
Epidemiology and treatment modalities for the management of benign prostatic hyperplasia
Introduction
Benign prostatic hyperplasia (BPH) is defined by the American Urological Association (AUA) as a histologic diagnosis referring to the proliferation of smooth muscle and epithelial cells within the prostatic transition zone (1). The prostatic transition zone makes up about 5% of the prostate and is the portion that surrounds the proximal urethra. This zone is the site of continual growth throughout life (2). The presence of BPH in older men is strongly linked to the development of lower urinary tract symptoms (LUTS), which is defined by several symptoms including urgency, nocturia, frequency, dysuria, difficulty emptying the bladder, difficulty initiating micturition, and weak or interrupted stream during micturition (3). Although some LUTS is defined as “LUTS independent of BPH”, BPH and its downstream effects lead to chronic LUTS in many men. BPH with LUTS has also been linked to erectile dysfunction (ED) (4).
The prostate was first anatomically described by Nicolo Massa of Padua in 1550 (5). It was another hundred years later, in 1649, when the enlarged prostate was proposed to cause urinary retention by Herr (6). Since then, BPH and its role in causing symptomology has been extensively studied. As the prostatic gland enlarges, due to hyperplasia, it may lead to bladder outlet obstruction (BOO). BOO can cause LUTS by two mechanisms: (I) thickening of the prostate which physically narrows the urethra (static component), and (II) the effect of augmented smooth muscle tone (dynamic component) (7). Normally, a mixture of both mechanisms cause BOO related LUTS. Unlike the pathophysiology of LUTS in BPH patients, ED and its link with BPH is not universally understood. However, recent studies have shown that BPH may increase ED through LUTS symptoms, fibrous muscle growth, and/or through its recommended treatments (8).
Although the diagnosis of BPH is histological, physicians utilize a multi-faceted approach in evaluating men for possible BPH. Symptom scoring, patient history, physical exam including DRE, diagnostic imaging, including ultrasound or prostate MRI, and laboratory studies are commonly used amongst physicians. Proper diagnosis also warrants an understanding of prostate size and prostatic growth rates. The average prostate is commonly described to patients as being the size of a walnut, weighing 11 grams on average, in younger adult men. The mean range falls between 7–16 grams (9). The mean doubling time for prostatic volume is 32.6 years, with an average growth rate of around 2.2% per year (10). The objective of this narrative review is to briefly highlight the epidemiology and pathophysiology of BPH, focus on the current treatment options for patients with symptomatic BPH, and touch on future potential directions for management. Treatments are summarized in Table 1.
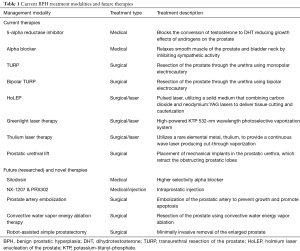
Full table
Epidemiology and pathophysiology
BPH and its associated symptomatology affect many men worldwide: as of 2010, the prevalence is over 210 million men (11). Up to 50% of men over the age of 50 and up to 80% of men over the age of 80 experience LUTS from BPH (12). Furthermore, BPH prevalence is on the rise, due to an increase in modifiable metabolic risk factors, such as obesity (13). Male obesity has been linked to increased risk of BPH and an increased severity of LUTS in the men affected by BPH (14). Obesity causes a number of systemic effects including increased inflammatory processes as well as increased intra-abdominal pressures (15). These systemic affects may be a factor in the increased prevalence of BPH and may increase the reported severity of LUTS. Similar to the inflammatory effects of obesity, infection has also been linked to increased severity of BPH symptoms (16). The reverse is also true, with BPH leading to unique infectious complications in patients (17).
Since the late 1800s, BPH, previously known as Benign Prostatic Hypertrophy, has been linked to two factors: age and the presence of testosterone (18). However, the exact pathophysiology is still yet to be identified. The pathophysiology of BPH has been linked to many factors including sex hormones, neurotransmitters, inflammation, diet, microorganisms and cellular effects on epithelial as well as stromal tissue (19). Although androgen levels have long been studied as one of the largest influencers on prostatic growth (20), estrogen may also play a role. It may seem counterintuitive that as men age and testosterone levels fall, the prostate still continues to grow. However, with the help of estrogen, prostatic hyperplasia is allowed to continue even with diminishing androgen levels. Estrogen signaling increases the level of androgen receptors in the prostatic gland leading to signal amplification and stimulation of hyperplasia, even with reducing levels of androgen (21). Additionally, estradiol has been found to induce epithelial-to-mesenchymal transition in benign prostatic epithelial cells (22). Epithelial-to-mesenchymal transition causes prostatic hyperplasia, and is evidenced by the loss of E-cadherin, increased pSmad3 and high Snail markers in BPH samples. This affirmed the notion that the accumulation of mesenchymal-like cells derived from prostatic epithelium causes BPH rather than prostatic stromal proliferation (23). With an understanding of the mechanisms involved in BPH formation and the increasing number of cases of symptomatic BPH, it is imperative to explore the current management of symptomatic BPH.
Methods
A scientific literature search (utilizing databases of PubMed, Medline, ScienceDirect, etc.) on the most commonly used treatment modalities was performed to determine which management techniques or medications to include in this narrative. Keywords including “benign prostatic hyperplasia epidemiology and management”, “lower urinary tract symptoms”, “urethral lift”, “greenlight therapy”, “thulium laser”, and “transurethral resection of prostate” were used to specify searches. Emphasis was placed on management techniques mentioned in the AUA guidelines on BPH management. Only peer reviewed articles available in the English language were included. A total of about 100 studies, reviews, narratives or trials were incorporated into this narrative. Studies included were not limited to those performed or published in the United States. Those studies that included randomized control trials (RCTs) or were published with higher impact were weighted heavier in the current therapies section of this review.
