatic seminoma and PET positive lesions. The median residual tumor size was 4.9 cm and the median time from last dose of chemotherapy to PET scan was 6.9 weeks. Observation of PET positive lesions was performed in 51 (57%) patients, 26 (29%) had surgical resection, 9 (10%) were biopsied, and 4 (4%) received radiation therapy. Despite the enhancement on PET scan, resected lesions showed necrosis in 21 (81%) and viable seminoma in only 5 (19%). None of the biopsied masses showed seminoma. This revealed a PPV for PET scan of 23% in predicting viable seminoma. Of the 51 patients undergoing surveillance, progression occurred in 11 (22%) and relapse in 2 (4%) after resection. These results are in line with practice at our institution, where we feel it is safe to monitor most PC seminoma with CT scans with indications for surgery remaining growing residual masses with negative markers or residual but resectable lesions after salvage chemotherapy.
Nerve sparing and template guided dissections
Classic descriptions of RPLND included removal of all nodal tissue in the retroperitoneum, from the suprahilar region down to the common iliac vessels bilaterally (1). This was irrespective of imaging and the sympathetic chain and post-sympathetic nerves that are responsible for antegrade ejaculation (23). It wasn’t until separate publications by Jewett in 1988 and Donahue in 1990 that it became clear that preservation of the post-ganglionic sympathetic nerves with a full bilateral dissection could preserve antegrade ejaculation while still providing excellent cancer control (24,25). At the same time, it was well known from mapping studies that the spread of disease in testis cancer occurs in a predictable pattern with right sided tumors spreading to the paracaval and interaortocaval regions first with rare right to left cross over and left sided tumors primarily spreading to the preaortic and paraaortic regions (26-28). A desire to decrease the morbidity of RPLND and preserve ejaculation thus led to the development of modified templates that called for dissection only of the ipsilateral side of tumor involvement. Initially favored only in the primary setting before chemotherapy (29), feasibility in the PC setting has been shown. Beck et al. reported on 100 patients who underwent PC RPLND with a unilateral modified template. Right sided modified template dissection was performed in 43 patients. This included removal of the right common iliac nodes medial to the ureter, paracaval, precaval and interaortocaval nodes. A left sided modified template was performed in 39 patients and included removal of the preaortic, paraaortic and left common iliac nodes. A “full” left modified template was performed in 18 patients and included removal of the interaortocaval nodes in addition to those in a left modified template. This was a well-selected cohort as all patients had NSGCT, normal serum tumor markers after chemotherapy and metastatic disease that was limited to the primary landing zone. At a median follow up of 31.9 months, the 2- and 5-year disease free survival was 95% with only four patients developing recurrent disease. All recurrences were outside the boundaries of a full bilateral template RPLND (30). Cho et al. later reported on the long term follow up of this cohort with a median follow up 125 months. Only 3 additional patients developed recurrence and they were again all outside the boundaries of a full bilateral template RPLND. Ten year overall survival in the series was 99% (31). Still, some advocate for full bilateral dissection in the postchemotherapy setting due to concerns of incomplete resection and an ability to preserve antegrade ejaculation with bilateral nerve sparing when possible. Carver et al. reported on their experience with 269 patients who had viable tumor after postchemotherapy RPLND for NSGCT. Their full dissection included infrahilar removal of precaval, paracaval, interaortocaval, preaortic, paraortic and ipsilateral common iliac nodes. They found that 7–32% of patients had evidence of extratemplate retroperitoneal disease, depending on the boundaries of modified template used. The rate of extratemplate disease was 29% for residual masses up to 5 cm when using the modified template as defined by Beck et al. and Cho et al. (32). Based on these results, we favor a modified “extended” template PC RPLND in select patients without bulky residual disease that is limited to the primary landing zones. On the right side this would include all peri renal hilar, paracaval, inter aorto-caval and para-aortic nodes down to the inferior mesenteric artery. On the left it includes all of the left renal hilar, para-aortic, interaortocaval and retrocaval areas. Nerve sparing is performed whenever possible. In a recent report of our experience with post chemotherapy resection in masses with a median size of 3.65 cm, nerve sparing was accomplished in 74% of cases (33).
