
An open-label, single-arm pilot study to evaluate the efficacy of daily low dose tadalafil on depression in patients with erectile dysfunction
Introduction
Erectile dysfunction (ED) can have a negative effect on the quality of life in males. In addition to the physical effects, ED can cause psychological issues, like lower emotional satisfaction and general happiness (1,2). Especially, previous studies have reported the association between ED and depressive symptoms (3). Many studies have reported not only that depression and antidepressant medications can cause ED, but also that having ED may increase the risk of depression (4-6). Therefore, the simultaneous treatment of depression and ED is needed.
Phosphodiesterase (PDE) 5 inhibitors, which regulate certain signaling pathways by elevating cyclic guanosine monophosphate (cGMP) levels, have been widely used to treat ED. PDE5 inhibitors such as tadalafil increase cGMP by blocking its breakdown at its catalytic site (7,8). Increased cGMP facilitates postsynaptic action in the brain, and activates downstream effectors resulting in changes in neuronal activities (9). Though many clinical studies have suggested a role of other PDE5 inhibitors, like sildenafil and vardenafil, as possible antidepressant medications (10-13), there have been only few animal studies exploring tadalafil (14), but no clinical studies to our knowledge.
Furthermore, accumulating evidence suggests that brain-derived neurotrophic factor (BDNF) is associated with the pathophysiology of depressive disorders (15). And, the cyclic adenosine monophosphate (cAMP) response element-binding protein (CREB) was found among the transcription factors regulating BDNF expression (16). So, we also investigated whether tadalafil would increase BDNF levels in men with depression through nitric oxide (NO)/cGMP/protein kinase G (PKG)/CREB/BDNF signaling.
Therefore, the aim of this study was to investigate the efficacy of daily low dose tadalafil on depression and BDNF levels in patients with ED.
Methods
Study design
This open-label, single-arm pilot study was conducted at single medical center in Korea. The subjects were prescribed a low-dose PDE5 inhibitor (tadalafil, 5 mg) once daily for 8 weeks and instructed to take the tablet before bedtime. Written informed consent was received from each patient prior to their participation in the study.
Subjects
Male patients aged 50–75 years with at least a three-month history of ED [International Index of Erectile Function (IIEF)-5 score ≤21] and depression [the Korean version of the Patient Health Questionnaire (PHQ)-9 score ≥5] were included in the study. PHQ-9 is one of the representative tools used for assessing general depressive symptoms in a primary health care setting (17,18).
Patients who had the following symptoms were excluded from the study: (I) serious cerebrovascular or cardiovascular conditions within the previous 6 months; (II) uncontrolled hypertension or hypotension; (III) uncontrolled diabetes; (IV) uncontrolled arrhythmias; (V) major psychiatric disorder or neurological disorder; (VI) history of glaucoma; and (VII) history of major hematological, renal, or hepatic abnormalities. Patients currently taking tricyclic antidepressants (e.g., amitriptyline, imipramine, desipramine, or amoxapine), selective serotonin reuptake inhibitors (e.g., citalopram, escitalopram, fluoxetine, paroxetine, or sertraline), or monoamine oxidase inhibitors (e.g., isocarboxazid, phenelzine, selegiline, or tranylcypromine) were also excluded.
Efficacy variables
Patients were assessed by a psychiatrist at baseline, and at 4 and 8 weeks after starting the medication. PHQ-9 (primary outcome) and PHQ-15 to evaluate somatization scores (19,20) were completed at every visit during the 8 weeks of the study. Blood samples for measuring serum BDNF levels were taken at baseline and after 8 weeks of treatment. BDNF levels were quantified using the Quantikine® Total BDNF Immunoassay Kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Patients reported the presence or absence of adverse events at each visit. The incidence, type, and severity of each adverse event were reported. A vital sign assessment and physical examination were performed for analysis at the beginning of the study, and at 4 and 8 weeks.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS version 20.0 for Windows; SPSS Inc., Chicago, IL, USA). Data are presented as means ± standard deviations. Changes in PHQ-9 and PHQ-15 scores, and serum BDNF levels between the two time-points (before and after the administration of 8 weeks of tadalafil) were assessed by Wilcoxon signed-rank tests. P<0.05 were considered statistically significant.