Management
A middle aged or older man presenting with LUTS is often evaluated in the context of BPH contributing to these symptoms and is therefore treated on the presumption of BPH. The initial diagnostics, as stated above, for a man presenting with LUTS is to take a detailed history of the symptoms as well as to perform a comprehensive physical exam. Physical exam should also include a digital rectal exam to assess the size of the prostate. Laboratory tests should include a urinalysis to evaluate for bacteriuria, pyuria and hematuria (24). Although previously recommended, the AUA guidelines specifically state that routine creatinine tests are not indicated for patients presenting with LUTS secondary to BPH (1). While there is no obvious correlation between clinically significant prostate cancer and LUTS (25,26), patients with certain risk factors such as family history of prostate cancer, African American race, or unexplained lower back pain should be spoken to about prostate cancer screening including a PSA. A patient’s medications should also be listed and evaluated as many common medications such as diuretics, antidepressants, and others may contribute to LUTS (27).
Once a patient has undergone diagnostic evaluation for his LUTS secondary to BPH, his symptomatology can be quantified by the International Prostate Symptom Scorer or the almost identical AUA-Symptom Index (28). These scores are composed of self-administered questions that assess the severity of three symptom groups: frequency, nocturia and urgency. The aggregate score aids in primary treatment approach. Those patients with a score of ≤8 indicate mild symptoms and may choose observation (29), which is also an option for those with moderate symptoms without impairment to overall quality of life. Observation involves patient education and the modification of risk factors that may increase LUTS secondary to BPH or lead to Acute Urinary Retention. Strategies to reduce risk and severity of BPH include, weight loss, increased physical activity, and a reduction in the consumption of caffeine and alcohol (30). A change in a patient’s voiding position may improve their urodynamic parameters (31). Patients with symptom scores above 8 are considered to have moderate to severe symptoms and have several available treatment options. These treatment options vary in approach, invasiveness and outcomes.
Current treatment options
Medication therapy
After lifestyle modifications, medication is generally first line in the treatment of symptomatic BPH (32). Two drug classes became accepted standard of care in the late 1980s early 1990s; 5-alpha-reductase inhibitors such as finasteride and Alpha-blockers like terazosin (33). Androgens play a significant role in prostate growth throughout a patient’s life. Normal functioning of the prostate and other components of the male reproductive system, are dependent on the reduction of testosterone to dihydrotestosterone (DHT) by the enzyme 5-alpha reductase. 5-alpha reductase inhibitors block the conversion of testosterone to DHT and thus reduce the growth effects of androgens on the prostate (34). Finasteride, a 5-alpha reductase inhibitor, was the first FDA approved medication for BPH and is commonly prescribed to patients for other conditions besides BPH such as androgenetic alopecia (35). Finasteride works by inhibiting type II 5-alpha reductase, and reduces prostate volume and the symptoms associated with BPH (36). As monotherapy, it has been found to reduce prostate volume by over 20% (37) and to reduce clinical symptomatic progression by 34% (38). Similar to finasteride, dutasteride is a 5 alpha reductase inhibitor that inhibits both type I and type II 5 alpha reductase (36). Although dutasteride has a greater inhibition of DHT (>90% compared to >70% for finasteride), both drugs have similar reductions in clinical symptoms (39). In addition, both drugs have comparable side effect profiles with sexual side effects of impotence, decreased libido and ejaculatory dysfunction being the most common (40).
Alpha-blockers are also utilized in the treatment of BPH and work by relaxing the smooth muscle of the prostate and bladder neck by inhibiting sympathetic activity (41). Prazosin was the first selective alpha-1 blocker but has largely been replaced by FDA-approved longer acting alpha blockers. These include terazosin, doxazosin, tamsulosin and alfuzosin (41). Alpha blockers offer a more rapid onset and efficacy in the first year than finasteride, however, only 5 alpha reductase inhibitors cause prostate regression and reduced risk of BPH complications over time (42). Alpha-blockers also have side effects, including most commonly fatigue, dizziness, and hypotension (41). A more selective alpha blocker Silodosin, however, an analysis of 19 unique studies with over 4,000 patients showed that the efficacy of silodosin was comparable to other alpha blockers currently in use (43).
Although monotherapy, with alpha blockers and 5 alpha reductase inhibitors, is beneficial, the combination of these drugs is highly effective. One study found that men with BPH had a 34% clinical risk reduction on finasteride (P=0.002), a 39% clinical risk reduction on doxazosin (P<0.001) and a 66% clinical risk reduction with combination therapy (P<0.001) (38). Additionally, combination therapy was found to reduce BPH-related symptoms more successfully than either drug alone (44).
Patient’s with BPH may also experience concomitant overactive bladder symptoms or may develop symptoms similar to overactive bladder due to BPH treatment. In addition to Alpha-blockers and 5-alpha reductase inhibitors, a few studies have shown the benefit of anti-muscarinics and beta-3 adrenergic agonists in improving storage symptoms in patients experiencing overactive bladder symptomology (45,46).
Surgical management
The interventional management of BPH is another option for patients who are suitable for surgical procedure and is generally offered to patients with persistent or severe BPH refractory to medical therapy. While surgery may be viewed as more expensive in the short term, the out of pocket cost of 5 years of continuous medication has been shown to exceed the costs of early surgery (mean ‘out-of-pocket’ cost of medical treatment versus surgical treatment was $1,742 and $1,436 respectively P=0.005) (47). Additionally, some experts believe BPH has become more of a chronic condition causing long term economic burden due to the proportional increase in BPH cases managed by medical therapy alone (48). Highlighted here are some of the available surgical interventions for BPH.