Extraretroperitoneal resection
While modified templates are feasible for patients with disease in a unilateral retroperitoneal landing zone, up to 40% of patients with metastatic disease may have disease outside the standard boundaries of RPLND (34). Again, a complete resection should always be the goal in the management of PC testis cancer and this should include resection of extraretroperitoneal masses whenever possible. This should be done regardless of pathology in the retroperitoneum, as extraretroperitoneal pathology may be discordant up to 30% of the time (35).
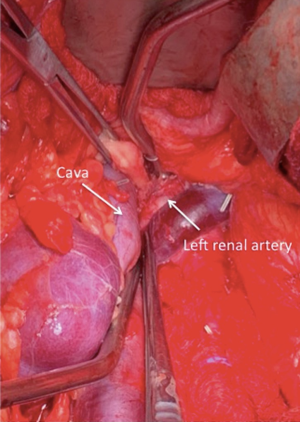
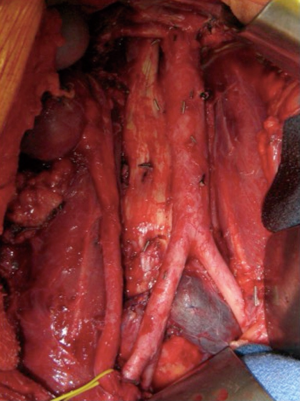
The spread of metastases typically follows lymphatics from the retroperitoneum to the cysterna chili and through the posterior mediastinum to the thoracic duct and ultimately the junction of the subclavian and internal jugular veins (36). Therefore, common extraretroperitoneal sites of disease include the suprahilar regions, retrocrural space or posterior mediastinum and cervical nodal region. Concurrent resection at the time of PC RPLND is feasible for residual masses in all of these locations. We have performed resection of suprahilar and retrocrural nodes through a thoracoabdominal approach, a separate thoracotomy, but also through the same midline incision as RPLND. After dissection of the suprahilar region, the crus of the diaphragm is identified and split to enter the posterior mediastinum (Figure 1). Concurrent resection of cervical nodes can also be performed at the same time as RPLND with a two-team approach as we have previously described (37).
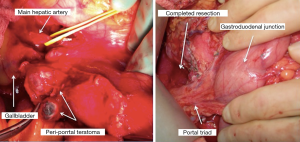
Pulmonary and hepatic metastases are also common and may be resected at the same time as RPLND, often with a thoracoabdominal incision (38). However, we prefer to defer these more morbid resections in case retroperitoneal pathology determines a need for further chemotherapy. We have occasionally performed peri-portal and mesenteric lymphadenectomy at the same time as RPLND (Figures 2,3).
Pelvic nodal metastases are rare in testis cancer but can occur almost 2% of the time and more commonly in patients with a prior history of groin surgery (39,40). A study by Jacob et al. of 134 patients with NSGCT and residual disease in the pelvis found a viable tumor rate of 20% and teratoma rate of 55% (39). Clearly, if present on preoperative imaging, resection of pelvic nodes is essential to prevent disease recurrence.
Adjunctive procedures
Surgeons performing PC RPLND must be prepared for the need for adjunctive procedures, including nephrectomy and vascular reconstruction, in up to 30% of cases (41,42). The risk of such procedures comes from the need for a complete resection coupled with the intense desmoplastic reaction that can occur after chemotherapy. Rarely other procedures such as bowel resection or ureteral resection and repair are needed.
A review from our institution of 85 patients undergoing PC RPLND found 13 (15%) who required vascular procedures including cavotomy, caval resection, aortic resection, common iliac vessel resection and renal vessel resection or reimplantation. Nephrectomy was performed in 12 (14%) patients and almost all (10/12) were left sided. One patient had ureteral resection with appendiceal substitution and one had partial duodenectomy (42).
