
Therapeutic strategies for uncommon testis cancer histologies: teratoma with malignant transformation and malignant testicular sex cord stromal tumors
Introduction
Testicular cancer accounts for less than 1% of malignancies in men in the United States, but with an estimated 9,310 new cases in 2018 it remains the most common solid tumor in men aged 20–34 years (1). Testicular germ cell tumors (GCTs), including seminomas and non-seminomas, comprise the majority of testicular neoplasms (>90%) whereas gonadal-stromal tumors represent less than 5% of adult testis tumors (2). While prognosticators and therapeutic strategies for GCTs are well-established and associated with excellent outcomes, patients with rare testicular cancer histologies such as teratomas with somatic-type malignancy and testicular sex cord stromal tumors (TSCST) experience much less favorable outcomes and optimal management paradigms are lacking. The current manuscript will review management strategies for patients with uncommon testicular cancer histologies.
Teratoma with malignant transformation (TMT)
Epidemiology and physiopathology
Teratoma is a common form of GCT containing elements derived from two or more germ-cell layers (endoderm, mesoderm, and ectoderm) and can be classified as mature or immature based on the differentiation of its components (3). TMT is a rare form of teratoma containing somatic type malignant elements encountered in other organs and tissues (4). This entity represents a spectrum of neoplasms including sarcomas [e.g., rhabdomyosarcoma (RMS), malignant nerve sheath tumor], carcinomas [adenocarcinoma (ADC), squamous cell carcinoma (SCC)], hematopoietic malignancies (leukemia) and others [carcinoid, nephroblastoma, primitive neuroectodermal tumors (PNET)] (4). TMT may present as primary or metastatic GCT and can also arise in extra-gonadal sites such as the mediastinum, retroperitoneum, and intracranial cavity (5,6). TMT accounts for less than 5% of metastatic testicular tumors and most commonly affects younger men, although pediatric cases have been reported (7-9).
Over the years, several debated theories have been postulated to explain the malignant transformation occurring within a GCT. According to Ulbright, malignant transformation arises from either differentiation of totipotent germ-cell elements into somatic tissues with subsequent malignant transformation, or malignant transformation of pre-existing teratomatous components (8). Oosterhuis et al. speculate that since mature teratoma in metastases is derived from primary tumors with mature components, the apparent induction of differentiation in metastases is actually the result of selective destruction of non-teratomatous elements by chemotherapy, and thus does not occur through differentiation of totipotent germ-cells (10). This process consequently allows for selective growth of the chemo-resistant teratomatous elements. Moreover, the presence of chromosome 12p abnormalities in these tumors, including isochromosome 12 in most cases, reflects common GCT clonality (11,12).
Histopathology
TMT are characterized by invasion of adjacent germ-cell elements by highly atypical somatic cells (13). Authors suggest that the most significant feature for the diagnosis of TMT is expansile proliferation of somatic malignant elements and clinically significant TMT is considered when the somatic-type component fills a field of view at low magnification (4× lens). Thus, assessment of the degree of atypia and the growth pattern of the somatic malignant proliferation by expert genitourinary pathologists is critical for accurate diagnosis and management of patients with TMT.
Outcomes
Experience regarding TMT remains scarce and relies on small case series mainly from high-volume cancer centers (11,14-23) (Table 1). Patients with stage I disease have a favorable prognosis while those with metastatic disease have dismal oncological outcomes despite aggressive surgery and conventional cisplatin-based GCT systemic therapy with historic series from referral centers reporting cancer-specific survival rates in the 50% range (11,15,24).
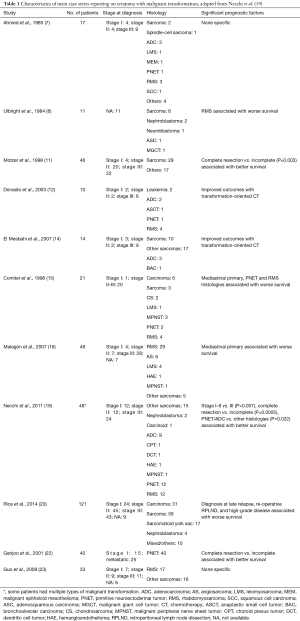
Full table
The largest single-institution TMT series included patients with sarcoma, carcinoma, sarcomatoid yolk sac tumor and nephroblastoma (20). Approximately 75% of the patients presented with stage II–III disease and overall the estimated 5-year cancer-specific survival was 64% (median follow-up 71 months). Predictors of worse survival included high-grade disease, sarcomatoid yolk sac tumor histology, re-operative retroperitoneal lymphadenectomy, and diagnosis of malignant transformation at late relapse. There were no differences in outcomes between sarcoma and carcinoma patients although recurrence patterns differed in that patients with carcinomas relapsed several years later than patients with sarcomas following initial GCT diagnosis.
TMT patients often present with locally advanced disease involving surrounding organs and/or major vessels, which require complex surgical resections and reconstructions in order to achieve durable cure. In the series reported by Rice et al., more than half of the patients (56.2%) required collateral resections, such as nephrectomy, lobectomy, colectomy, neck dissection and aorta/inferior vena cava replacement/grafting (20). Aggressive resection is supported by evidence that negative margins are paramount to achieving long-term remission and superior oncological outcomes (11). Furthermore, compared to patients with GCT, local relapse after initial surgery is much more common with TMT (greater than systemic recurrences), particularly if patients underwent initial treatments in non-specialized centers (37% vs. 13%) (20). When suspected or confirmed, consideration should be given towards referring patients with TMT to high-volume comprehensive cancer centers with expert multidisciplinary care.