Transurethral resection of the prostate (TURP)
TURP has long been considered the historical gold standard for the surgical treatment of BPH (49). TURP at one point was the second most commonly performed operation in the United States (50). The conventional monopolar TURP has been in use for several decades and has been extensively studied for its morbidity and mortality (51). A study of 10,654 patients investigated outcomes after monopolar TURP operations and found a cumulative short-term morbidity rate of 11.1%, of which 1.4% was due to Transurethral resection (TUR) syndrome (52)—water intoxication from the irrigation solution causing hyponatremia and other acid-base imbalances in the body (53). TUR syndrome along with side effects from surgery, led to the introduction of bipolar TURP in the late 1990s. Unlike monopolar TURP, bipolar TURP allows for the utilization of isotonic irrigation solutions and reduces the risk of electrolyte imbalances including TUR syndrome (54). The differences in outcomes and efficacy of bipolar versus monopolar TURP have been studied with mixed results. One study found that bipolar resection with 0.9% NaCl had minimal effects on serum sodium when compared with monopolar resection (55). A systematic review and meta-analysis of 16 RCTs (1,406 patients) found no clinical differences in the short-term efficacy of either TURP but concluded bipolar TURP is more preferable due to its reduced side effect profile (56). Furthermore, other studies also stress the clinical equivalency between monopolar and bipolar TURP (57). Both types of TURP procedures cause a large proportion of patients to experience retrograde ejaculation (58). In 1999, TURP represented 81% of all surgical treatment for BPH but by 2005, this number fell to 39% of all BPH procedures (59). This is due to the introduction of newer procedures in recent years.
Holmium laser enucleation of the prostate (HoLEP)
The holmium laser is a pulsed laser, utilizing a solid medium that combines both carbon dioxide and neodymium:YAG lasers to deliver simultaneous tissue cutting and cauterization. The wavelength can also be transmitted down an optical fiber and has a thermal injury zone of 0.5 to 1 mm, making it suitable for endoscopic urologic surgery (60). HoLEP was first reported in 1996 as a viable technique for the management of BPH (61).
Many studies have compared HoLEP to TURP. A systematic review and meta-analysis compared the efficacy and safety of Bipolar TURP to HoLEP. Four trials, including three RCTs, were assessed and the analysis concluded that both techniques were safe with similar symptomatic relief for patients (62). A Brazilian institution compared their outcomes from HoLEP with TURP and reported that HoLEP was as effective as TURP in terms of patient outcomes and operative time (63). A Canadian RCT compared HoLEP to TURP in 80 patients. The study reported that HoLEP patients had shorter catheterization times and hospital stays and had greater symptomatic improvement than in patients treated with TURP (64). Another RCT compared HoLEP to TURP in the treatment of prostates larger than 40 grams (65). The study found that HoLEP had less perioperative morbidity and superior urodynamic outcomes, and at 24-month follow-up, HoLEP was equivalent to TURP. However, HoLEP is not devoid of its own side effect profile. HoLEP has been associated with higher rates of early postoperative urgency urinary incontinence compared to TURP (44% versus 38.6% respectively) (66). HoLEP, similar to TURP, also has high rates of post-operative retrograde ejaculation (67).
HoLEP has been described as the emerging gold standard for BPH surgical management in the twenty-first century however the implementation of this surgical technique faces the obstacle of a steep learning curve (68,69). Today, with many institutions having at least 10 years of training and experience with HoLEP, the procedure appears to be a viable and effective treatment for BPH (70). However, the difficulty of performing HoLEP still bars its use in many smaller or referral centers.
Greenlight
Greenlight is a commercially marketed and manufactured high-powered potassium-titanyl-phosphate (KTP) 532-nm wavelength photo selective vaporization system. Greenlight laser energy is considered photo selective because it transmits fully through aqueous irrigant but is absorbed by tissue with a high oxyhemoglobin content, such as prostatic tissue (71). The original 80 watt (W) laser was improved to the 120-W laser and currently a 180-W laser is also available (72). The employment of these higher powered and more efficient lasers depends on physician experience (73). Although experience and practice are required for its effective use, Greenlight, especially at lower power, has been reported to have a smaller learning curve than that of HoLEP (74). Additionally, Greenlight and HoLEP have been shown to have comparable efficacies and outcomes (75,76).
Multiple institutions have compared Greenlight to the benchmark standard, TURP. One RCT with 220 subjects reported that when compared to TURP, Greenlight had similar International Prostate Symptom Score (IPSS) and peak flow improvement but had better clinical outcomes in terms of recovery experience and endpoints (77). Greenlight has also been shown to have lower bleeding complication rates when compared to TURP (78). RCTs are currently being designed to further study the use of Greenlight in those patients taking anticoagulants (79). A multicenter RCT reported that Greenlight 120-W had comparable uroflowmetry parameters and complications with TURP but had significantly lower length hospital stays for patients (80). With comparable outcomes and effectiveness to that of TURP highlighted in multiple studies, the main benefit of Greenlight appears to be the reduction in the length of hospital stay (81-83). The economic advantage of this reduced length of hospital stay was further exemplified in a cost analysis performed in Greece comparing Greenlight to TURP (84). This study reported that, the average cost (including the cost of equipment, anesthesia, medications, consumables, inpatient hospitalization and complication management within 1 year of operation) was $2,371 for Greenlight and $2,935 for TURP. Similarly, a cost analysis in Canada was performed comparing Greenlight to TURP and bipolar TURP. With a cohort of 202 patients, the study reported a mean per-patient cost for all cases (including both the day of surgery and inpatient cases) to be $3,836 for Greenlight, $4,978 for bipolar TURP and $4,963 for TURP (85).
