
Infantile synchronous primary bilateral testicular germ cell tumor: a case report and review of the literature
Introduction
Testicular tumors are uncommon in adults, accounting for <1% of all cancers (1). Testicular germ cell tumors (TGCTs) represent the majority of the testicular tumors (>95%) (1), with >18,000 new cases diagnosed annually in Europe (2) and >9,000 newly diagnosed cases annually in the United States (3). Prepubertal TGCTs are extremely rare, representing only 1% of all pediatric solid tumors and having an annual incidence of 0.5–2/100,000 boys (4,5). In general, the prepubertal TGCTs are fundamentally distinct from the adult TGCTs, both in clinical and biological features (6). Yolk sac tumors (YSTs) and teratomas are the most frequent forms of prepubertal TGCTs, whereas seminomas are the most common in adults.
Bilateral TGCTs (BTGCTs) account for 0.5% to 5% of all testicular tumors (1), and the majority of these are metachronous. Synchronous BTGCTs account for approximately 0.5% to 1% (7). Reports of BTGCTs in children are particularly rare, regardless of whether the case is metachronous or synchronous. Because of the overall low incidence of BTGCTs, knowledge about their epidemiology, clinical features, and treatment outcomes has come primarily from clinical reports and case series, which mainly represent adult cases. Unfortunately, current guidelines of testicular cancer contain little information related to bilateral disease.
Herein, we report a case of synchronous primary BTGCT with testes-specific histologies in a 16-month-old child treated with left testicle-sparing surgery and right high radical orchiectomy. We also provide a review of the literature on BTGCTs from the PubMed and Embase databases, which were searched using the following key words: testicular OR testis OR testicle AND bilateral AND tumor. Only English-language articles were considered. The literature search found only 8 cases of prepubertal synchronous primary BTGCTs, and only 3 cases with testes-specific histologies (8-15).
Case presentation
Chief complaints
A 16-month-old boy was admitted to the Urology Department of our hospital, with the complaint of a painless scrotal mass that had appeared 2-week prior.
History of present illness
Two weeks prior to presentation, the patient’s parents noticed the newly expansive nature of the child’s scrotum, which had occurred without any testicular pain or rapidity.
History of past illness
The patient had a clear medical history.
Physical examination
At hospital admission, the patient’s temperature was 36.7 °C, heart rate was 110 beats per minute, respiratory rate was 26 breaths per minute, blood pressure was 98/68 mmHg, and oxygen saturation in room air was 100%. Physical examination showed normal appearance of the external male genitalia. The right testis was descended, while the left scrotum was empty. A medium firm mass was palpable within the right scrotum; he left testis had a palpable mass at the external inguinal ring but appeared normal in size (Figure 1A). Physical examination of the abdomen provided normal findings.

Laboratory examinations
Blood analysis revealed a mild leukocytosis (13.19×109/L; normal range, 4−12×109/L), with predominant lymphocytes (55.5%; normal range, 20–40%), normal hematocrit, and mild thrombocytosis (503×109/L; normal range, 100–400×109/L). The blood biochemistries were normal, as were the findings from urine analysis and fecal analysis. Analysis of serum testicular tumor markers showed alpha-fetoprotein (AFP) of 12,567 ng/mL (normal range, 0–20 ng/mL), β-human chorionic gonadotrophin (HCG) of 0 mIU/mL (normal range, 0–5.3 mIU/mL), and total testosterone of <0.35 nmol/L (normal range, 0–1.04 nmol/L).
Imaging examinations
Scrotal ultrasonography revealed a homogeneous hyperechoic solid mass (3.0 cm × 2.0 cm × 2.0 cm) with rich blood supply in the right testis and no discernable testicular tissue. Furthermore, a well-delineated heterogeneous echo mass (0.96 cm × 0.63 cm) was located within the left testis (Figure 1B,C). Retroperitoneal ultrasound revealed that there was no enlargement of the retroperitoneal lymph node. Contrast-enhanced computed tomography (CT) scan revealed a markedly enhanced mass in the right testis and a mildly enhanced mass in the left testis (Figure 2). Magnetic resonance imaging (non-contrast-enhanced) of the right testis revealed a hypointense solid mass on the T1-weighted image and a hyperintense mass on the T2-weighted image of the right testis; imaging of the left testis revealed a hyperintense cystic neoplasm on the T1-weighted image and relatively heterogeneous signal intensity on the T2-weighted image (Figure 3). Electrocardiogram and chest X-ray showed no abnormities.
