
Neurogenic bladder: management of the severely impaired patient with complete urethral destruction: ileovesicostomy, suprapubic tube drainage or urinary diversion—is one treatment modality better than another?
Introduction
In patients with neurologic impairment, a segment of the population exists that are unable to be compliant with clean intermittent catheterization (CIC) due to either; cognitive impairment, physical limitations, inadequate social support for surrogate CIC or the patient choosing to be noncompliant with medical directives. In these problematic patients, management of the neurogenic bladder (NGB) is challenging. Indeed, in spite of the physician informing these patients regarding the risks, alternative and benefits of various treatment modalities that are available, the patient will often choose to simplify management by use of an indwelling urethral catheter (1,2). Although patients may successfully be managed for several years by a urethral catheter, long term complications leading to chronic pericatheter urinary incontinence will occasionally arise (2,3). Classically these complications will occur due to either the Foley balloon inducing pressure necrosis of the bladder neck and/or inadvertent repetitive dislodgment of the Foley catheter devastating the urinary outlet (4,5). In any event, once chronic urinary incontinence around the indwelling urethral catheter is established, maceration of the perineal skin occurs resulting in an increased risk for the development of high-grade decubitus ulcers and osteomyelitis (4,5).
In patients where a chronic indwelling catheter has resulted in urethral erosion, and use of intermittent catheterization is not a possibility, we classically pursued treatment by one of three modalities: closure of the bladder neck and suprapubic tube (SPT) placement, closure of the bladder neck with an incontinent ileovesicostomy “ileal chimney” or cystectomy with urinary conduit diversion. The purpose of this paper is to test the hypothesis that an ileovesicostomy is the best treatment alternative (1,6-11).
Methods
A prospective patient registry that includes individuals seen for management of a NGB has been maintained since 1986. The permission to maintain the database and the study has been approved by the Mayo Ethical and Research Committee (IRB 07-003450) and informed consent for inclusion obtained from all patients.
Only individuals with urethral erosion and intractable urinary incontinence arising as a consequence of a long term indwelling urethral catheter (>1 yr) are included in the study.
All 29 female patients had previously failed management of their intractable urinary incontinence with occlusive slings and concurrent placement of a SPT. All 19 of the men included in this study had previously been managed with a limited sphincterotomy and condom catheter drainage. With advancing age, the male patients had lost their ability to maintain the condom catheter and all were eventually managed with an indwelling urethral catheter. In the men, this management plan became a non-viable option due to persistent pericatheter urinary incontinence in 11 patients, and intractable urinary incontinence associated with repetitive life-threatening autonomic dysreflexia secondary to the recurrent dislodgment of the Foley balloon into the urethra in eight patients.
At the time of initial evaluation, we performed a baseline physical examination, renal function determination, urodynamic and radiographic studies. All patients admitted to the study had to have a baseline renal function ≥75 mL/min/m2 (12). Patients noted to have developed stage 3 chronic renal failure during follow-up, had three separate renal clearance studies each separated by a minimum of a month time interval where repetitive renal clearance values were <60 mL/min/m2.
Video urodynamic studies were performed using either a 5 or 8 F double-lumen urethral catheter with urodynamic fill rates of 25 mL/min. If urinary incontinence was established at low leak point pressures (<15 cmH2O) at volumes of <150 mL, a Foley catheter was placed, and a balloon was used to occlude the bladder neck. Only patients without evidence of vesicoureteral reflux at the time of preoperative videourodynamics evaluation were included in this study.
If the baseline renal ultrasound suggested the presence of hydronephrosis, not associated with vesicoureteral reflux, nuclear scintigraphy using MAG 3 Lasix washout renal scans were performed to rule out obstruction. No patient with radiographic evidence of upper tract obstruction was admitted into the study.
In patients where baseline radiographic studies suggested the presence of a renal or bladder calculi, a non-contrast computed tomography (CT) scan was performed. Individuals with pre-existing renal calculi were excluded from the study. If renal ultrasonography suggested the presence of renal scars either a dimercaptosuccinic acid (DMSA) evaluation or CT urogram was performed to evaluate for the presence of pre-existing renal scarring. Patients with a pre-existing renal scar were admitted into the study only after their scar had been characterized by radiographic studies.