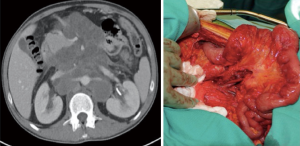
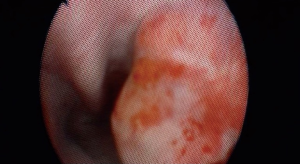
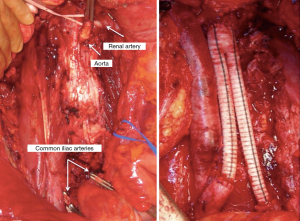
Several risk factors exist for adjunctive surgery, including elevated markers and risk group, but residual tumor size is the greatest risk factor. A residual tumor size greater 10 cm has been shown to carry an odds ratio of 7.2 in predicting the need for any adjunctive procedure (41). Preoperative imaging may show circumferential vessel involvement. A recent report found that circumferential involvement of the cava >135 degrees and of the aorta >330 degrees predicts the need for resection or reconstruction (43). We have utilized intraoperative vena cavoscopy using a flexible cystoscope to either rule out caval involvement or to confirm complete tumor thrombus resection (Figure 4). We typically favor resection of the vena cava when involved as long-standing obstruction leads to the development of well-established venous collaterals (Figure 5) (44). However, aortic resection obviously does require grafting so coordination with vascular surgeons is important and must be anticipated preoperatively (Figure 6).
Modern efforts to minimize morbidity
While nerve-sparing techniques and modified templates are examples of two early attempts to minimize the morbidity of RPLND, several modern efforts have also been made. Minimally invasive techniques have been employed in the PC setting with proposed benefits of decreased pain, blood loss and hospital stay. The largest series of laparoscopic PC RPLND comes from Steiner et al. (45). They performed surgery in 100 patients who were well selected to exclude any with bulky residual masses (median 1.4 cm) or with encasement of the great vessels. Results were favorable as median estimated blood loss was 84 cc, median hospital stay 3.9 days and 95% had preserved antegrade ejaculation. The majority of these patients however had residual masses <1 cm (57%) and would not meet the indications for any surgery in most centers. One patient required conversion to open surgery and only one had recurrence. Though the series were smaller, Cheney et al. and Kamel et al. have each shown the feasibility of robotic assisted PC-RPLND (46,47). The series by Cheney et al. included 9 patients undergoing PC-RPLND. They had a median of 18 nodes removed, estimated blood loss of 313 cc and length of stay 2.2 days with 2 cases converted to an open procedure. Kamel et al. evaluated 12 PC-RPLNDs and found similar blood loss (mean 475 cc) and hospital stays (mean 3.2 days). Only one patient was converted to an open procedure and there were no recurrences at a median follow up of 31 months. A more recent report of 13 patients undergoing robotic PC-RPLND by Singh et al. (48) included 12 unilateral cases and one bilateral that required repositioning. The median length of stay in the series was 4 days and median node count was 20. Final pathology showed necrosis only in 10 patients and 4 developed a chyle leak.
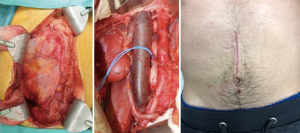
We have developed and reported on our novel approach to RPLND. This involves a midline extraperitoneal (EP) incision that aims to minimize the morbidity of a transabdominal approach. Our incision starts several centimeters below the xiphoid process and extends 4–5 cm below the umbilicus (Figure 6). The extraperitoneal space between the peritoneum and transversalis fascia is developed first in the inferior portion of the incision and carried cephalad over Gerotas fascia so that the retroperitoneal dissection template can be exposed (Figure 7). From this incision we are able to perform either modified or full bilateral template dissections that can include suprahilar and retrocrural dissections. A recent report of our experience with 131 cases from 2010–2017 included 72 patients undergoing EP PC RPLND. The median residual mass size was 3.65 cm and nerve sparing was performed in 53 (74%) of cases. Median estimated blood loss was 475 cc with a median of 32 nodes removed. Adjunctive procedures were performed in 31% of cases and the median hospital stay was 3 days with 90 day complications occurring in 4 (4.5%) of patients (33). Patients are given clear liquids on postoperative day 0 and advanced to a regular diet the next day, with some being able to go home on postoperative day 1 regardless of bowel activity given the absence of complication of ileus.