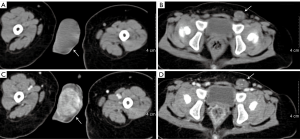
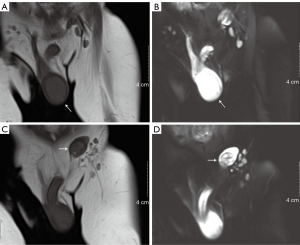
Final diagnosis
The final diagnosis was bilateral testicular tumor and left side cryptorchidism.
Treatment
Exploratory surgery of the bilateral testes was performed via left scrotal incision and right inguinal incision. The intraoperative findings were a nodular, well-delineated, white-colored, solid cystic mass in the left testis and an ovoid, ill-delineated, white/yellow-colored, solid mass in the right (Figure 4). Histological analysis showed the tumors to be completely enucleated, and the frozen section diagnoses were teratoma in the left testis and YST in the right testis. The decision was made to proceed with left testicle-sparing surgery and right radical inguinal orchiectomy with high ligation of the spermatic cord.
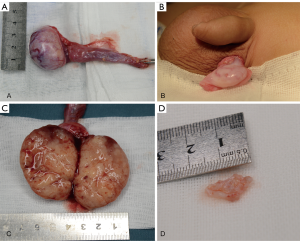
Outcome and follow-up
Final pathology confirmed a cystic mature teratoma from the left testis and a pure YST from the right testis (spermatic cord not invaded). Immunohistochemical staining of the right testicular tumor showed positivity for AFP, SALL4, PLAP, GPC3, Ki67 and CD117 but negativity for CD30 and HCG (Figure 5). No further treatment was given, as patient was at clinical stage I for the YST. There were no intraoperative or postoperative complications.
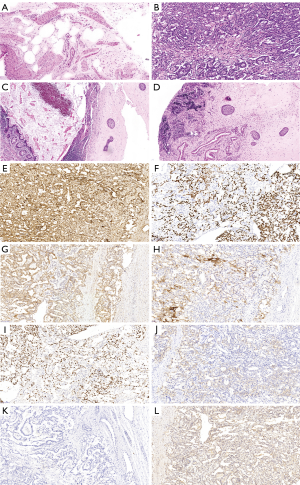
The 14-month follow-up included ultrasonography examinations at 1, 2, 4, 5, 6, 9, 10 and 14 months postoperatively and showed that the left testis had normal contour; no atrophy or residual tumor was observed. The right scrotum was empty and no evidence of recurrence was observed. AFP levels at 5, 13, 21, 29 and 44 d postoperatively were 4,626, 1,048, 351, 101.9 and 8.61 ng/mL, respectively. During the entire follow-up period, AFP levels remained within normal range (<10 ng/mL).
Discussion
Clinical manifestations of prepubertal TGCTs are various and nonspecific, indicating the lack of differences between clinical presentations of benign and early malignant TGCTs. Generally, a painless scrotal mass or swelling is most common in a child with a testicular tumor (4). Once a testicular tumor is suspected, a physical examination and ultrasonography examination should be undertaken. A high frequency testicular ultrasound—which can detect small, asymptomatic, non-palpable testicular masses and provide findings to distinguish benign from malignant masses—represents the initial modality for confirmation of the presence of a testicular mass and for assessment of the contralateral testis (16).
It is reported that a lesion presenting on sonography with a hyperechoic and homogeneous pattern is more likely a YST, with teratomas being frequently more echogenic and heterogeneous (17,18). In our case, the patient presented a painless swelling of the right scrotum, with a contralateral undescended testicle. The preoperative scrotal ultrasound demonstrated bilateral testicular tumors. According to the ultrasound characteristics, the right testicular YST and left testicular teratoma were highly suspected, and each was confirmed by the final pathology. The high frequency testicular ultrasound helped to demonstrate the salvageable parenchyma of the affected testis (19). CT and magnetic resonance imaging examinations have emerged as valuable second-line assistant diagnostic tools for the analysis of testicular tumor pathology; moreover, they are especially recommended in cases of discrepancies between ultrasound findings and clinical presentations, so that radical orchiectomy can be avoided in cases of benign lesions (20,21).
Evaluation of serum tumor markers, such as AFP, β-HCG and testosterone, plays an important role in both diagnosis and follow-up. AFP is a YST-specific marker, and many previous studies have indicated that AFP is useful in diagnosing YSTs, monitoring the treatment response, and detecting recurrence (6). In our case, the patient had an elevated AFP level (12,567 ng/mL) preoperatively, and the follow-up AFP level had decreased to normal by postoperative day 44. Although the AFP level can be physiologically elevated in infants, it will rarely exceed >100 ng/mL in infants older than 6 months (22,23).