Full resection with negative margins is paramount to achieving long term remission. In a 1998 single-institution series, 46 TMT patients with complete resection had significantly improved oncological outcomes during follow-up compared to those with incomplete resection and positive margins (P=0.003) (11).
With a sparse body of literature, there is no established standard of care for TMT, but rather management guidance from high-volume centers. Patients with clinical stage I disease should strongly be considered for primary retroperitoneal lymph node dissection (RPLND) while those with advanced, but resectable disease, are best managed with post-chemotherapy RPLND, multidisciplinary surgical involvement and en bloc resection of visceral and vascular structures when required.
Systemic therapy
TMT patients can present with systemic progressive disease and normal serum tumor markers despite adequate treatment with cisplatin-based regimens. Systemic treatment of such cases remains challenging due to their chemo-resistant phenotype. Indeed patients with TMT are unresponsive to conventional GCT regimens and have a propensity for late systemic failure (25). Histology-specific systemic chemotherapy regimens have been suggested as a more effective management strategy for TMT by several authors. In a case series of 10 TMT patients treated with regimens tailored to the histology of the somatic malignancy, seven had a partial response including three with a long-term response (12). A similar European study evaluated 8 TMT patients treated with chemotherapy regimens directed to the non-GCT component at relapse and reported a 50% partial response (14). Hence, the optimal management strategy for TMT should comprise resection of all sites of disease with malignant transformation-oriented systemic therapy.
Nonetheless, treatment of TMT remains challenging and effective therapeutic options in advanced disease are still lacking. Gene expression profiling represents a novel approach to better understand molecular mechanisms and identify potential actionable targets in refractory TMT cases (26,27).
Sarcomas
GCT transformation with sarcomatous components (SC) is the most common histology among TMT arising from testicular neoplasms and include cases of undifferentiated sarcoma, RMS, and sarcomatoid yolk sac, although other rarer types have been reported [leiomyosarcoma (LMS), angiosarcoma and malignant nerve sheath tumor] (4,11,15,28).
In rare cases, sarcomatous (or sarcomatoid) transformation occurred in GCT patients without teratomatous elements (29). SC can arise in primary testicular tumor, metastases or both, and their GCT clonal origin has been demonstrated (30). Development of SC differentiation after GCT diagnosis often occurs within 2 years after initial treatments (12,20).
When confined to the testis, patients with SC enjoy a favorable prognosis comparable to that of their pure GCT counterparts (23). Conversely, survival rates in patients with metastatic SC have been reported to range from 40–50% with only 10% of patients alive without disease at last follow-up (16). Rice et al. found that tumor grade in sarcomas or sarcomatoid yolk sac cases was an important prognosticator of survival and suggested that more aggressive treatments and more stringent postoperative surveillance be considered for patients with high-grade sarcomatous/sarcomatoid TMT (20). SC are known to be resistant to conventional cisplatin GCT agents and a SC directed regimen consisting of doxorubicin-based chemotherapy is preferred (12). TMT with SC is radio-resistant, thus radiation has little value and is limited to palliation purposes (31).
PNET
PNET are categorized as peripheral or central (32). Peripheral PNET carry the Ewing sarcoma (EWS) family of tumors translocations on chromosome 22. Central PNET affects the central nervous system mostly in pediatric patients and does not express the EWS translocations. PNET arising from GCT typically exhibit features of central PNET and can be incorrectly diagnosed as immature teratomas (33,34). Neuroectodermal elements may resemble neuroblastoma, medulloepithelioma, peripheral neuroepithelioma or ependymoblastoma (35). Identification of isochromosome 12 in resected tumors supports its GCT origin (36).
TMT patients with PNET do not respond to cisplatin-based chemotherapy but can benefit from a PNET-based systemic regimen of cyclophosphamide, doxorubicin, and vincristine (CAV) alternating with ifosfamide plus etoposide (IE) after retroperitoneal surgery or in patients with unresectable disease (37,38). PNET histology seems to have the best response to systemic therapy in advanced TMT and patients with recurrent advanced PNET may respond to high-dose chemotherapy with stem cell support (12).
Carcinomas
Carcinomas such as ADCs, SCC and neuroendocrine carcinomas represent a rare subset of TMT patients (39). Some tumors stain for cytokeratins and carcinoembryonic antigen (CEA) but are negative for GCT markers such as placental alkaline phosphatase (PLAP), alpha-fetoprotein (AFP) and human chorionic gonadotropin (HCG) (35). Outcomes are based upon stage and resectability of disease (4). Compared to other TMT histologies, carcinomas have a propensity for delayed relapse of 5 years or more from initial GCT diagnosis and seldom respond to fluorouracil-based chemotherapy regimens or radiation (20).
Malignant TSCST
Epidemiology
TSCST are the second largest group among primary testicular tumors and comprise approximately 5% of all testicular neoplasms (2,40). TSCST originate from Leydig cells, Sertoli cells and other cells from mesenchymal or hematopoietic origin. Pre-pubertal TSCST are usually benign tumors and have no metastatic potential with the exception of undifferentiated tumors (41). Approximately 10% of adult TSCST can be malignant and criteria for malignancy include unfavorable histopathologic features and presence of metastasis (Table 2) (42-46).