Key patient characteristics that could impact long term results were recorded. Specifically, patients were divided into patients with quadriplegia (injuries to C1-7), or people with paraplegia (injuries from T1 to L5), and all patients were either American Spinal Cord Injury Association Scale A or B (13). The number of years that the indwelling urethral catheter had been in situ prior to treatment is noted. Body mass index (BMI) at the time of our surgical intervention, and the presence of metabolic syndrome, that is the presence of the triad of hypertension, hyperlipidemia, and elevation in fasting blood sugar was documented. Morbid obesity was defined as a BMI of >35–40 and concurrent metabolic syndrome or a BMI of >40 (14).
Patients were offered the options of SPT placement with the closure of the bladder neck, ileovesicostomy with the closure of the bladder neck or cystectomy with supra vesical urinary diversion with an enteric conduit (4,5). Three critical points regarding the selection of operative procedure are noteworthy: (I) all patients that had undergone a previous transurethral resection of an inflammatory urothelial polyp underwent cystectomy and enteric conduit diversion (15). Cystectomy was recommended in this patient population due to concerns for urothelial cancer development (16). (II) All patients with an end filled detrusor pressure of >40 cm of water at a capacity of ≤125 mL were deemed to have an end-stage bladder, and due to concerns we would not have enough bladder volume to perform a successful bladder neck closure (BNC) cystectomy and urinary conduit recommended (5). (III) All patients who were morbidly obese who did not undergo a cystectomy due to the above criteria, were treated with the closure of the bladder neck-proximal urethra and SPT placement. In this patient population, closure of the bladder neck/proximal urethra was invariably accomplished via a perineal/vaginal approach. This approach was used due to the patient’s obesity making a retropubic approach an extremely demanding technical procedure (3).
Postoperatively patients were encouraged to maintain a urine output of >2,400 mL per day. In individuals with an indwelling SPT who did not meet this minimal daily requirement, we irrigated the bladder with 250 mL saline daily to aid in the prevention of stone formation (17).
We routinely obtained a cystogram and renal ultrasound at three months post BNC to evaluate for the presence of vesicoureteral reflux or upper tract obstruction, secondary to BNC. After that, radiographic assessments consisted of yearly renal-bladder ultrasound and kidney, ureter, and bladder (KUB). In patients who had undergone a BNC, additional studies with both cystography and cystoscopy were obtained if follow up renal ultrasounds revealed the new onset of, or worsening hydronephrosis, if ≥4 symptomatic urinary tract infections had occurred within a years’ time span or if preliminary radiographic studies suggested the presence of a bladder stone. In the presence of new onset or progressive hydronephrosis and the absence of reflux, both a CT urogram and MAG 3 Lasix washout renal studies were performed.
In patients with an ileovesicostomy and BNC with new onset, hydronephrosis or new onset of urolithiasis, we also performed a videourodynamic study to verify the ileovesicostomy was draining under low pressure. A detrusor leak point pressure of <40 cm of water was considered normal (6,16,18).
All upper and lower tract calculi were reported. If screening radiographic studies suggested the new onset of and or progression of renal scarring, a DMSA renal scan or CT scan with contrast was obtained.
Yearly evaluations consisted of a review of the patient’s interval medical history, serum creatinine, cystatin C (from 2003 to date). Renal clearance was determined by iothalamate, from 1986–2005, and by cystatin C, 2003 to date (12,19-21). All patients with ileum used as part of their reconstructive procedures had yearly serum B12 levels measured. Individuals were considered to be below normal if a serum B12 levels of <200 ng/mL were obtained (22).
We defined an uroseptic episode as a hospitalization where intravenous antibiotics were administrated for treatment of positive blood culture, with the same bacteria species noted to be present in both blood and urine cultures. All patients with a diagnosis of urosepsis had either a concurrent temperature of >38.5 °C and/or a significant alteration in mental status at the time of hospitalization. It is noteworthy that this paper does not review perioperative complications that occurred, ≤30 days post procedure.
Statistical evaluations used either chi-squared contingency table analysis, Fisher’s exact 2-tailed tests, or Kaplan-Meier curve analysis where indicated, P values of <0.05 were considered significant.
Results
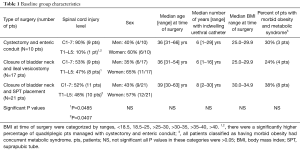
Table 1 delineates the baseline values of the study populations at the time of their surgical procedure. The only difference between the study groups is the significantly increased incidence of people with quadriplegia (C1-7 spinal cord injuries) in the patients managed by cystectomy and enteric conduit, vs. those maintained by the closure of the bladder neck and ileovesicostomy or SPT placement, P=0.0485 and 0.0407, respectively.