Thulium laser therapy
In contrast to the pulsed holmium laser, the thulium laser utilizes a rare elemental metal, thulium, to provide a continuous wave laser that produces a clean and fast cut-through vaporization. A multitude of techniques using the thulium laser have been described for BPH including ablation, enucleation and resection (86). The thulium laser was first investigated for its benefit over the holmium laser in canine prostatic tissue in 2005 where it was concluded that the thulium fiber laser may be potentially advantageous due to its ability for pulsed waves, smaller size and more efficient operation than that of the holmium laser (87).
Several studies have compared the thulium laser to TURP for BPH management. An RCT of 106 patients compared thulium laser enucleation of the prostate (TmLEP) to TURP and found that in a 3-month observation the two procedures were comparable in terms of safety and efficiency (88). A systematic review and meta-analysis assessing seven trials comparing the efficacy and safety of the thulium laser versus TURP, reported that the two procedures had similar efficacy in symptom scores. It noted that thulium laser prostatectomy had the advantage in amount of required blood transfusions, serum sodium decreases, catheterization time and hospital stay while TURP was shorter in terms of operative time (89).
Thulium has also been compared to other laser-based modalities. In comparison to Greenlight laser 120-W, thulium laser was equivalent in terms of complications, patient reoperations and PSA levels a year after surgery (90). Thulium vapoenucleation of the prostate (ThuVEP) was compared to HoLEP in an RCT of 94 patients. The study found that the procedures had low perioperative morbidity and were equivalent and satisfactory for immediate micturition improvement (91).
Current research supports that each laser modality is on par or superior to the standard TURP. The main drawback of these laser modalities is the learning curve involved in their use and outcomes dependent on physician expertise.
Prostatic urethral lift (PUL)
With its FDA approval for the use as a BPH treatment modality in 2013, PUL has been increasing in popularity amongst clinicians (92). The procedure is a minimally invasive surgery that involves placing mechanical implants through the urethra, which retract the obstructing prostatic lobes and hold them in place away from the prostatic urethra (93). Patient selection for the procedure involves extensive exclusion criteria including patients with a history of urinary retention, those with prostates larger than 100 mL, those who have had previous pelvic surgeries or those with obstructive median lobes (94).
A systematic review and meta-analysis evaluated the outcomes of PUL. The analysis comprised 10 trials covering an estimated 650 patients. The evidence showed overall symptom improvement (IPSS difference of ‒7.2 to ‒8.7 points) and preservation of sexual health (standardized mean gain range of 0.3 to 0.4) in patients during the first 12 months of follow-up (95). An RCT compared PUL to a sham procedure to investigate sexual function in the 3- and 12-month follow-up. The trial reported that there was no evidence of ejaculatory dysfunction following the procedure and that SHIM (Sexual health inventory for men) scores were significantly improved from baseline at the 12-month follow-up (P=0.016). In particular, the Ejaculatory-bother score was improved by 40% from baseline (96) in patients who underwent the procedure. PUL may be ideal in younger patients or any patient, who meets criteria, wishing to preserve sexual functioning.
PUL has also been compared to the benchmark standard TURP. A multicenter randomized study of 80 patients compared the two-year follow-up outcomes of patients receiving TURP versus those receiving PUL. The study reported that TURP was superior to PUL in IPSS and maximum urinary flow rate (Qmax) improvements but that PUL was superior in recovery quality, ejaculatory functional preservation and performance on the BPH6 index (97).
In summary, the main advantages of PUL are its preservation of sexual function, minor complication profile and ability to be performed under local anesthesia (98). The main drawbacks of PUL are its highly selective patient inclusion criteria and its reoperation rate of 1.4–16% (99).
Future therapies
Within the past decade, a multitude of novel treatment modalities have emerged for the treatment of BPH. These newer therapies share a similar goal in optimizing quality of life while maintaining or improving efficacy from current therapies. On the medical side of treatment, novel drug therapies are being investigated. Intraprostatic injections are also being investigated including, botulinum neurotoxin A (BoNT-A), NX-1207 and PRX302 (100). BoNT-A has shown efficacy by its downregulation of alpha 1a receptors thereby causing a reduction in contractility. This reduction in contractility is two-fold, reducing obstruction by elevated smooth muscle tone and reducing obstruction by the prostatic mass itself. NX-1207 causes atrophy of the prostate by inducing prostatic apoptosis. PRX302 contains a pore forming toxin which causes prostatic involution. These injectable medications must be further investigated in clinical trials for efficacy and side effect profile (101). Prostate artery embolization has shown promising results and may be a viable treatment pending long term follow-up data (102). In comparison to TURP, one study reported lower improvements in functional outcomes for PAE patients but fewer complications in a 12-week period (103). From the surgical standpoint, minimally invasive prostate convective water vapor ablation or steam therapy has been found to deliver improvements in BPH with preservation of ejaculatory function (104). Three-year outcomes for Rezūm, convective radiofrequency water vapor thermal therapy, were promising with large IPSS improvements and consistent symptomatic relief (105). Rezūm is also associated with benefits including expedited recovery time however the procedure requires catheterization for a few days after surgery and benefits are not fully realized until a few months post-operatively. Finally, robot-assisted prostatectomy for BPH has also increased in popularity in recent years (106). Robot-assisted simple prostatectomy has also been shown to have shorter hospital stay and a lower morbidity profile when compared to open simple prostatectomy (107).