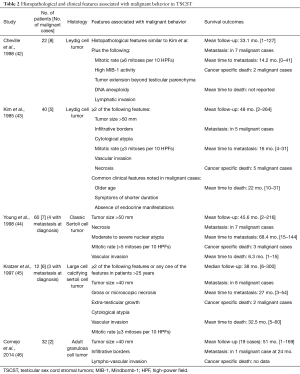
Full table
A retrospective analysis from National Cancer Database (NCDB) demonstrated that patients with stage I TSCST with malignant features had a lower 5-year survival rate compared to those with stage I testicular GCT (47). Patients with metastatic TSCST have poor survival and these tumors are usually resistant to chemotherapy and radiotherapy (48). Rare occurrence of TSCST and limited clinical data make optimization of treatment strategies for advanced disease challenging. Nevertheless, a recent comprehensive molecular profiling study detected potentially actionable mutations in approximately 40% of adult granulosa cell tumors of the ovary (49). With advancements in the use of high-throughput genomic, epigenomic and proteomic assays, future patients with TSCST could benefit from novel treatments and molecular-guided therapies.
Malignant Leydig cell tumors
Leydig cell tumors comprise approximately 3% of all testicular tumors (43). Although they can be seen over a wide age range (2–90 years), they mostly occur in patients between the ages of 30 and 60 years (42,43). Most patients develop a palpable mass (up to 90%), yet 15% of patients present with gynecomastia. Endocrine abnormalities such as precocious puberty in children and sexual dysfunction in adults can be associated with Leydig cell tumors. Bilateral tumors occur in about 2% of all patients and approximately 5% have a history of Klinefelter’s syndrome (50). Most Leydig cell tumors are benign and the prognosis of benign cases is very favorable (51,52). However approximately 10% of Leydig cell tumors are malignant. The aggressive morphologic features associated with metastatic progression in Leydig cell tumors are cytologic atypia, necrosis, angiolymphatic invasion, increased mitotic activity, atypical mitotic figures, infiltrative margins, extension beyond the testicular parenchyma, DNA aneuploidy, and increased Mindbomb-1 (MIB-1) activity (2,42,43).
Testis-sparing surgery (TSS) is a reasonable treatment option for benign Leydig cell tumors (50). In a multicenter retrospective study that included 56 patients with benign Leydig cell tumors with a mean size of less than 15 mm, overall survival rates for patients treated by either radical orchiectomy or TSS were similar. Local recurrence was noted in only 2 (9.5%) patients, both successfully treated with subsequent radical orchiectomy. No metastatic recurrences were observed after a median follow-up of 43 months. TSS is highly recommended for small, ultrasound-detected, non-palpable intraparenchymal testicular lesions according to the European Association of Urology (EAU) guidelines (53). In patients with symptoms of hormonal disorders, immediate orchiectomy was not recommended due to the possibility of an existing benign TSCST. For stage I TSCST cases with rare or no malignant features, treatment with orchiectomy was sufficient and no metastatic recurrence was observed (54). Likewise, patients in a single institutional cohort that consisted of a total of 48 men with TSCST tumors [Leydig cell (28), Sertoli cell (13), granulosa cell (2) and unclassified (5) tumors], 34 patients with no malignant features at partial or radical orchiectomy were successfully observed without undergoing primary RPLND and experienced no tumor recurrence, albeit with limited follow-up (median 14.5 months) (55). Moreover, the accuracy of frozen section examination to differentiate malignant from benign testicular tumor is excellent as reported in a large case series that included 14 patients with Leydig cell tumors (56). A small case series consisting of 18 patients with Leydig cell (16) and Sertoli cell tumors (2) confirmed that none of the patients with low malignant potential on pathological examination relapsed during follow-up (57).
Although 1- and 5-year overall survival rates of patients with malignant appearing stage I Leydig cell tumors are reported as 98% and 91% following orchiectomy, malignant Leydig cell tumors can be lethal when patients present with distant metastases or experience metastatic progression. In one retrospective case series that included 35 benign and 5 malignant testicular Leydig cell tumor cases, death due to disease occurred in 5 (16%) patients after a median follow-up of 4 years (43,47). Older age, symptoms of shorter duration and absence of endocrine manifestations were the clinical features associated with metastasis. Of note, malignant histopathologic features may not be present at the time of orchiectomy in patients that experience metastatic progression following surgery (58,59). Likewise, in a retrospective analysis of 52 patients, age and favorable pathology in the orchiectomy specimens were not absolute for prognosis as one patient with no unfavorable pathological features at the time of orchiectomy died of metastatic disease progression (60). Thus, the presence of metastasis might be the only reliable indicator of malignant behavior in Leydig cell tumors, whereas the presence of favorable histopathological features in the primary tumor does not always preclude the risk of metastatic progression.
There currently exist no standardized treatment algorithms for malignant Leydig cell tumors (47). For patients with clinical stage I TSCST with two or more malignant features, primary RPLND is a recognized management strategy. Among six patients who underwent RPLND for clinical stage I disease, four were alive with no evidence of disease at a median follow-up of 6.6 years (55). RPLND revealed pathologic lymph node involvement (pN2) in only one of six cases, however the pN2 patient and one pN0 patient died of disease 24 months following surgery. Mosharafa et al. reported that five clinical stage I patients with two or more malignant features who underwent primary RPLND remained alive with no evidence of disease, none of whom had pathologic node-positive disease at the time of surgery (61). Conversely, four patients with clinical stage I malignant Leydig cell tumors, who were initially observed but later developed retroperitoneal recurrence and subsequently managed with RPLND for clinical stage II–III tumors, died of disease progression (61). Thus, evidence suggests (albeit limited) that a delayed RPLND for retroperitoneal recurrence affords little therapeutic value, although there are reports of patients with low volume stage II TSCST at presentation where RPLND resulted in durable responses (55).