Full table
Table 2 reflects the complications found following our surgical intervention. Not revealed in this table is the finding that all bladder neck ligations were initially successful with no revisions required. Of note, the number of hospitalizations needed for the treatment of urosepsis was significantly higher in patients managed with cystectomy and enteric conduit formation and ligation of the bladder neck and ileovesicostomy than bladder neck ligation and SPT P=0.025 and 0.006, respectively. It is noteworthy that despite the increased episodes of urosepsis in patients managed with an enteric urinary conduit or ileovesicostomy we did not find a significant difference in the incidence of renal scarring or the onset of stage 3 chronic renal failure between the study populations.

Full table
Irrespective of what type of treatment we employed the incidence of urolithiasis was quite elevated with approximately 30–50% of our patients developing either bladder or kidney stones during a combined median follow-up interval of 8 yrs, range 2–30 yrs. All of the stones that were evaluated were struvite in composition.
In patients managed by an ileovesicostomy, 82% (14/17) had a history of urosepsis, urolithiasis, or new onset of hydronephrosis prompting performance of a video urodynamic study in the upright sitting position. A detrusor leak point pressure of <20 cm of water was found in 65% (11/17) (16,18). An abnormal detrusor leak point pressure >40 cmH2O was found in 18% (3/17) two were elevated due to stomal stenosis, and one was associated with a parastomal hernia all required surgical revision (16,18). See Tables 2,3.

Full table
A total of 41% (39/96) of the kidneys that were at risk developed new renal scars. Over 62% (24/39) of the renal scarring produced was in association with urolithiasis, 31% (12/39) were associated with anatomic outlet obstruction, e.g., stomal stenosis, parastomal hernia, ureteroenteric stricture. It is noteworthy that 21% (8/39) of the renal scars occurred in the presence of vesicoureteral reflux. The vesicoureteral reflux in all of these patients arose as a secondary response to either outlet obstruction (stomal stenosis, parastomal hernia) in 50% (4/8) or bladder calculi in 50% (4/8). In essence, 92% (36/39) of the patients with new onset of renal scarring were associated with the development of either urolithiasis, anatomic obstruction (stomal stenosis, parastomal hernia or ureteral-enteric obstruction) and/or vesicoureteral reflux. In 8% (3/39) of the patients, the new scar occurred after a febrile UTI, with no known association with the three factors above. See Table 2.
A total of 35% (17/48 pts) developed the new onset of stage 3 chronic renal failure. In 59% (10/17 pts), of the individuals, stage 3 renal failure developed following an episode of urolithiasis and urosepsis and in 35% (6/17) it occurred following the development of anatomic outlet obstruction (stomal or ureteroenteric stenosis). In the one remaining patient with new onset of ≥ grade 3 renal failure, no underlying urologic cause could be identified; the patient was however morbidly obese with concurrent metabolic syndrome with documented poorly controlled diabetes mellitus and hypertension.
Table 3 reflects the need for surgical intervention not related to the treatment of urolithiasis. When excluding the need for surgery due to urolithiasis, individuals undergoing either an enteric conduit or ileovesicostomy required significantly more surgical interventions than individuals experiencing placement of a SPT, P=0.0126 and 0.0023, respectively. When evaluating the need for surgical intervention due to either urolithiasis or other complications, a total of 50% (5/10) of the patients managed by an ileal conduit required operative intervention, 40% (4/10) required more than one intervention. In patients managed by BNC and ileovesicostomy, 88% (15/17) of the patients required a significant surgical intervention, and 76% (13/17) required more than one intervention. In patients managed by BNC, and placement of a SPT 52% (11/21) patients required surgical intervention, chiefly for stone disease, 19% (4/21) required more than one intervention. It is noteworthy that patients undergoing BNC and ileovesicostomy required significantly more surgical interventions than patients managed with either a cystectomy and ileal conduit (P=0.0285) or BNC and SPT management (P=0.0180). No significant difference in the need for additional surgery between cystectomy and ileal conduit or BNC and SPT management are noted (P=0.901).