Conclusions
The increase in the prevalence of BPH in recent decades has been met with a growth of treatment options. Physicians today must determine which management modality is optimal for their particular patient. However, first physicians must decide if a patient should be managed on medical therapy or undergo surgical intervention. This decision is based on both patient and physician preference and surgical candidacy. With the changing nature of modifiable risk factors for BPH, further research into optimizing treatment is ongoing.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Management of Benign Prostatic Hyperplasia. American Urological Association 2010. Available online: https://www.auanet.org/guidelines/benign-prostatic-hyperplasia-(bph)-guideline/benign-prostatic-hyperplasia-(2010-reviewed-and-validity-confirmed-2014)
- McNeal JE. The zonal anatomy of the prostate. Prostate 1981;2:35-49. [Crossref] [PubMed]
- Parsons JK. Benign Prostatic Hyperplasia and Male Lower Urinary Tract Symptoms: Epidemiology and Risk Factors. Curr Bladder Dysfunct Rep 2010;5:212-8. [Crossref] [PubMed]
- De Nunzio C, Roehrborn CG, Andersson KE, et al. Erectile Dysfunction and Lower Urinary Tract Symptoms. Eur Urol Focus 2017;3:352-63. [Crossref] [PubMed]
- Chapple CR. Anatomy and Innervation of the Prostate Gland. In: Chapple CR. editor. Prostatic Obstruction: Pathogenesis and Treatment. London: Springer London, 1994:5-76.
- Herr HW. The enlarged prostate: a brief history of its surgical treatment. BJU Int 2006;98:947-52. [Crossref] [PubMed]
- McVary KT, Roehrborn CG, Avins AL, et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J Urol 2011;185:1793-803. [Crossref] [PubMed]
- Dutkiewicz S, Skawiński D, Duda W, et al. Assessing the Influence of Benign Prostatic Hyperplasia (BPH) on Erectile Dysfunction (ED) among patients in Poland. Cent European J Urol 2012;65:135-8. [Crossref] [PubMed]
- Leissner KH, Tisell LE. The weight of the human prostate. Scand J Urol Nephrol 1979;13:137-42. [Crossref] [PubMed]
- Lieber MM, Rhodes T, Jacobson DJ, et al. Natural history of benign prostatic enlargement: long-term longitudinal population-based study of prostate volume doubling times. BJU int 2010;105:214-9. [Crossref] [PubMed]
- Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163-96. [Crossref] [PubMed]
- Egan KB. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am 2016;43:289-97. [Crossref] [PubMed]
- Patel ND, Parsons JK. Epidemiology and etiology of benign prostatic hyperplasia and bladder outlet obstruction. Indian J Urol 2014;30:170-6. [Crossref] [PubMed]
- Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 2012;110:540-5. [Crossref] [PubMed]
- Parikesit D, Mochtar CA, Umbas R, et al. The impact of obesity towards prostate diseases. Prostate Int 2016;4:1-6. [Crossref] [PubMed]
- Nickel JC. Inflammation and benign prostatic hyperplasia. Urol Clin North Am 2008;35:109-15. vii. [Crossref] [PubMed]
- Abdul-Muhsin HM, Jakob NJ, McLemore RM, et al. Infectious complications associated with the use of temporary prostatic urethral stents in patients with benign prostatic hyperplasia. Can J Urol 2016;23:8465-70. [PubMed]
- Rand HW. V. A Contribution to the Surgery of the Hypertrophied Prostate. Ann Surg 1895;22:217-25. [Crossref] [PubMed]
- Lee C, Kozlowski JM, Grayhack JT. Intrinsic and extrinsic factors controlling benign prostatic growth. Prostate 1997;31:131-8. [Crossref] [PubMed]
- Bruchovsky N, Lesser B, Van Doorn E, et al. Hormonal effects on cell proliferation in rat prostate. Vitam Horm 1975;33:61-102. [Crossref] [PubMed]
- Wilson JD. The pathogenesis of benign prostatic hyperplasia. Am J Med 1980;68:745-56. [Crossref] [PubMed]
- Shi X, Peng Y, Du X, et al. Estradiol promotes epithelial-to-mesenchymal transition in human benign prostatic epithelial cells. Prostate 2017;77:1424-37. [Crossref] [PubMed]
- Alonso-Magdalena P, Brössner C, Reiner A, et al. A role for epithelial-mesenchymal transition in the etiology of benign prostatic hyperplasia. Proceedings of the National Academy of Sciences of the United States of America 2009;106:2859-63. [Crossref] [PubMed]
- Alawamlh OAH, Goueli R, Lee RK. Lower Urinary Tract Symptoms, Benign Prostatic Hyperplasia, and Urinary Retention. Med Clin North Am 2018;102:301-11. [Crossref] [PubMed]
- Østerø Í, Jákupsstovu J, Brodersen J. Do men with lower urinary tract symptoms have an increased risk of advanced prostate cancer?. BMJ 2018;361:k1202. [Crossref] [PubMed]