It is well known that TGCTs spread by the retroperitoneal pathway of lymphatic drainage (24), and reports of metastases describe involvement of only nodal or distant sites that do not include the contralateral testis (25). Because there are no lymphatic or vascular connections between the testes, bilateral testicular tumors are likely to have different histologic types (25). YSTs represent the most common malignant lesions of the testes, while the most common benign lesions are teratomas. Cases of synchronous primary BTGCTs with testes-specific histologies in children have been reported rarely. To our knowledge, there are only 8 reported cases of prepubertal synchronous primary BTGCTs in the English language literature to date, and including only 3 cases with testes-specific histology (8-15); our case is the 4th one.
In 1994, Royal et al. (15) reported a case of synchronous mature teratoma of the left testis and mixed germ cell tumor (95% YST) of the right testis in a 2-year-old white male treated by bilateral radical inguinal orchiectomy. In 1998, Luo et al. (13) reported a case of synchronous mature teratoma of the right testis and YST of the left testis in a 7-month-old infant treated by bilateral radical inguinal orchiectomy. Then, in 2018, Dong et al. (8) reported a case of synchronous YST (stage I) of the left testis and mature teratoma of the right testis in a 7-month-old infant treated by right testicle-sparing surgery and left high radical orchiectomy. Reports from the American Academy of Pediatrics Prepubertal Testis Registry have indicated that YSTs account for 62% of all tumors, whereas teratomas have comprised only 25% (26). A report by Maizlin et al. (5) indicated that YSTs accounted for 42%, with teratomas accounting for 17.3%. Some recent single-institution studies have found the most common histology to be that of teratoma (6,27).
The treatment of patients with BTGCTs should be based upon the histopathologic type, clinical stage, and patient age. Radical orchiectomy remains the standard of care for resection in cases of prepubertal malignant TGCTs (5). According to the Associazione Italiana Ematologia Oncologia Pediatrica protocol guideline (28), radical orchiectomy is sufficient for patients with stage I YST, without subsequent chemotherapy; however, long-term postoperative surveillance of serum AFP level and ultrasonography examination is necessary. For advanced stage YST or recurrence, chemotherapy or retroperitoneal lymph node dissection should be performed (28).
On the other hand, testicle-sparing surgery is presently advocated, especially for benign lesions or bilateral and/or multiple lesions, depending on the reliability of both preoperative ultrasound and intraoperative frozen section diagnosis (29). The testicle-sparing surgery could preserve the salvageable testicular parenchyma for fertility and provide freedom from long-term androgen supplementation. With potential psychologic, cosmetic and functional advantages, testicle-sparing surgery should be used in children with TGCTs in which the normal testicular tissue seems salvageable according to findings from ultrasonography or magnetic resonance imaging, and when the AFP concentration is normal (4,30). Previous studies have revealed that, with proper enucleation of the benign TGCTs, recurrence is rare (9).
Conclusions
Teratomas and YSTs constitute most of the prepubertal TGCTs. Patients with synchronous primary BTGCTs with teratoma and YST respectively are extremely rare. Radical orchiectomy is the standard of care for YSTs. For patients whose biological markers are negative and intraoperative frozen specimens are suggestive of a benign pathology, testicle-sparing surgery is the preferred treatment option.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Informed consent was obtained from the patient in surgical consents to use surgical/pathologic imaging and to present case.
References
- Zequi Sde C, da Costa WH, Santana TB, et al. Bilateral testicular germ cell tumours: a systematic review. BJU Int 2012;110:1102-9. [Crossref] [PubMed]
- Litchfield K, Thomsen H, Mitchell JS, et al. Quantifying the heritability of testicular germ cell tumour using both population-based and genomic approaches. Sci Rep 2015;5:13889. [Crossref] [PubMed]
- Cancer Facts & Figures 2018. American Cancer Society, 2018.