Metastatic spread of malignant Leydig tumors usually occurs within 2 years following primary surgical resection (48). The most common site of metastasis is within the retroperitoneal lymph nodes, whereas distant sites include the lungs, mediastinal and cervical lymph nodes, pelvis, liver, and bone (48,61). For patients with high-volume nodal and distant metastases, management options are limited and survival is dismal. RPLND and metastasectomy offer little therapeutic value and advanced disease is resistant to radiotherapy and cisplatin-based chemotherapy. In a retrospective study that consisted of eight patients with bulky stage II and stage III metastatic tumors who underwent RPLND, median survival was only 1.2 years in spite of aggressive surgical therapy (abdominal and pelvic mass excisions, bowel resection, hepatic lobectomy, and splenectomy) and additional postoperative therapy (radiotherapy in one patient and cisplatin-based combination chemotherapy in five patients) (61). In addition to resistance observed with combination chemotherapy, resistance to other single chemotherapeutic agents was also reported in advanced TSCST cases (48). A steroidogenesis inhibitor (mitotane) was utilized for one patient with increased estradiol (105 pg/mL) and testosterone (2,112 ng/dL) levels at the time of metastatic recurrence. However, response to mitotane was partial and the patient eventually died of disease within nine months (59).
Malignant sertoli cell tumors
Sertoli cell tumors comprises approximately 0.5% to 1.5% of all testicular tumors (62). The mean age at diagnosis is 45 years (range, 15–80 years) (44). Patients most often manifest a testicular mass whereas hormonal disorders are usually infrequent. Hormonal abnormalities might occur in patients with androgen insensitivity and Peutz-Jeghers syndrome (44,62). In contrast, large-cell calcifying tumor is a distinct subtype of Sertoli cell tumors and it shows a tendency to occur in younger patients (13 to 34 years) and in patients with complex genetic dysplastic syndromes (63). This subtype demonstrates high tumor bilaterality (44%) and tumor multifocality (28%). Other previously defined subtypes of Sertoli cell tumors such as sclerosing and lipid cell variant, however, are no longer considered distinct subtypes in the recent molecular-based tumor classification (40).
Histopathological criteria associated with malignancy in Sertoli cell tumors are quite similar to malignant Leydig cell tumors: tumor size of 50 mm or greater, necrosis, moderate to severe nuclear atypia, vascular invasion and a mitotic rate of more than five mitoses per 10 high-power fields (HPFs) (44,45). Moreover, Leydig cell tumors with abundant sclerosing stroma appear to metastasize less frequently than more cellular forms (40,62). In a large case series that consisted of 60 patients with Sertoli cell tumors, four patients with malignant tumors had metastasis at initial diagnosis. Additional three patients with malignant tumors developed metastasis subsequently. Among patients with at least 5 years of follow-up, all of the nine patients with benign tumors still remained free of disease and four patients with malignant tumors were still alive with disease. However, two patients who had metastasis at presentation and one patient who developed subsequent metastasis eventually died of disease. Although extremely rare, malignant Sertoli cell tumors have been reported in pre-pubertal patients (64,65). Metastatic progression can occur in the absence of malignant features such as significant pleomorphism or mitosis (64). Thus, neither pre-pubertal status nor histopathological features are sufficient enough to exclude metastasis risk.
Most clinical data regarding management and survival outcomes of malignant Sertoli cell tumors come from small retrospective studies that incorporate all TSCTS types (53,54,61). Treatment, malignancy criteria and follow-up of Sertoli cell tumors are all similar to Leydig cell tumors. In a retrospective analysis of the NCDB, 84% of patients diagnosed with clinical stage I malignant Sertoli cell tumors were treated with radical orchiectomy alone, and the remaining 16% were additionally treated either RPLND, chemotherapy or radiotherapy (47). For 14 patients with stage II/III Sertoli cell tumors, management varied greatly and involved RPLND, chemotherapy or radiotherapy. However, the 5-year overall survival rate for patients with malignant stage I Sertoli cell tumors (77%) was significantly lower than the 5-year overall survival rate for patients with malignant stage I Leydig cell tumors (91%) and stage I seminomas (98%) (47,66). Thus, it was suggested that primary RPLND should be the mainstay of therapy for malignant Sertoli cell tumors, even for stage I disease (47). Whether Sertoli cell tumors exhibit more aggressive biological behavior than other TSCST is undetermined. However, overall survival rates reveal an inferior trend. Thus, patients diagnosed with malignant Sertoli cell tumors should receive sufficient treatment and meticulous follow-up.