Ethics statement
This study was approved by the Institutional Review Board of the Catholic University of Korea (HC17MISI0012). This is a randomized clinical trial on the second phase, registered at the Clinical Research Information Service (CRIS, http://cris.nih.go.kr), number KCT0003306.
Results
Subjects
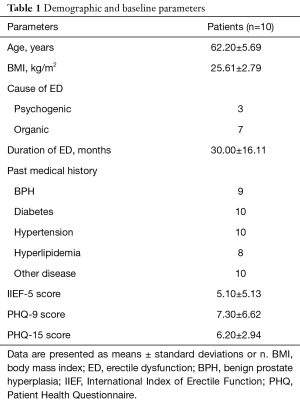
Overall, 10 male patients were assigned to the treatment group in this study, and all of them completed the eight-week treatment course without any adverse events. The demographic data and baseline characteristics of subjects are shown in Table 1. The average age of the 10 patients was 62.20±5.69 years, and the mean duration of ED was 30.00±16.11 months. The mean baseline IIEF, PHQ-9, and PHQ-15 scores were 5.10±5.13, 7.30±6.62, and 6.20±2.94, respectively.
Full table
Efficacy variables
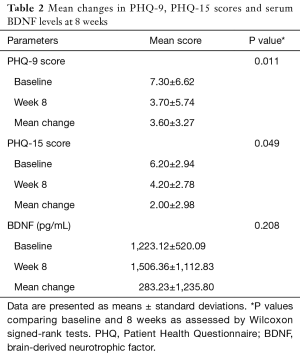
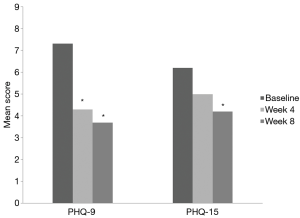
Changes in efficacy variables are shown in Table 2. The PHQ-9 and PHQ-15 scores at 8 weeks were 3.70±5.74 and 4.20±2.78, respectively. Mean changes in the PHQ-9 and PHQ-15 scores were 3.60±3.27 and 2.00±2.98, respectively (Figure 1). Analyses of the mean changes in PHQ-9 scores revealed that subject depressive symptoms were significantly improved after administration of 4 weeks of tadalafil (P=0.018) and 8 weeks of tadalafil (P=0.011), respectively. And, there was also a statistically significant increase in PHQ-15 scores (P=0.049). The serum levels of BDNF showed a slight increase after 8 weeks of treatment (1,506.36±1,112.83 pg/mL) compared to baseline (1,223.12±520.09 pg/mL). However, this difference was not statistically significant (P=0.208).
Full table
Discussion
The main findings of this prospective study were daily low-dose tadalafil administration significantly increased PHQ-9 score (decreased depression) and improved serum BDNF levels (but not significantly).
Depression represents a tremendous burden to individuals suffering from the disorder and to the global health economy. Although current antidepressants have focused on modulating monoaminergic signaling, the relationships between changes in monoamine levels and the therapeutic effects of a given antidepressant have not yet been fully elucidated. Moreover, the monoaminergic hypothesis does not provide a sufficient explanation for the mechanisms underlying the development of depression. Furthermore, the delayed onset of a therapeutic effect, partial or inadequate treatment response, and the development of various side effects are significant limitations of current therapies (21). Therefore, there has been an increasing interest in the development of PDE5 inhibitors for the treatment of major depressive disorder.
Many clinical studies have suggested a role for other PDE5 inhibitors, like sildenafil and vardenafil, as antidepressant medications. Seidman et al. randomized 152 men with ED into 12 weeks of treatment with sildenafil citrate and placebo groups, and assessed the effects of each on depression. 85.8% were given sildenafil in 58 treatment responders and mean decreases of 10.6 in Hamilton Depression Rating Scale score were seen in treatment responders (11). In another study, patients who underwent 6 weeks of double-blind treatment with sildenafil also had significantly greater changes from baseline on Beck Depression Inventory II scores compared with the placebo group (13). Rosen et al. found that vardenafil was well tolerated and highly efficacious in men with ED and untreated mild major depressive disorder compared to the placebo group through a 12-week, multicenter, randomized, flexible-dose, parallel-group, double-blind study (10).