- Martin RM, Vatten L, Gunnell D, et al. Lower urinary tract symptoms and risk of prostate cancer: the HUNT 2 Cohort, Norway. Int J Cancer 2008;123:1924-8. [Crossref] [PubMed]
- Wuerstle MC, Van Den Eeden SK, Poon KT, et al. Contribution of common medications to lower urinary tract symptoms in men. Arch Intern Med 2011;171:1680-2. [Crossref] [PubMed]
- Barry MJ, Fowler FJ Jr, O'leary MP, et al. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J Urol 2017;197:S189-97. [Crossref] [PubMed]
- Oelke M, Bachmann A, Descazeaud A, et al. EAU guidelines on the treatment and follow-up of non-neurogenic male lower urinary tract symptoms including benign prostatic obstruction. Eur Urol 2013;64:118-40. [Crossref] [PubMed]
- Parsons JK. Modifiable risk factors for benign prostatic hyperplasia and lower urinary tract symptoms: new approaches to old problems. J Urol 2007;178:395-401. [Crossref] [PubMed]
- Aghamir SM, Mohseni M, Arasteh S. The effect of voiding position on uroflowmetry findings of healthy men and patients with benign prostatic hyperplasia. Urol J 2005;2:216-21. [PubMed]
- Bishr M, Boehm K, Trudeau V, et al. Medical management of benign prostatic hyperplasia: Results from a population-based study. Can Urol Assoc J 2016;10:55-9. [Crossref] [PubMed]
- Lepor H. Medical treatment of benign prostatic hyperplasia. Rev Urol 2011;13:20-33. [PubMed]
- Goldenberg L, So A, Fleshner N, et al. The role of 5-alpha reductase inhibitors in prostate pathophysiology: Is there an additional advantage to inhibition of type 1 isoenzyme? Can Urol Assoc J 2009;3:S109-14. [Crossref] [PubMed]
- Mysore V, Shashikumar BM. Guidelines on the use of finasteride in androgenetic alopecia. Indian J Dermatol Venereol Leprol 2016;82:128-34. [Crossref] [PubMed]
- Rittmaster RS. 5α-reductase inhibitors in benign prostatic hyperplasia and prostate cancer risk reduction. Best Pract Res Clin Endocrinol Metab 2008;22:389-402. [Crossref] [PubMed]
- Smith AB, Carson CC. Finasteride in the treatment of patients with benign prostatic hyperplasia: a review. Ther Clin Risk Manag 2009;5:535-45. [PubMed]
- McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med 2003;349:2387-98. [Crossref] [PubMed]
- Pirozzi L, Sountoulides P, Castellan P, et al. Current Pharmacological Treatment for Male LUTS due to BPH: Dutasteride or Finasteride? Curr Drug Targets 2015;16:1165-71. [Crossref] [PubMed]
- Nickel JC. Comparison of clinical trials with finasteride and dutasteride. Rev Urol 2004;6 Suppl 9:S31-9. [PubMed]
- Lepor H. Alpha blockers for the treatment of benign prostatic hyperplasia. Rev Urol 2007;9:181-90. [PubMed]
- Eri LM, Tveter KJ. Treatment of benign prostatic hyperplasia. A pharmacoeconomic perspective. Drugs Aging 1997;10:107-18. [Crossref] [PubMed]
- Jung JH, Kim J, MacDonald R, et al. Silodosin for the treatment of lower urinary tract symptoms in men with benign prostatic hyperplasia. Cochrane Database Syst Rev 2017;11:CD012615. [PubMed]
- McVary KT. A review of combination therapy in patients with benign prostatic hyperplasia. Clin Ther 2007;29:387-98. [Crossref] [PubMed]
- Moss MC, Rezan T, Karaman UR, et al. Treatment of Concomitant OAB and BPH. Curr Urol Rep 2017;18:1. [Crossref] [PubMed]
- Knutson T, Edlund C, Fall M, et al. BPH with coexisting overactive bladder dysfunction--an everyday urological dilemma. Neurourol Urodyn 2001;20:237-47. [Crossref] [PubMed]
- Ahn HS, Kim SJ, Choi JB, et al. Long-term cost comparison between surgical and medical therapy for benign prostatic hyperplasia: a study using hospital billing data. BJU Int 2019;123:E79-85. [Crossref] [PubMed]
- Hollingsworth JM, Wei JT. Economic impact of surgical intervention in the treatment of benign prostatic hyperplasia. Rev Urol 2006;8 Suppl 3:S9-15. [PubMed]
- Young MJ, Elmussareh M, Morrison T, et al. The changing practice of transurethral resection of the prostate. Ann R Coll Surg Engl 2018;100:326-9. [Crossref] [PubMed]
- Wei JT, Calhoun E, Jacobsen SJ. Urologic diseases in America project: benign prostatic hyperplasia. J Urol 2005;173:1256-61. [Crossref] [PubMed]
- Reich O, Gratzke C, Stief CG. Techniques and long-term results of surgical procedures for BPH. Eur Urol 2006;49:970-8; discussion 978. [Crossref] [PubMed]
- Reich O, Gratzke C, Bachmann A, et al. Morbidity, mortality and early outcome of transurethral resection of the prostate: a prospective multicenter evaluation of 10,654 patients. J Urol 2008;180:246-9. [Crossref] [PubMed]
- Chambers A. Transurethral resection syndrome--it does not have to be a mystery. AORN J 2002;75:156-64, 166, 168-70; quiz 171-8.