Conclusions
The surgical management of disseminated testis cancer has always been and will remain essential to provide long-term survival. Cisplatin-based chemotherapy has changed the indications for PC RPLND, but surgical resection remains essential. The surgical management of PC testis cancer is complex and challenging. With strict indications for surgery, adequate preoperative assessment and surgeon familiarity with the need for extraretroperitoneal resection, adjunctive procedures and methods to minimize morbidity, both excellent functional and oncologic outcomes can be obtained.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Chevassu MA. Tumeurs du testicule 1906.
- Tandstad T, Kollmannsberger CK, Roth BJ, et al. Practice Makes Perfect: The Rest of the Story in Testicular Cancer as a Model Curable Neoplasm. J Clin Oncol 2017;35:3525-8. [Crossref] [PubMed]
- Ghodoussipour S, Daneshmand S. Postchemotherapy Resection of Residual Mass in Nonseminomatous Germ Cell Tumor. Urol Clin North Am 2019;46:389-98. [PubMed]
- Flechon A, Bompas E, Biron P, et al. Management of post-chemotherapy residual masses in advanced seminoma. J Urol 2002;168:1975-9. [Crossref] [PubMed]
- Ravi R, Ong J, Oliver RT, et al. The management of residual masses after chemotherapy in metastatic seminoma. BJU Int 1999;83:649-53. [Crossref] [PubMed]
- National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Testicular Cancer (Version 1.2019).
- Hugen CM, Hu B, Jeldres C, et al. Utilization of retroperitoneal lymph node dissection for testicular cancer in the United States: Results from the National Cancer Database (1998-2011). Urol Oncol 2016;34:487.e7-487.e11. [Crossref] [PubMed]
- Staubitz WJ, Early KS, Magoss IV, et al. Surgical management of testis tumor. J Urol 1974;111:205-9. [Crossref] [PubMed]
- Sago AL, Ball TP, Novicki DE. Complications of retroperitoneal lymphadenectomy. Urology 1979;13:241-3. [Crossref] [PubMed]
- Macleod LC, Rajanahally S, Nayak JG, et al. Characterizing the Morbidity of Postchemotherapy Retroperitoneal Lymph Node Dissection for Testis Cancer in a National Cohort of Privately Insured Patients. Urology 2016;91:70-6. [Crossref] [PubMed]
- Subramanian VS, Nguyen CT, Stephenson AJ, et al. Complications of open primary and post-chemotherapy retroperitoneal lymph node dissection for testicular cancer. Urol Oncol 2010;28:504-9. [Crossref] [PubMed]
- Shayegan B, Carver BS, Stasi J, et al. Clinical outcome following post-chemotherapy retroperitoneal lymph node dissection in men with intermediate- and poor-risk nonseminomatous germ cell tumour. BJU Int 2007;99:993-7. [Crossref] [PubMed]
- Karam JA, Raj GV. Growing teratoma syndrome. Urology 2009;74:783-4. [Crossref] [PubMed]
- Rice KR, Magers MJ, Beck SD, et al. Management of germ cell tumors with somatic type malignancy: pathological features, prognostic factors and survival outcomes. J Urol 2014;192:1403-9. [Crossref] [PubMed]
- Pfannenberg AC, Oechsle K, Bokemeyer C, et al. The role of [(18)F] FDG-PET, CT/MRI and tumor marker kinetics in the evaluation of post chemotherapy residual masses in metastatic germ cell tumors--prospects for management. World J Urol 2004;22:132-9. [Crossref] [PubMed]
- Spermon JR, De Geus-Oei LF, Kiemeney LA, et al. The role of (18)fluoro-2-deoxyglucose positron emission tomography in initial staging and re-staging after chemotherapy for testicular germ cell tumours. BJU Int 2002;89:549-56. [Crossref] [PubMed]