Granulosa cell tumors
There are less than 150 reported cases of testicular Granulosa cell tumor (46,67,68). Based on disease onset, they are grouped in two distinct types. Almost 90% of juvenile Granulosa cell tumors occur in infants six months of age or younger (67). All juvenile subtypes show benign behavior despite frequent mitotic activity and they are amenable to TSS. For adult Granulosa cell tumors, mean age at presentation is 40 years (range, 14–87 years) and they mostly occur without endocrine-related symptoms (46). Adult Granulosa cell testicular tumors can show aggressive behavior. Lymphovascular invasion, infiltrative borders, and tumor size of more than 4 cm are features associated with malignant biology. However, most testicular Granulosa cell tumor cases can be managed with radical orchiectomy alone, yet regional lymph node metastasis when present has been reported to respond completely to induction cisplatin-based chemotherapy similarly to conventional GCT types (46,68).
Miscellaneous tumors
Some tumors consist of two or more sex cord stromal elements (mixed sex cord stromal tumors) and can show malignant behavior (40). Gonadoblastoma consists of two distinct components, small sex cord cells and germ cell nests, and 40% of such cases involve both testicles (40,69). Over 50% of gonadoblastoma cases progress to dysgerminoma/seminoma and some of them were reported to have malignant pelvic masses at presentation, thus immediate bilateral orchiectomy/gonadectomy is recommended soon after diagnosis (70,71). Testicular fibrothecomas are other distinct TSCST types that are uniformly benign and no testicular fibrothecoma recurrence has been reported following surgical resection (72). There are also several miscellaneous testicular tumors which are extremely rare and difficult to distinguish from other testicular tumors by imaging and clinical examination (40,73). Tumor-like lesions of ovarian common epithelial types can arise from testis, tunica vaginalis and paratesticular tissues; and some types such as clear cell ADC can show malignant behavior (73,74). Albeit rare, 50% of patients with rete testis carcinomas present with metastasis (75). Management includes radical orchiectomy and RPLND after confirmation of diagnosis; however approximately 40% of patients die within one year and chemotherapy is ineffective.
Surveillance strategies
The optimal follow-up strategy for TSCST remains unknown. Although most metastatic events are reported to occur within 2 years in patients with malignant Leydig cell tumors, metastases have occurred as late as nine years following excision of the primary tumor (48,59). Delayed metastases (seven years following orchiectomy) have even been reported in cases where the primary tumor is devoid of malignant features (59). Although endocrinological abnormalities are more common in benign cases, some patients with malignant Leydig cell tumors have altered steroidogenesis involving hormones such as testosterone, luteinizing hormone, estrone and androstenedione (43,76-78). Moreover, delayed recurrence of malignant testicular Leydig cell cancer confirmed by RPLND was detected resulting from elevated serum estradiol levels (79). Thus, assessment of the pituitary/gonadal axis at both initial and also follow-up visits is reasonable. Of note, GCT markers [AFP, HCG and lactate dehydrogenase (LDH)] were reported as negative for all TSCST in previous case series and routine assessment during follow-up is not indicated (52,62,80).
In summary, cancer surveillance in patients with malignant TSCST includes physical examination, radiographic evaluation of the chest, abdomen and pelvis, and consideration towards intermittent assessment of serum testosterone, estradiol, LH and FSH levels—particularly in patients with functional tumors (59,80). Some authors recommend extending follow-up to 10 to 15 years after surgical resection as late metastatic events, albeit rare, have occurred in patients with TSCST (52,80). Essentially, optimal surveillance for patients with malignant TSCST should be tailored according to the aggressiveness of the disease, health status of the patient and previous treatments received (81).
Conclusions
TMT represents an uncommon type of cisplatin-based chemo-refractory GCT with dismal prognosis in patients with advanced stage disease and a propensity for late recurrence. Optimal management for TMT patients involves a multidisciplinary approach at high-volume referral centers with aggressive surgery and histology-tailored systemic therapy. Surgery can be curative in patients with early stage malignant TSCST while those with high-volume metastatic disease have poor outcomes as these tumors are considered chemoradiation resistant. Further prospective studies are critical to better elucidate the carcinogenesis of such uncommon malignant tumors in order to identify actionable targets, new prognosticators and novel therapeutic approaches.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Dilworth JP, Farrow GM, Oesterling JE. Non-germ cell tumors of testis. Urology 1991;37:399-417. [Crossref] [PubMed]
- Carver BS, Al-Ahmadie H, Sheinfeld J. Adult and pediatric testicular teratoma. Urol Clin North Am 2007;34:245-51. abstract x. [Crossref] [PubMed]
- Mikuz G, Colecchia M. Teratoma with somatic-type malignant components of the testis. A review and an update. Virchows Arch 2012;461:27-32. [Crossref] [PubMed]
- Moran CA, Suster S. Primary germ cell tumors of the mediastinum: I. analysis of 322 cases with special emphasis on teratomatous lesions and a proposal for histopathologic classification and clinical staging. Cancer 1997;80:681-90. [Crossref] [PubMed]