Discussion
The preservation of long term renal function in patients with a NGB is stated to be directly related to five factors; the presence of an indwelling catheter, high detrusor storage pressures, the development of hydroureteronephrosis (usually due to vesicoureteral reflux), urolithiasis, and a BMI >35 associated with metabolic syndrome (1,14,23-28). In this regard, it is noteworthy that all of our three treatment categories were statistically similar regarding BMI, the presence of metabolic syndrome and the length that a urethral catheter had been in situ before our surgical procedure, see Table 1. Also, it should be noted that all patients included in the study were free from urolithiasis at the time of our surgical intervention. It is, therefore, our assumption that any significant alteration in renal function or new onset of renal scars that developed post-surgery is primarily due to our intervening surgical procedure. This paper only discusses the long term complications following the operative intervention; we did not discuss or review immediate perioperative complications. In this regard, it is imperative to mention that Clavien grade 3 or higher complications arising within the first 30 days following surgery are reported to occur in up to 30% of the patients following a cystectomy and diversion for benign conditions. Clavien grade 3 or higher complications are also reported to occur in 10–20% of patients undergoing BNC and ileovesicostomy or SPT placement (5,6,24,29).
Popularization of the ileovesicostomy
Over the past thirty years, we have managed several individuals who developed a devastated bladder outlet due to an indwelling urethral catheter. From 1986 to 1994, we routinely offered two options for management, either bladder neck ligation, and SPT placement or cystectomy with a urinary enteric conduit diversion (4,5,15). In 1994 we added a third alternative treatment modality, closure of the bladder neck and ileovesicostomy (6,7). The addition of this operative procedure to our surgical armamentarium was due to the hypothesis that ileovesicostomy could provide several benefits over the other two alternatives. Specifically it was believed this procedure would reduce the complications of stone disease (no catheter serving as a nidus for stone formation), result in fewer episodes of urosepsis, reduce the complications of vesicoureteral reflux induced by indwelling catheter and would be free from the ureteroenteric strictures associated with an enteric urinary conduit (1,6-11). Due to the purported advantages and the assumed low risk, multiple authors suggested that ileovesicostomy should be considered the treatment of choice in patients who are nonresponsive to pharmacologic treatment and unable to perform intermittent catheterization. Certainly, it was believed that this treatment would be far superior to an indwelling SPT (6,7,9,10,18,23,27-31).
Ileovesicostomy and obesity
Unfortunately, as can be seen from our data, the purported hypothetical advantages of the ileovesicostomy were far from our experience. See Tables 2,3. The question arises why the high incidence of complications following this procedure? In this regard, four specific problems are noteworthy; obesity, stomal complications, urolithiasis, and urosepsis. First and foremost, when considering the performance of an ileovesicostomy in a patient with neuropathy, the problem of progressive obesity found in the neurologically impaired patient population needs to be addressed. This patient population will experience up to a 50% decrease in basal energy metabolism due to the significant loss of muscle and skeletal mass found with a progressive neuropathy or spinal cord injury (14,32). The decrease in basal metabolic rate combined with a decrease in physical activity related to the ongoing neurologic process results in substantial weight gain as the patient's age. Indeed, a 2.5-fold higher incidence of obesity is found in the neurologically impaired patient population compared to an age; race and sex-matched nondisabled controls (14,32). This increase in BMI correlates to not only problems with stomal complications but a high risk of obesity-related disease that can impact renal function, e.g., hypertension, dyslipidemia, diabetes mellitus (14,28,32-34).