However, to our knowledge there have only been a few preclinical studies, but no clinical studies, investigating the effects of tadalafil in ED and depression. Baek et al. reported that tadalafil improves depressive symptoms and alleviates memory impairment by suppressing apoptotic neuronal cell death and enhancing cell proliferation in maternal-separated rat pups (14). Our present clinical study also revealed increasing PHQ-9 scores and serum BDNF levels after tadalafil administration as compared with baseline.
Some reports have demonstrated the antidepressant effect of PDE5 inhibitors through NO/cGMP/PKG/CREB signaling (22). And, CREB was found among the transcription factors regulating BDNF expression (16). Chronic unpredictable mild stress (CUMS) decreases phosphorylation of CREB, which normally regulates several factors involved in activity-dependent synaptic modulation, such as BDNF (23). Accumulating evidence suggests that BDNF is associated with the pathophysiology of depressive disorder (15). Reduced CREB/BDNF signaling may contribute to the pathophysiology of depression and increasing CREB/BDNF signaling in depressive disorder might be one of the mechanisms underlying the effectiveness of PDE5 inhibitors for the treatment of depression (24). Although there was no statistically significant change in serum BDNF levels after tadalafil treatment, this preliminary study demonstrated a trend towards increasing serum BDNF levels after tadalafil administration. We hypothesize that the lack of statistical significance may be due to limitations afforded by the small sample size of this study.
In addition, the improvement in depressive symptoms seen after treatment with PDE5 inhibitors may be explained by some other mechanism. There is a correlation between improved erections and the improvement of depression. While the exact mechanism underlying this correlation remains unclear, it may partly be explained by a corresponding improvement in self-confidence that follows the improvement of erectile function (25).
The present study has some limitations that deserve mention. First, this study is an open-label, single-arm pilot study comparing changes in depressive symptoms before and after treatment with tadalafil, rather than a randomized, placebo-controlled study. And, our study had a relatively small sample size, which rendered it underpowered to show differences in treatment response and symptom severity. Therefore, a large-sized, randomized, placebo-controlled study is needed to confirm the effectiveness of daily low-dose tadalafil for the treatment of depression. Second, it is also necessary to adjust for severity of depressive symptoms, which was not done in this study. Any other factors that have been shown to be associated with differences in BDNF levels should also be adjusted. Third, we did not diagnose depression via DSM-IV criteria; rather, we screened for the presence and severity of depressive symptoms by using PHQ-9 scores.
Conclusions
The results of this prospective, clinical study suggest that daily low dose tadalafil may have a potential role in the treatment of depression in patients with ED. However, a randomized placebo-controlled study using a larger sample size is required to clearly elucidate the mechanisms underlying the improvement of depressive symptoms in ED patients seen with tadalafil treatment.
Acknowledgments
None.
Footnote
Conflicts of Interest: The study was sponsored by Hanmi Pharm Co., Ltd.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Catholic University of Korea (HC17MISI0012). Written informed consent was received from each patient prior to their participation in the study.