- Preissig SH, Smith MT, Huntington HW. Rhabdomyosarcoma arising in a pineal teratoma. Cancer 1979;44:281-4. [Crossref] [PubMed]
- Ahmed T, Bosl GJ, Hajdu SI. Teratoma with malignant transformation in germ cell tumors in men. Cancer 1985;56:860-3. [Crossref] [PubMed]
- Ulbright TM, Loehrer PJ, Roth LM, et al. The development of non-germ cell malignancies within germ cell tumors. A clinicopathologic study of 11 cases. Cancer 1984;54:1824-33. [Crossref] [PubMed]
- Terenziani M, D'Angelo P, Bisogno G, et al. Teratoma with a malignant somatic component in pediatric patients: the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr Blood Cancer 2010;54:532-7. [PubMed]
- Oosterhuis JW, Suurmeyer AJ, Sleyfer DT, et al. Effects of multiple-drug chemotherapy (cis-diammine-dichloroplatinum, bleomycin, and vinblastine) on the maturation of retroperitoneal lymph node metastases of nonseminomatous germ cell tumors of the testis. No evidence for De Novo induction of differentiation. Cancer 1983;51:408-16. [Crossref] [PubMed]
- Motzer RJ, Amsterdam A, Prieto V, et al. Teratoma with malignant transformation: diverse malignant histologies arising in men with germ cell tumors. J Urol 1998;159:133-8. [Crossref] [PubMed]
- Donadio AC, Motzer RJ, Bajorin DF, et al. Chemotherapy for teratoma with malignant transformation. J Clin Oncol 2003;21:4285-91. [Crossref] [PubMed]
- Davey DD, Ulbright TM, Loehrer PJ, et al. The significance of atypia within teratomatous metastases after chemotherapy for malignant germ cell tumors. Cancer 1987;59:533-9. [Crossref] [PubMed]
- El Mesbahi O, Terrier-Lacombe MJ, Rebischung C, et al. Chemotherapy in patients with teratoma with malignant transformation. Eur Urol 2007;51:1306-11; discussion 1311-2. [Crossref] [PubMed]
- Comiter CV, Kibel AS, Richie JP, et al. Prognostic features of teratomas with malignant transformation: a clinicopathological study of 21 cases. J Urol 1998;159:859-63. [Crossref] [PubMed]
- Malagón HD, Valdez AM, Moran CA, et al. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol 2007;31:1356-62. [Crossref] [PubMed]
- Spiess PE, Pisters LL, Liu P, et al. Malignant transformation of testicular teratoma: a chemoresistant phenotype. Urol Oncol 2008;26:595-9. [Crossref] [PubMed]
- Athanasiou A, Vanel D, El Mesbahi O, et al. Non-germ cell tumours arising in germ cell tumours (teratoma with malignant transformation) in men: CT and MR findings. Eur J Radiol 2009;69:230-5. [Crossref] [PubMed]
- Necchi A, Colecchia M, Nicolai N, et al. Towards the definition of the best management and prognostic factors of teratoma with malignant transformation: a single-institution case series and new proposal. BJU Int 2011;107:1088-94. [Crossref] [PubMed]
- Rice KR, Magers MJ, Beck SD, et al. Management of germ cell tumors with somatic type malignancy: pathological features, prognostic factors and survival outcomes. J Urol 2014;192:1403-9. [Crossref] [PubMed]
- Colecchia M, Necchi A, Paolini B, et al. Teratoma with somatic-type malignant components in germ cell tumors of the testis: a clinicopathologic analysis of 40 cases with outcome correlation. Int J Surg Pathol 2011;19:321-7. [Crossref] [PubMed]
- Ganjoo KN, Foster RS, Michael H, et al. Germ cell tumor associated primitive neuroectodermal tumors. J Urol 2001;165:1514-6. [Crossref] [PubMed]
- Guo CC, Punar M, Contreras AL, et al. Testicular germ cell tumors with sarcomatous components: an analysis of 33 cases. Am J Surg Pathol 2009;33:1173-8. [Crossref] [PubMed]
- Little JS Jr, Foster RS, Ulbright TM, et al. Unusual neoplasms detected in testis cancer patients undergoing post-chemotherapy retroperitoneal lymphadenectomy. J Urol 1994;152:1144-9. [Crossref] [PubMed]
- Giannatempo P, Pond GR, Sonpavde G, et al. Treatment and clinical outcomes of patients with teratoma with somatic-type malignant transformation: an international collaboration. J Urol 2016;196:95-100. [Crossref] [PubMed]
- Wang J, Kazmi SA. Teratoma with malignant transformation: a case report with pathological, cytogenetic, and immunohistochemistry analysis. Sarcoma 2011;2011:450743. [Crossref] [PubMed]
- Sugimura J, Foster RS, Cummings OW, et al. Gene expression profiling of early- and late-relapse nonseminomatous germ cell tumor and primitive neuroectodermal tumor of the testis. Clin Cancer Res 2004;10:2368-78. [Crossref] [PubMed]
- Little JS Jr, Foster RS, Ulbright TM, et al. Unusual neoplasms detected in testicular cancer patients undergoing postchemotherapy retroperitoneal lymphadenectomy. World J Urol 1994;12:200-6. [Crossref] [PubMed]
- True LD, Otis CN, Delprado W, et al. Spermatocytic seminoma of testis with sarcomatous transformation. A report of five cases. Am J Surg Pathol 1988;12:75-82. [Crossref] [PubMed]
- Korski K, Breborowicz D, Filas V, et al. A case of primary testicular germ cell tumor with rhabdomyosarcoma metastases as an example of applying the FISH method to diagnostic pathology. APMIS 2007;115:1296-301. [Crossref] [PubMed]
- Pantoja E, Roswit B. Radiotherapy in malignant teratomas. Cancer Lett 1975;1:103-7. [Crossref] [PubMed]
- de Alava E, Gerald WL. Molecular biology of the Ewing's sarcoma/primitive neuroectodermal tumor family. J Clin Oncol 2000;18:204-13. [Crossref] [PubMed]
- Michael H, Hull MT, Ulbright TM, et al. Primitive neuroectodermal tumors arising in testicular germ cell neoplasms. Am J Surg Pathol 1997;21:896-904. [Crossref] [PubMed]
- Azizi M, Peyton CC, Spiess PE. Primitive neuroectodermal tumor arising from an untreated congenital undescended testicle. Can J Urol 2018;25:9530-3. [PubMed]
- Eble JN, Sauter G, Epstein JJ. World Health Organization classification of tumors. Pathology and genetics tumors of the urinary system and male genital organs. Lyon: IARC Press, 2004.