When the concept of ileovesicostomy was initially popularized, there were concerns, raised that stomal complications could become problematic during long term follow up (6). Specifically, stomal complications following urinary diversion for benign conditions are reported to range from 8–48% depending upon; the length of follow-up, the patients BMI and the level and completeness of the spinal cord injury (33-35). In this regard, an obese patient (BMI >30), with complete spinal cord injuries above T-10 will be at an increased risk for both stomal complications and problems with collecting appliance adhesion (33,34). The increased problems with stomal complications are related to denervation atrophy of the lower abdominal rectus muscles, resulting in an increased risk of parastomal hernias and conduit prolapse. Compounding these stomal problems in obese paraplegic and quadriplegic patients is the irregular folding contour of the lower abdomen that almost invariably causes a poor fit of the collection appliance (28,33,34). In fact, in obese patients with spinal cord injuries at T-10 or above, the preferred location of a stoma is usually in the upper abdominal quadrants, this position allowing for a better fit of the collection appliance (33,34). Unfortunately, when performing an ileovesicostomy, a short ileal loop to the lower abdominal quadrant is highly recommended (6,7,16,18). The shortened length helps prevent redundancy and kinking that could inhibit urinary drainage (16,18). However, placement of the stoma in the lower quadrant leads to progressive stomal appliance problems as the neuropathically impaired patient gains weight. Indeed, long term follows up reveals that 25% of ileovesicostomy patients will develop a poor fit for the appliance when the stoma is placed in the lower quadrants (6,11,28). In our ileovesicostomy patient population, approximately 24% (4/17) of the patients met the high-risk criteria for stomal complications specifically, a spinal cord injury classified as ASIA A or B, above or at the T 10 level, with a BMI of >30 (28,33,34). All four of our patients that fell in this high-risk category required revision surgery related to their ileovesicostomy. Our finding that obesity is related to ileovesicostomy complications is not novel and has been reported on previously (28).
Ileovesicostomy, urolithiasis and vesicoureteral reflux
One of our most disturbing findings is the high incidence of urolithiasis and urosepsis found with ileovesicostomy. These complications were noted despite our documentation that 82% of our ileovesicostomy patients had low-pressure leakage in the sitting position (<20 cmH2O). In our experience, we found that the ileovesicostomy, predisposes to stagnant, poorly drained urine, with mucous and debris accumulating in the depths of the bladder. Indeed, the presence of both calcified and non-calcified mucous debris was almost invariably seen during follow up radiologic studies. Unfortunately, we believe that the retained mucous debris or bladder stones served as a nidus for bladder mucosal irritation and resulted in the new onset of vesicoureteral reflux in 21% (7/34) of our renal units at risk. This poorly drained system in combination with persistent mucous debris, bladder calculi, and the frequent occurrence of concurrent vesicoureteral reflux, resulted in 82% (14/17) of our patients having repetitive urosepsis episodes. Our experience is not unique, and other authors have seen this sequale and attempted to manage this problem with bladder irrigations (28,31). Unfortunately, attempts to have patients who had already documented that they were either unable or unwilling to be compliant with CIC, place a catheter in the stoma and irrigate out the mucous was universally unsuccessful in our hands. Due to multiple recurrent urosepsis episodes, we took down the ileovesicostomy in 29% (5/17) of our patients.
Is ileovesicostomy really better than SPT diversion?
To our knowledge, this is the first publication to directly compare and contrast management of a neuropathic bladder with an ileovesicostomy versus a SPT. It is interesting to note that our findings are opposed to the leading hypothesis regarding ileovesicostomy specifically that a patient managed by an ileovesicostomy would have significant advantages over one managed with a SPT. Prior publications hypothesized that there would be fewer complications of stone disease, fewer episodes of urosepsis, and a reduction in the incidence of vesicoureteral reflux (1,6-11). Our data fail to confirm that hypothesis. Indeed, we provide evidence that management with an ileovesicostomy did not decrease the incidence of urolithiasis, did not prevent the development of vesicoureteral reflux and was associated with a higher risk for urosepsis, see Table 2. Especially when compared to management with a SPT, the ileovesicostomy was associated with a significantly increased need for hospitalizations for recurrent urosepsis, (P=0.006) and the need for surgical intervention not related to urolithiasis (P=0.0023) compared to management with a SPT. In addition to these concerns, 12% (2/17) of the patients managed by an ileovesicostomy developed a small bowel obstruction requiring reoperation and 12% (2/17) developed a B12 deficiency, two risks not associated with patients managed by a SPT. In essence, in our experience, we had significantly worse outcomes, as defined by more complications, in patients managed with an ileovesicostomy compared those managed with a SPT. Indeed it is our opinion that an indwelling catheter is not the worst of the alternative treatment methods used to manage a NGB (2). In fact, in individuals who are either physically unable or socially unwilling to perform intermittent catheterization, we have abandoned offering these patients an ileovesicostomy as an alternative procedure preferring to manage patients with a SPT.