- Hueber PA, Al-Asker A, Zorn KC. Monopolar vs. bipolar TURP: assessing their clinical advantages. Can Urol Assoc J 2011;5:390-1. [Crossref] [PubMed]
- Karadeniz MS, Bayazit E, Aksoy O, et al. Bipolar versus monopolar resection of benign prostate hyperplasia: a comparison of plasma electrolytes, hemoglobin and TUR syndrome. SpringerPlus 2016;5:1739. [Crossref] [PubMed]
- Mamoulakis C, Ubbink DT, de la Rosette JJ. Bipolar versus monopolar transurethral resection of the prostate: a systematic review and meta-analysis of randomized controlled trials. Eur Urol 2009;56:798-809. [Crossref] [PubMed]
- Méndez-Probst CE, Nott L, Pautler SE, et al. A multicentre single-blind randomized controlled trial comparing bipolar and monopolar transurethral resection of the prostate. Can Urol Assoc J 2011;5:385-9. [Crossref] [PubMed]
- Pavone C, Abbadessa D, Scaduto G, et al. Sexual dysfunctions after transurethral resection of the prostate (TURP): evidence from a retrospective study on 264 patients. Arch Ital Urol Androl 2015;87:8-13. [Crossref] [PubMed]
- Rocco B, Albo G, Ferreira RC, et al. Recent advances in the surgical treatment of benign prostatic hyperplasia. Ther Adv Urol 2011;3:263-72. [Crossref] [PubMed]
- Wollin TA, Denstedt JD. The holmium laser in urology. J Clin Laser Med Surg 1998;16:13-20. [Crossref] [PubMed]
- Gilling PJ, Cass CB, Cresswell MD, et al. The use of the holmium laser in the treatment of benign prostatic hyperplasia. J Endourol 1996;10:459-61. [Crossref] [PubMed]
- Qian X, Liu H, Xu D, et al. Functional outcomes and complications following B-TURP versus HoLEP for the treatment of benign prostatic hyperplasia: a review of the literature and Meta-analysis. Aging Male 2017;20:184-91. [PubMed]
- Barboza LE, Malafaia O, Slongo LE, et al. Holmium Laser enucleation of the prostate (HoLEP) versus Transurethral Resection of the Prostate (TURP). Rev Col Bras Cir 2015;42:165-70. [Crossref] [PubMed]
- Eltabey MA, Sherif H, Hussein AA. Holmium laser enucleation versus transurethral resection of the prostate. Can J Urol 2010;17:5447-52. [PubMed]
- Wilson LC, Gilling PJ, Williams A, et al. A randomised trial comparing holmium laser enucleation versus transurethral resection in the treatment of prostates larger than 40 grams: results at 2 years. Eur Urol 2006;50:569-73. [Crossref] [PubMed]
- Rigatti L, Naspro R, Salonia A, et al. Urodynamics after TURP and HoLEP in urodynamically obstructed patients: are there any differences at 1 year of follow-up? Urology 2006;67:1193-8. [Crossref] [PubMed]
- Kuebker JM, Miller NL. Holmium Laser Enucleation of the Prostate: Patient Selection and Outcomes. Curr Urol Rep 2017;18:96. [Crossref] [PubMed]
- Michalak J, Tzou D, Funk J. HoLEP: the gold standard for the surgical management of BPH in the 21(st) Century. Am J Clin Exp Urol 2015;3:36-42. [PubMed]
- Razzak M. BPH: HoLEP--a steep learning curve but better for patients. Nat Rev Urol 2013;10:66. [Crossref] [PubMed]
- Shigemura K, Fujisawa M. Current status of holmium laser enucleation of the prostate. Int J Urol 2018;25:206-11. [Crossref] [PubMed]
- Te AE. The Next Generation in Laser Treatments and the Role of the GreenLight High-Performance System Laser. Rev Urol 2006;8 Suppl 3:S24-30. [PubMed]
- Zhang X, Shen P, He Q, et al. Different lasers in the treatment of benign prostatic hyperplasia: a network meta-analysis. Scientific reports 2016;6:23503. [Crossref] [PubMed]
- Bastard C, Zorn K, Peyronnet B, et al. Assessment of Learning Curves for 180-W GreenLight XPS Photoselective Vaporisation of the Prostate: A Multicentre Study. Eur Urol Focus 2019;5:266-72. [Crossref] [PubMed]
- de la Rosette J, Alivizatos G. Lasers for the treatment of bladder outlet obstruction: are they challenging conventional treatment modalities? Eur Urol 2006;50:418-20. [Crossref] [PubMed]
- Elshal AM, Elkoushy MA, El-Nahas AR, et al. GreenLight laser (XPS) photoselective vapo-enucleation versus holmium laser enucleation of the prostate for the treatment of symptomatic benign prostatic hyperplasia: a randomized controlled study. J Urol 2015;193:927-34. [Crossref] [PubMed]
- Cho SY, Park J, Yoo S, et al. One-year Surgical Outcomes of Complete or Incomplete Enucleation of Prostate by Monopolar Electrocoagulation, Photoselective Vapoenucleation of 120-W GreenLight Laser, and Holmium Laser. Urology 2017;108:142-8. [Crossref] [PubMed]
- Cimino S, Voce S, Palmieri F, et al. Transurethral resection of the prostate (TURP) vs GreenLight photoselective vaporization of benign prostatic hyperplasia: analysis of BPH6 outcomes after 1 year of follow-up. Int J Impot Res 2017;29:240-3. [Crossref] [PubMed]
- Bruyère F, Huglo D, Challacombe B, et al. Blood loss comparison during transurethral resection of prostate and high power GreenLight(™) laser therapy using isotopic measure of red blood cells volume. J Endourol 2011;25:1655-9. [Crossref] [PubMed]
- Charbonneau H, Pasquié M, Peyronnet B, et al. Stopping or maintaining oral anticoagulation in patients undergoing photoselective vaporization of the prostate (SOAP) surgery for benign prostate obstruction: study protocol for a multicentre randomized controlled trial. Trials 2018;19:705. [Crossref] [PubMed]
- Lukacs B, Loeffler J, Bruyere F, et al. Photoselective vaporization of the prostate with GreenLight 120-W laser compared with monopolar transurethral resection of the prostate: a multicenter randomized controlled trial. Eur Urol 2012;61:1165-73. [Crossref] [PubMed]