- Wang X, Xu S, Tang D, et al. Prepubertal testicular and paratesticular tumors in China: a single-center experience over a 10-year period. J Pediatr Surg 2012;47:1576-80. [Crossref] [PubMed]
- Maizlin II, Dellinger M, Gow KW, et al. Testicular tumors in prepubescent patients. J Pediatr Surg 2018;53:1748-52. [Crossref] [PubMed]
- Wu D, Shen N, Lin X, et al. Prepubertal testicular tumors in China: a 10-year experience with 67 cases. Pediatr Surg Int 2018;34:1339-43. [Crossref] [PubMed]
- Watson RA, Morgan RD, Joseph J, et al. Bilateral Testicular Germ Cell Tumors: A Case-Series From a UK-Based Tertiary Referral Center Over 19 Years. Clin Genitourin Cancer 2018;16:e513-6. [Crossref] [PubMed]
- Dong S, Zhao L, Pei G, et al. Testicular Yolk Sac Tumor and Mature Teratoma: Synchronous Bilateral Occurrence in an Infant. Urology 2018;117:142-4. [Crossref] [PubMed]
- Madden-Fuentes R, Wiener JS, Ross SS, et al. Partial orchiectomy for bilateral synchronous testicular masses in a prepubescent boy: a case report. Urology 2012;80:1144-6. [Crossref] [PubMed]
- Koski ME, Thomas JC. Successful bilateral testicular sparing surgery for benign teratoma. J Pediatr Urol 2009;5:72-4. [Crossref] [PubMed]
- Herek O, Ulman I, Ozcan C, et al. Bilateral testicular teratoma in infancy: report of a rare case treated by testis-sparing surgery. Eur J Pediatr Surg 2004;14:209-11. [Crossref] [PubMed]
- Tasaki Y, Nakagawa M, Hanada T, et al. Testis sparing surgery for infantile synchronous bilateral teratoma of the testis. Int J Urol 1998;5:501-3. [Crossref] [PubMed]
- Luo C, Lin J, Huang C. Bilateral testicular tumors in an infant. Pediatr Surg Int 1998;13:69-70. [Crossref] [PubMed]
- Mansfield JT, Cartwright PC. Bilateral testis tumors in an infant: synchronous teratoma and epidermoid cyst. J Urol 1995;153:1077-9. [Crossref] [PubMed]
- Royal SA, Joseph DB, Galliani CA. Bilateral testicular teratoma. AJR Am J Roentgenol 1994;163:1194. [Crossref] [PubMed]
- Tsili AC, Bertolotto M, Rocher L, et al. Sonographically indeterminate scrotal masses: how MRI helps in characterization. Diagn Interv Radiol 2018;24:225-36. [Crossref] [PubMed]
- Song QD. Ultrasound Appearances of Pediatric Testicular Yolk Sac Tumors: Twenty-one Cases in a Single Institution. J Ultrasound Med 2018;37:2457-63. [Crossref] [PubMed]
- Bertolotto M, Grenier N, Hamm B, et al. Imaging of Bilateral Synchronous Testicular Tumors of Different Histologic Types and Implications for Surgical Management. J Ultrasound Med 2016;35:2511-6. [Crossref] [PubMed]
- Dell'Atti L. Efficacy of ultrasound-guided testicle-sparing surgery for small testicular masses. J Ultrasound 2015;19:29-33. [Crossref] [PubMed]
- Tsili AC, Sofikitis N, Stiliara E, et al. MRI of testicular malignancies. Abdom Radiol (NY) 2019;44:1070-82. [Crossref] [PubMed]
- Mathur M, Spektor M. MR Imaging of the Testicular and Extratesticular Tumors: When Do We Need? Magn Reson Imaging Clin N Am 2019;27:151-71. [Crossref] [PubMed]
- Blohm ME, Vesterling-Horner D, Calaminus G, et al. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol 1998;15:135-42. [Crossref] [PubMed]
- Wu JT, Book L, Sudar K. Serum alpha fetoprotein (AFP) levels in normal infants. Pediatr Res 1981;15:50-2. [Crossref] [PubMed]
- Paño B, Sebastià C, Buñesch L, et al. Pathways of lymphatic spread in male urogenital pelvic malignancies. Radiographics 2011;31:135-60. [Crossref] [PubMed]
- Thomas JP, Davis-Dao C, Lewinger JP, et al. Null Association Between Histology of First and Second Primary Malignancies in Men With Bilateral Testicular Germ Cell Tumors. Am J Epidemiol 2013;178:1240-5. [Crossref] [PubMed]
- Ross JH, Rybicki L, Kay R. Clinical behavior and a contemporary management algorithm for prepubertal testis tumors: a summary of the Prepubertal Testis Tumor Registry. J Urol 2002;168:1675-8; discussion 1678-9.
- Oottamasathien S, Thomas JC, Adams MC, et al. Testicular tumours in children: a single-institutional experience. BJU Int 2007;99:1123-6. [Crossref] [PubMed]
- Terenziani M, De Pasquale MD, Bisogno G, et al. Malignant testicular germ cell tumors in children and adolescents: The AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) protocol. Urol Oncol 2018;36:502.e7-502.e13. [Crossref] [PubMed]
- Dell'Atti L. Successful management of an asymptomatic bilateral synchronous testicular carcinoid tumor with a testicular-sparing surgery. Asian J Androl 2017;19:507-8. [Crossref] [PubMed]
- Pediatrique JSVftGDEeU. Testis-sparing surgery for benign testicular tumors in children. J Urol 2001;165:2280-3. [Crossref] [PubMed]