Cystectomy and ileal conduit urinary diversion as an option
In patients with an end-stage NGB that are not candidates for BNC, cystectomy and ileal conduit urinary diversion does remain a viable alternative but is far from ideal. In our experience, 50% (5/10) of the patients required surgical intervention due to complications, during a median follow up of 11 yrs, range 2–30 yrs. This incidence of complication is well within the range of those previously published that reveal approximately 60% of patients at 10 years of follow up will require a surgical intervention following an ileal conduit. Surgical interventions predominantly revolve around the treatment of urolithiasis, stomal/peristomal complications, ureteral-enteric stenosis, incisional hernias, and small bowel obstruction (35-37).
Conclusions
In conclusion, although there is no perfect treatment modality, for the patient with a NGB who refuses or is unable to perform intermittent catheterization, we currently prefer treatment with SPT placement or where indicated cystectomy and urinary conduit formation. Due to the significant long-term complications, we have seen following ileovesicostomy we have dropped this procedure from our surgical armamentarium.
Acknowledgments
The authors would like to thank Sue Rathbun, who has helped maintain the prospective database on patients with neurogenic bladder impairment.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The permission to maintain the database and the study has been approved by the Mayo Ethical and Research Committee (IRB 07-003450) and informed consent for inclusion obtained from all patients.
References
- Cameron AP, Wallner LP, Tate DG, et al. Bladder management after spinal cord injury in the United States 1972 to 2005. J Urol 2010;184:213-7. [Crossref] [PubMed]
- Weld KJ, Dmochowski RR. Effect of bladder management on urological complications in spinal cord injured patients. J Urol 2000;163:768-72. [Crossref] [PubMed]
- Stoffel JT, McGuire EJ. Outcome of urethral closure in patients with neurologic impairment and complete urethral destruction. Neurourol Urodyn 2006;25:19-22. [Crossref] [PubMed]
- Shpall AI, Ginsberg DA. Bladder neck closure with lower urinary tract reconstruction: technique and long-term followup. J Urol 2004;172:2296-9. [Crossref] [PubMed]
- Colli J, Lloyd LK. Bladder neck closure and suprapubic catheter placement as definitive management of neurogenic bladder. J Spinal Cord Med 2011;34:273-7. [Crossref] [PubMed]
- Schwartz SL, Kennelly MJ, McGuire EJ, et al. Incontinent ileo-vesicostomy urinary diversion in the treatment of lower urinary tract dysfunction. J Urol 1994;152:99-102. [Crossref] [PubMed]
- Rivas DA, Karasick S, Chancellor MB. Cutaneous ileocystostomy (a bladder chimney) for the treatment of severe neurogenic vesical dysfunction. Paraplegia 1995;33:530-5. [PubMed]
- Stoffel JT. Paediatric urology: first study of incontinent ileovesicostomy in children. Nat Rev Urol 2013;10:621-2. [Crossref] [PubMed]
- Mutchnik SE, Hinson JL, Nickell KG, et al. Ileovesicostomy as an alternative form of bladder management in tetraplegic patients. Urology 1997;49:353-7. [Crossref] [PubMed]
- Gauthier AR Jr, Winters JC. Incontinent ileovesicostomy in the management of neurogenic bladder dysfunction. Neurourol Urodyn 2003;22:142-6. [Crossref] [PubMed]
- Gudziak MR, Tiguert R, Puri K, et al. Management of neurogenic bladder dysfunction with incontinent ileovesicostomy. Urology 1999;54:1008-11. [Crossref] [PubMed]
- Jenkins MA, Brown DJ, Ierino FL, et al. Cystatin C for estimation of glomerular filtration rate in patients with spinal cord injury. Ann Clin Biochem 2003;40:364-8. [Crossref] [PubMed]
- Young W. Spinal cord injury levels and classification. Available online: https://www.sci-info-pages.com/levels-and-classification/
- Gater DR. Weight after SCI: the good, the bad and the ugly. J Spinal Cord Med 2017;40:138-40. [Crossref] [PubMed]
- Brown ET, Osborn D, Mock S, et al. Perioperative complications of conduit urinary diversion with concomitant cystectomy for benign indications: a population-based analysis. Neurourol Urodyn 2017;36:1411-6. [Crossref] [PubMed]