References
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44. [Crossref] [PubMed]
- Litwin MS, Nied RJ, Dhanani N. Health-related quality of life in men with erectile dysfunction. J Gen Intern Med 1998;13:159-66. [Crossref] [PubMed]
- Liu Q, Zhang Y, Wang J, et al. Erectile Dysfunction and Depression: A Systematic Review and Meta-Analysis. J Sex Med 2018;15:1073-82. [Crossref] [PubMed]
- Shiri R, Koskimaki J, Tammela TL, et al. Bidirectional relationship between depression and erectile dysfunction. J Urol 2007;177:669-73. [Crossref] [PubMed]
- Lemogne C, Ledru F, Bonierbale M, et al. Erectile dysfunction and depressive mood in men with coronary heart disease. Int J Cardiol 2010;138:277-80. [Crossref] [PubMed]
- Chou PS, Chou WP, Chen MC, et al. Newly diagnosed erectile dysfunction and risk of depression: a population-based 5-year follow-up study in Taiwan. J Sex Med 2015;12:804-12. [Crossref] [PubMed]
- Coward RM, Carson CC. Tadalafil in the treatment of erectile dysfunction. Ther Clin Risk Manag 2008;4:1315-30. [Crossref] [PubMed]
- Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res 2004;16 Suppl 1:S4-7. [Crossref] [PubMed]
- Reierson GW, Guo S, Mastronardi C, et al. cGMP Signaling, Phosphodiesterases and Major Depressive Disorder. Curr Neuropharmacol 2011;9:715-27. [Crossref] [PubMed]
- Rosen R, Shabsigh R, Berber M, et al. Efficacy and tolerability of vardenafil in men with mild depression and erectile dysfunction: the depression-related improvement with vardenafil for erectile response study. Am J Psychiatry 2006;163:79-87. [Crossref] [PubMed]
- Seidman SN, Roose SP, Menza MA, et al. Treatment of erectile dysfunction in men with depressive symptoms: results of a placebo-controlled trial with sildenafil citrate. Am J Psychiatry 2001;158:1623-30. [Crossref] [PubMed]
- Hatzichristou D, Cuzin B, Martin-Morales A, et al. Vardenafil improves satisfaction rates, depressive symptomatology, and self-confidence in a broad population of men with erectile dysfunction. J Sex Med 2005;2:109-16. [Crossref] [PubMed]
- Kennedy SH, Dugre H, Defoy I. A multicenter, double-blind, placebo-controlled study of sildenafil citrate in Canadian men with erectile dysfunction and untreated symptoms of depression, in the absence of major depressive disorder. Int Clin Psychopharmacol 2011;26:151-8. [Crossref] [PubMed]
- Baek SB, Bahn G, Moon SJ, et al. The phosphodiesterase type-5 inhibitor, tadalafil, improves depressive symptoms, ameliorates memory impairment, as well as suppresses apoptosis and enhances cell proliferation in the hippocampus of maternal-separated rat pups. Neurosci Lett 2011;488:26-30. [Crossref] [PubMed]
- Kishi T, Yoshimura R, Ikuta T, et al. Brain-Derived Neurotrophic Factor and Major Depressive Disorder: Evidence from Meta-Analyses. Front Psychiatry 2018;8:308. [Crossref] [PubMed]
- Zuccato C, Cattaneo E. Role of brain-derived neurotrophic factor in Huntington's disease. Prog Neurobiol 2007;81:294-330. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [Crossref] [PubMed]
- Han C, Jo SA, Kwak JH, et al. Validation of the Patient Health Questionnaire-9 Korean version in the elderly population: the Ansan Geriatric study. Compr Psychiatry 2008;49:218-23. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The PHQ-15: validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med 2002;64:258-66. [Crossref] [PubMed]
- Han C, Pae CU, Patkar AA, et al. Psychometric properties of the Patient Health Questionnaire-15 (PHQ-15) for measuring the somatic symptoms of psychiatric outpatients. Psychosomatics 2009;50:580-5. [PubMed]
- Esposito K, Reierson GW, Luo HR, et al. Phosphodiesterase genes and antidepressant treatment response: a review. Ann Med 2009;41:177-85. [Crossref] [PubMed]
- Halene TB, Siegel SJ. PDE inhibitors in psychiatry--future options for dementia, depression and schizophrenia? Drug Discov Today 2007;12:870-8. [Crossref] [PubMed]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem 2003;10:86-98. [Crossref] [PubMed]
- Wang C, Zhang J, Lu Y, et al. Antidepressant-like effects of the phosphodiesterase-4 inhibitor etazolate and phosphodiesterase-5 inhibitor sildenafil via cyclic AMP or cyclic GMP signaling in mice. Metab Brain Dis 2014;29:673-82. [Crossref] [PubMed]
- Shim YS, Pae CU, Cho KJ, et al. Effects of daily low-dose treatment with phosphodiesterase type 5 inhibitor on cognition, depression, somatization and erectile function in patients with erectile dysfunction: a double-blind, placebo-controlled study. Int J Impot Res 2014;26:76-80. [Crossref] [PubMed]