- De Santis M, Becherer A, Bokemeyer C, et al. 2-18fluoro-deoxy-D-glucose positron emission tomography is a reliable predictor for viable tumor in postchemotherapy seminoma: an update of the prospective multicentric SEMPET trial. J Clin Oncol 2004;22:1034-9. [Crossref] [PubMed]
- Becherer A, De Santis M, Karanikas G, et al. FDG PET is superior to CT in the prediction of viable tumour in post-chemotherapy seminoma residuals. Eur J Radiol 2005;54:284-8. [Crossref] [PubMed]
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in the postchemotherapy management of patients with seminoma: systematic review and meta-analysis. Biomed Res Int 2014;2014:852681. [Crossref]
- Bachner M, Loriot Y, Gross-Goupil M, et al. 2-(1)(8)fluoro-deoxy-D-glucose positron emission tomography (FDG-PET) for postchemotherapy seminoma residual lesions: a retrospective validation of the SEMPET trial. Ann Oncol 2012;23:59-64. [Crossref] [PubMed]
- Becherer A. PET in testicular cancer. Methods Mol Biol 2011;727:225-41. [Crossref] [PubMed]
- Cathomas R, Klingbiel D, Bernard B, et al. Questioning the Value of Fluorodeoxyglucose Positron Emission Tomography for Residual Lesions After Chemotherapy for Metastatic Seminoma: Results of an International Global Germ Cell Cancer Group Registry. J Clin Oncol 2018.Jco1800210. [Epub ahead of print]. [PubMed]
- Large MC, Sheinfeld J, Eggener SE. Retroperitoneal lymph node dissection: reassessment of modified templates. BJU Int 2009;104:1369-75. [Crossref] [PubMed]
- Jewett MA, Kong YS, Goldberg SD, et al. Retroperitoneal lymphadenectomy for testis tumor with nerve sparing for ejaculation. J Urol 1988;139:1220-4. [Crossref] [PubMed]
- Donohue JP, Foster RS, Rowland RG, et al. Nerve-sparing retroperitoneal lymphadenectomy with preservation of ejaculation. J Urol 1990;144:287-91; discussion 291-2. [Crossref] [PubMed]
- Busch FM, Sayegh ES. Roentgenographic visualization of human testicular lymphatics: a preliminary report. J Urol 1963;89:106-10. [Crossref] [PubMed]
- Ray B, Hajdu SI, Whitmore WF Jr. Proceedings: Distribution of retroperitoneal lymph node metastases in testicular germinal tumors. Cancer 1974;33:340-8. [Crossref] [PubMed]
- Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol 1982;128:315-20. [Crossref] [PubMed]
- Weissbach L, Boedefeld EA. Localization of solitary and multiple metastases in stage II nonseminomatous testis tumor as basis for a modified staging lymph node dissection in stage I. J Urol 1987;138:77-82. [Crossref] [PubMed]
- Beck SD, Foster RS, Bihrle R, et al. Is full bilateral retroperitoneal lymph node dissection always necessary for postchemotherapy residual tumor? Cancer 2007;110:1235-40. [Crossref] [PubMed]
- Cho JS, Kaimakliotis HZ, Cary C, et al. Modified retroperitoneal lymph node dissection for post-chemotherapy residual tumour: a long-term update. BJU Int 2017;120:104-8. [Crossref] [PubMed]
- Carver BS, Shayegan B, Eggener S, et al. Incidence of metastatic nonseminomatous germ cell tumor outside the boundaries of a modified postchemotherapy retroperitoneal lymph node dissection. J Clin Oncol 2007;25:4365-9. [Crossref] [PubMed]
- Burg ML, Jacob L, Ghodoussipour S, et al. MP49-16: A contemporary review of midline extraperitoneal retroperitoneal lymph node dissection for testicular germ cell tumor. Journal of Urology 2019;201:e711-2.