- Telli O, Okutucu TM, Suer E, et al. A prospective, randomized comparative study of monopolar transurethral resection of the prostate versus photoselective vaporization of the prostate with GreenLight 120-W laser, in prostates less than 80 cc. Ther Adv Urol 2015;7:3-8. [Crossref] [PubMed]
- Capitán C, Blazquez C, Martin MD, et al. GreenLight HPS 120-W laser vaporization versus transurethral resection of the prostate for the treatment of lower urinary tract symptoms due to benign prostatic hyperplasia: a randomized clinical trial with 2-year follow-up. Eur Urol 2011;60:734-9. [Crossref] [PubMed]
- Bouchier-Hayes DM, Anderson P, Van Appledorn S, et al. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol 2006;20:580-5. [Crossref] [PubMed]
- Liatsikos E, Kyriazis I, Kallidonis P, et al. Photoselective GreenLight laser vaporization versus transurethral resection of the prostate in Greece: a comparative cost analysis. J Endourol 2012;26:168-73. [Crossref] [PubMed]
- Masucci L, Erman A, Krahn MD, et al. Cost analysis of Greenlight photoselective vaporization of the prostate compared to transurethral resection of the prostate for benign prostatic hyperplasia. Can Urol Assoc J 2018;12:382-7. [Crossref] [PubMed]
- Maheshwari PN, Joshi N, Maheshwari RP. Best laser for prostatectomy in the year 2013. Indian J Urol 2013;29:236-43. [Crossref] [PubMed]
- Fried NM, Murray KE. High-power thulium fiber laser ablation of urinary tissues at 1.94 microm. J Endourol 2005;19:25-31. [Crossref] [PubMed]
- Świniarski PP, Stepien S, Dudzic W, et al. Thulium laser enucleation of the prostate (TmLEP) vs. transurethral resection of the prostate (TURP): evaluation of early results. Cent European J Urol 2012;65:130-4. [Crossref] [PubMed]
- Zhu Y, Zhuo J, Xu D, et al. Thulium laser versus standard transurethral resection of the prostate for benign prostatic obstruction: a systematic review and meta-analysis. World J Urol 2015;33:509-15. [Crossref] [PubMed]
- Palmero-Martí JL, Panach-Navarrete J, Valls-Gonzalez L, et al. Comparative study between thulium laser (Tm: YAG) 150W and greenlight laser (LBO:ND-YAG) 120W for the treatment of benign prostatic hyperpplasia: Short-term efficacy and security. Actas Urol Esp 2017;41:188-93. [PubMed]
- Netsch C, Becker B, Tiburtius C, et al. A prospective, randomized trial comparing thulium vapoenucleation with holmium laser enucleation of the prostate for the treatment of symptomatic benign prostatic obstruction: perioperative safety and efficacy. World J Urol 2017;35:1913-21. [Crossref] [PubMed]
- Wang R. AB02. UroLift: a new surgical treatment for BPH without sexual side effect. Transl Androl Urol 2014;3:AB02.
- Garcia C, Chin P, Rashid P, et al. Prostatic urethral lift: A minimally invasive treatment for benign prostatic hyperplasia. Prostate Int 2015;3:1-5. [Crossref] [PubMed]
- Jones P, Rai BP, Aboumarzouk O, et al. UroLift: a new minimally-invasive treatment for benign prostatic hyperplasia. Ther Adv Urol 2016;8:372-6. [Crossref] [PubMed]
- Perera M, Roberts MJ, Doi SA, et al. Prostatic urethral lift improves urinary symptoms and flow while preserving sexual function for men with benign prostatic hyperplasia: a systematic review and meta-analysis. Eur Urol 2015;67:704-13. [Crossref] [PubMed]
- McVary KT, Gange SN, Shore ND, et al. Treatment of LUTS secondary to BPH while preserving sexual function: randomized controlled study of prostatic urethral lift. J Sex Med 2014;11:279-87. [Crossref] [PubMed]
- Gratzke C, Barber N, Speakman MJ, et al. Prostatic urethral lift vs transurethral resection of the prostate: 2-year results of the BPH6 prospective, multicentre, randomized study. BJU Int 2017;119:767-75. [Crossref] [PubMed]
- Larcher A, Broglia L, Lughezzani G, et al. Urethral lift for benign prostatic hyperplasia: a comprehensive review of the literature. Curr Urol Rep 2013;14:620-7. [Crossref] [PubMed]
- Kaplan SA. The Prostate Urethral Lift: Will It Be Uplifting or Downgraded as a Treatment Strategy for Benign Prostatic Hyperplasia? Eur Urol 2015;67:714-5. [Crossref] [PubMed]
- Magistro G, Stief CG, Gratzke C. New intraprostatic injectables and prostatic urethral lift for male LUTS. Nat Rev Urol 2015;12:461-71. [Crossref] [PubMed]
- Andersson KE. Intraprostatic injections for lower urinary tract symptoms treatment. Curr Opin Urol 2015;25:12-8. [Crossref] [PubMed]
- Mirakhur A, McWilliams JP. Prostate Artery Embolization for Benign Prostatic Hyperplasia: Current Status. Can Assoc Radiol J 2017;68:84-9. [Crossref] [PubMed]
- Abt D, Hechelhammer L, Müllhaupt G, et al. Comparison of prostatic artery embolisation (PAE) versus transurethral resection of the prostate (TURP) for benign prostatic hyperplasia: randomised, open label, non-inferiority trial. BMJ 2018;361:k2338. [Crossref] [PubMed]
- McVary KT, Gange SN, Gittelman MC, et al. Minimally Invasive Prostate Convective Water Vapor Energy Ablation: A Multicenter, Randomized, Controlled Study for the Treatment of Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hyperplasia. J Urol 2016;195:1529-38. [Crossref] [PubMed]
- McVary KT, Roehrborn CG. Three-Year Outcomes of the Prospective, Randomized Controlled Rezum System Study: Convective Radiofrequency Thermal Therapy for Treatment of Lower Urinary Tract Symptoms Due to Benign Prostatic Hyperplasia. Urology 2018;111:1-9. [Crossref] [PubMed]
- Ferretti M, Phillips J. Prostatectomy for benign prostate disease: open, laparoscopic and robotic techniques. Can J Urol 2015;22 Suppl 1:60-6. [PubMed]
- Shah AA, Gahan JC, Sorokin I. Comparison of Robot-Assisted Versus Open Simple Prostatectomy for Benign Prostatic Hyperplasia. Curr Urol Rep 2018;19:71. [Crossref] [PubMed]