- Kernek KM, Brunelli M, Ulbright TM, et al. Fluorescence in situ hybridization analysis of chromosome 12p in paraffin-embedded tissue is useful for establishing germ cell origin of metastatic tumors. Mod Pathol 2004;17:1309-13. [Crossref] [PubMed]
- Ehrlich Y, Beck SD, Ulbright TM, et al. Outcome analysis of patients with transformed teratoma to primitive neuroectodermal tumor. Ann Oncol 2010;21:1846-50. [Crossref] [PubMed]
- Al-Hader AA, Jain A, Al-Nasrallah N, et al. Metastatic malignant transformation of teratoma to primitive neuroectodermal tumor (PNET): results with PNET-based chemotherapy. Am J Clin Oncol 2015;38:364-6. [Crossref] [PubMed]
- Kasai T, Moriyama K, Tsuji M, et al. Adenocarcinoma arising from a mature cystic teratoma of the testis. Int J Urol 2003;10:505-9. [Crossref] [PubMed]
- Idrees MT, Ulbright TM, Oliva E, et al. The World Health Organization 2016 classification of testicular non-germ cell tumours: a review and update from the International Society of Urological Pathology Testis Consultation Panel. Histopathology 2017;70:513-21. [Crossref] [PubMed]
- Thomas JC, Ross JH, Kay R. Stromal testis tumors in children: a report from the prepubertal testis tumor registry. J Urol 2001;166:2338-40. [Crossref] [PubMed]
- Cheville JC, Sebo TJ, Lager DJ, et al. Leydig cell tumor of the testis: a clinicopathologic, DNA content, and MIB-1 comparison of nonmetastasizing and metastasizing tumors. Am J Surg Pathol 1998;22:1361-7. [Crossref] [PubMed]
- Kim I, Young RH, Scully RE. Leydig cell tumors of the testis. A clinicopathological analysis of 40 cases and review of the literature. Am J Surg Pathol 1985;9:177-92. [Crossref] [PubMed]
- Young RH, Koelliker DD, Scully RE. Sertoli cell tumors of the testis, not otherwise specified: a clinicopathologic analysis of 60 cases. Am J Surg Pathol 1998;22:709-21. [Crossref] [PubMed]
- Kratzer SS, Ulbright TM, Talerman A, et al. Large cell calcifying Sertoli cell tumor of the testis: contrasting features of six malignant and six benign tumors and a review of the literature. Am J Surg Pathol 1997;21:1271-80. [Crossref] [PubMed]
- Cornejo KM, Young RH. Adult granulosa cell tumors of the testis: a report of 32 cases. Am J Surg Pathol 2014;38:1242-50. [Crossref] [PubMed]
- Banerji JS, Odem-Davis K, Wolff EM, et al. Patterns of care and survival outcomes for malignant sex cord stromal testicular cancer: results from the national cancer data base. J Urol 2016;196:1117-22. [Crossref] [PubMed]
- Bertram KA, Bratloff B, Hodges GF, et al. Treatment of malignant Leydig cell tumor. Cancer 1991;68:2324-9. [Crossref] [PubMed]
- Rowland M, McMeekin S, Moore K, et al. Abstract A79: comprehensive genomic profiling (CGP) of adult granulosa cell tumors (aGCT) identifies clinically relevant genomic alterations (CRGA) and targeted therapy options. Molecular Cancer Therapeutics 2015;14:A79.