- Shah A, Nassiri N, Daneshmand S. Management of extraretroperitoneal masses in germ cell tumor. Curr Opin Urol 2019;29:33-41. [Crossref] [PubMed]
- Hartmann JT, Candelaria M, Kuczyk MA, et al. Comparison of histological results from the resection of residual masses at different sites after chemotherapy for metastatic non-seminomatous germ cell tumours. Eur J Cancer 1997;33:843-7. [Crossref] [PubMed]
- Lewinshtein DJ, Porter CR. The history and anatomy of urologic lymphadenectomy. Urol Clin North Am 2011;38:375-86. v. [Crossref] [PubMed]
- Hu B, Daneshmand S. Role of Extraretroperitoneal Surgery in Patients with Metastatic Germ Cell Tumors. Urol Clin North Am 2015;42:369-80. [Crossref] [PubMed]
- Skinner DG, Melamud A, Lieskovsky G. Complications of thoracoabdominal retroperitoneal lymph node dissection. J Urol 1982;127:1107-10. [Crossref] [PubMed]
- Jacob JM, Mehan R, Beck SD, et al. Management of Pelvic Metastases in Patients With Testicular Cancer. Urology 2017;102:159-63. [Crossref] [PubMed]
- Alanee SR, Carver BS, Feldman DR, et al. Pelvic Lymph Node Dissection in Patients Treated for Testis Cancer: The Memorial Sloan Kettering Cancer Center Experience. Urology 2016;95:128-31. [Crossref] [PubMed]
- Cary C, Masterson TA, Bihrle R, et al. Contemporary trends in postchemotherapy retroperitoneal lymph node dissection: Additional procedures and perioperative complications. Urol Oncol 2015;33:389.e15-21. [Crossref] [PubMed]
- Djaladat H, Nichols C, Daneshmand S. Adjuvant surgery in testicular cancer patients undergoing postchemotherapy retroperitoneal lymph node dissection. Ann Surg Oncol 2012;19:2388-93. [Crossref] [PubMed]
- Johnson SC, Smith ZL, Nottingham C, et al. Clinical and Radiographic Predictors of Great Vessel Resection or Reconstruction During Retroperitoneal Lymph Node Dissection for Testicular Cancer. Urology 2019;123:186-90. [Crossref] [PubMed]
- Duty B, Daneshmand S. Resection of the inferior vena cava without reconstruction for urologic malignancies. Urology 2009;74:1257-62. [Crossref] [PubMed]
- Steiner H, Leonhartsberger N, Stoehr B, et al. Postchemotherapy laparoscopic retroperitoneal lymph node dissection for low-volume, stage II, nonseminomatous germ cell tumor: first 100 patients. Eur Urol 2013;63:1013-7. [Crossref] [PubMed]
- Cheney SM, Andrews PE, Leibovich BC, et al. Robot-assisted retroperitoneal lymph node dissection: technique and initial case series of 18 patients. BJU Int 2015;115:114-20. [Crossref] [PubMed]
- Kamel MH, Littlejohn N, Cox M, et al. Post-Chemotherapy Robotic Retroperitoneal Lymph Node Dissection: Institutional Experience. J Endourol 2016;30:510-9. [Crossref] [PubMed]
- Singh A, Chatterjee S, Bansal P, et al. Robot-assisted retroperitoneal lymph node dissection: Feasibility and outcome in postchemotherapy residual mass in testicular cancer. Indian J Urol 2017;33:304-9. [Crossref] [PubMed]