- Leng WW, Faerber G, Del Terzo M, et al. Long-term outcome of incontinent ileovesicostomy management of severe lower urinary tract dysfunction. J Urol 1999;161:1803-6. [Crossref] [PubMed]
- Husmann DA. Long-term complications following bladder augmentations in patients with spina bifida: bladder calculi, perforation of the augmented bladder and upper tract deterioration. Transl Androl Urol 2016;5:3-11. [PubMed]
- Vainrib M, Reyblat P, Kong WG, et al. Supine and upright urodynamic evaluation of incontinent ileovesicostomy in wheelchair-bound adults with neurogenic bladder. Spinal Cord 2013;51:634-6. [Crossref] [PubMed]
- Thomassen SA, Johannesen IL, Erlandsen EJ, et al. Serum cystatin C as a marker of the renal function in patients with spinal cord injury. Spinal Cord 2002;40:524-8. [Crossref] [PubMed]
- Erlandsen EJ, Hansen RM, Randers E, et al. Estimating the glomerular filtration rate using serum cystatin C levels in patients with spinal cord injuries. Spinal Cord 2012;50:778-83. [Crossref] [PubMed]
- Goto T, Kawasaki Y, Takemoto J, et al. Evaluating estimated glomerular filtration rates of creatinine and cystatin C for male patients with chronic spinal cord injury. Spinal Cord 2018;56:447-52. [Crossref] [PubMed]
- Stein R, Schröder A, Thüroff JW. Bladder augmentation and urinary diversion in patients with neurogenic bladder: non-surgical considerations. J Pediatr Urol 2012;8:145-52. [Crossref] [PubMed]
- Elmelund M, Oturai PS, Toson B, et al. Forty-five-year follow-up on the renal function after spinal cord injury. Spinal Cord 2016;54:445-51. [Crossref] [PubMed]
- DeLong J, Tighiouart H, Stoffel J. Urinary diversion/reconstruction for cases of catheter intolerant secondary progressive multiple sclerosis with refractory urinary symptoms. J Urol 2011;185:2201-6. [Crossref] [PubMed]
- Weld KJ, Wall BM, Mangold TA, et al. Influences on renal function in chronic spinal cord injured patients. J Urol 2000;164:1490-3. [Crossref] [PubMed]
- El-Zoghby ZM, Lieske JC, Foley RN, et al. Urolithiasis and the risk of ESRD. Clin J Am Soc Nephrol 2012;7:1409-15. [Crossref] [PubMed]
- Feifer A, Corcos J. Contemporary role of suprapubic cystostomy in treatment of neuropathic bladder dysfunction in spinal cord injured patients. Neurourol Urodyn 2008;27:475-9. [Crossref] [PubMed]
- Tan HJ, Stoffel J, Daignault S, et al. Ileovesicostomy for adults with neurogenic bladders: complications and potential risk factors for adverse outcomes. Neurourol Urodyn 2008;27:238-43. [Crossref] [PubMed]
- Cohn JA, Large MC, Richards KA, et al. Cystectomy and urinary diversion as management of treatment-refractory benign disease: the impact of preoperative urological conditions on perioperative outcomes. Int J Urol 2014;21:382-6. [Crossref] [PubMed]
- Atan A, Konety BR, Nangia A, et al. Advantages and risks of ileovesicostomy for the management of neuropathic bladder. Urology 1999;54:636-40. [Crossref] [PubMed]
- Hellenthal NJ, Short SS, O'Connor RC, et al. Incontinent ileovesicostomy: long-term outcomes and complications. Neurourol Urodyn 2009;28:483-6. [Crossref] [PubMed]
- Gorgey AS, Gater DR Jr. Prevalence of obesity after spinal cord injury. Top Spinal Cord Inj Rehabil 2007;12:1-7. [Crossref] [PubMed]
- Thomason SS. Promoting outcomes for patients with spinal cord impairments and ostomies. Medsurg Nurs 2000;9:77-82. [PubMed]
- Turnbull GB. The ostomy files: special consideration for patients in a wheelchair. Ostomy Wound Manage 2007;53:8. [PubMed]
- Westney OL. The neurogenic bladder and incontinent urinary diversion. Urol Clin North Am 2010;37:581-92. [Crossref] [PubMed]
- Guillot-Tantay C, Chartier-Kastler E, Perrouin-Verbe MA, et al. Complications of non-continent cutaneous urinary diversion in adults with spinal cord injury: a retrospective study. Spinal Cord 2018;56:856-62. [Crossref] [PubMed]
- Husmann DA, McLorie GA, Churchill BM. Nonrefluxing colonic conduits: a long-term life-table analysis. J Urol 1989;142:1201-3. [Crossref] [PubMed]