- Laclergerie F, Mouillet G, Frontczak A, et al. Testicle-sparing surgery versus radical orchiectomy in the management of Leydig cell tumors: results from a multicenter study. World J Urol 2018;36:427-33. [Crossref] [PubMed]
- Giannarini G, Mogorovich A, Menchini Fabris F, et al. Long-term followup after elective testis sparing surgery for Leydig cell tumors: a single center experience. J Urol 2007;178:872-6. [Crossref] [PubMed]
- Suardi N, Strada E, Colombo R, et al. Leydig cell tumour of the testis: presentation, therapy, long-term follow-up and the role of organ-sparing surgery in a single-institution experience. BJU Int 2009;103:197-200. [Crossref] [PubMed]
- Albers P, Albrecht W, Algaba F, et al. Guidelines on testicular cancer: 2015 update. Eur Urol 2015;68:1054-68. [Crossref] [PubMed]
- Featherstone JM, Fernando HS, Theaker JM, et al. Sex cord stromal testicular tumors: a clinical series--uniformly stage I disease. J Urol 2009;181:2090-6; discussion 2096. [Crossref] [PubMed]
- Silberstein JL, Bazzi WM, Vertosick E, et al. Clinical outcomes of local and metastatic testicular sex cord-stromal tumors. J Urol 2014;192:415-9. [Crossref] [PubMed]
- Elert A, Olbert P, Hegele A, et al. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur Urol 2002;41:290-3. [Crossref] [PubMed]
- Conkey DS, Howard GC, Grigor KM, et al. Testicular sex cord-stromal tumours: the Edinburgh experience 1988-2002, and a review of the literature. Clin Oncol (R Coll Radiol) 2005;17:322-7. [Crossref] [PubMed]
- Muheilan MM, Shomaf M, Tarawneh E, et al. Leydig cell tumor in grey zone: a case report. Int J Surg Case Rep 2017;35:12-6. [Crossref] [PubMed]
- Schwarzman MI, Russo P, Bosl GJ, et al. Hormone-secreting metastatic interstitial cell tumor of the testis. J Urol 1989;141:620-2. [Crossref] [PubMed]
- Di Tonno F, Tavolini IM, Belmonte P, et al. Lessons from 52 patients with leydig cell tumor of the testis: the GUONE (North-Eastern Uro-Oncological Group, Italy) experience. Urol Int 2009;82:152-7. [Crossref] [PubMed]
- Mosharafa AA, Foster RS, Bihrle R, et al. Does retroperitoneal lymph node dissection have a curative role for patients with sex cord-stromal testicular tumors? Cancer 2003;98:753-7. [Crossref] [PubMed]
- Giglio M, Medica M, De Rose AF, et al. Testicular sertoli cell tumours and relative sub-types. Analysis of clinical and prognostic features. Urol Int 2003;70:205-10. [Crossref] [PubMed]
- Plata C, Algaba F, Andujar M, et al. Large cell calcifying Sertoli cell tumour of the testis. Histopathology 1995;26:255-9. [Crossref] [PubMed]
- Rosvoll RV, Woodard JR. Malignant Sertoli cell tumor of the testis. Cancer 1968;22:8-13. [Crossref] [PubMed]
- Kolon TF, Hochman HI. Malignant Sertoli cell tumor in a prepubescent boy. J Urol 1997;158:608-9. [Crossref] [PubMed]
- Mortensen MS, Lauritsen J, Gundgaard MG, et al. A nationwide cohort study of stage I seminoma patients followed on a surveillance program. Eur Urol 2014;66:1172-8. [Crossref] [PubMed]
- Kao CS, Cornejo KM, Ulbright TM, et al. Juvenile granulosa cell tumors of the testis: a clinicopathologic study of 70 cases with emphasis on its wide morphologic spectrum. Am J Surg Pathol 2015;39:1159-69. [Crossref] [PubMed]
- Elbachiri M, Taleb A, Derrabi N, et al. Adult-type granulosa cell tumor of the testis: report of a case and review of literature. Pan Afr Med J 2017;26:198. [Crossref] [PubMed]
- Roth LM, Cheng L. Classical gonadoblastoma: its relationship to the 'dissecting' variant and undifferentiated gonadal tissue. Histopathology 2018;72:545-55. [Crossref] [PubMed]
- Huang H, Wang C, Tian Q. Gonadal tumour risk in 292 phenotypic female patients with disorders of sex development containing Y chromosome or Y-derived sequence. Clin Endocrinol (Oxf) 2017;86:621-7. [Crossref] [PubMed]
- Scully RE. Gonadoblastoma. A review of 74 cases. Cancer 1970;25:1340-56. [Crossref] [PubMed]
- Zhang M, Kao CS, Ulbright TM, et al. Testicular fibrothecoma: a morphologic and immunohistochemical study of 16 cases. Am J Surg Pathol 2013;37:1208-14. [Crossref] [PubMed]
- Young RH, Scully RE. Testicular and paratesticular tumors and tumor-like lesions of ovarian common epithelial and mullerian types. A report of four cases and review of the literature. Am J Clin Pathol 1986;86:146-52. [Crossref] [PubMed]
- Jones MA, Young RH, Srigley JR, et al. Paratesticular serous papillary carcinoma. A report of six cases. Am J Surg Pathol 1995;19:1359-65. [Crossref] [PubMed]
- Klotz T, Schwindl B, Mathers MJ. Carcinoma of the rete testis with lymphogenous metastasis: multimodal treatment. Urologe A 2012;51:409-11. [Crossref] [PubMed]
- Davis S, Di Martino NA, Schneider G. Malignant interstitial cell carcinoma of the testis: report of two cases with steroid synthetic profiles, response to therapy, and review of the literature. Cancer 1981;47:425-31. [Crossref] [PubMed]
- Chen KT, Spaulding RW, Flam MS, et al. Malignant interstitial cell tumor of the testis. Cancer 1982;49:547-52. [Crossref] [PubMed]
- Gabrilove JL, Nicolis GL, Mitty HA, et al. Feminizing interstitial cell tumor of the testis: personal observations and a review of the literature. Cancer 1975;35:1184-202. [Crossref] [PubMed]
- Maeda T, Itoh N, Kobayashi K, et al. Elevated serum estradiol suggesting recurrence of Leydig cell tumor nine years after radical orchiectomy. Int J Urol 2002;9:659-61. [Crossref] [PubMed]
- Carmignani L, Colombo R, Gadda F, et al. Conservative surgical therapy for leydig cell tumor. J Urol 2007;178:507-11; discussion 511. [Crossref] [PubMed]
- Acar C, Gurocak S, Sozen S. Current treatment of testicular sex cord-stromal tumors: critical review. Urology 2009;73:1165-71. [Crossref] [PubMed]


