A systematic review of salvage focal therapies for localised non-metastatic radiorecurrent prostate cancer
Introduction
In the UK, around one-third of men newly diagnosed with clinically significant localised prostate cancer undergo radical external beam radiotherapy (EBRT) (1). The incidence of primary treatment with brachytherapy implantation has also increased over the last 20 years (2). Contemporary data suggests that 10–15% of men develop biochemical failure within 5 years (3). Traditional treatment options for these men have been limited and have consisted of either watchful waiting (WW) with or without delayed androgen deprivation therapy (ADT) or salvage radical prostatectomy (sRP) in those who are eligible and fit enough for treatment. Prolonged ADT use can lead to a castrate resistance state after a median of 2–3 years. Side-effects include vasomotor complications, sexual dysfunction and gynaecomastia, osteoporosis, metabolic syndrome and depression. Additionally, there may be an association with neurocognitive deficits, thromboembolism, and cardiovascular disease (4). Oncological outcomes after sRP are generally worse than for those undergoing primary treatment with 5-year biochemical disease-free survival (BDFS) estimated at 48% and cancer specific survival (CSS) of 92%. At 10 years, these fall to 37% and 83% respectively (5). In addition, wound healing after primary irradiation treatment is poor; complications after salvage surgery are common and include urinary incontinence (20–78.1%), anastomotic stricture (0–41.8%), rectal injury (0–12.5%) and erectile dysfunction (29–100%) (6). Whole gland alternatives to sRP include salvage brachytherapy (sBT), cryotherapy (sCT) and high-intensity focused ultrasound (sHIFU) (7,8).
Recently, there has been a shift towards a more focal ablative approach whereby only the areas of recurrent cancer within the prostate are treated rather than the whole gland. This can provide oncological control whilst limiting functional adverse events and preserving quality of life. Although primary prostate cancer is often multifocal, it has been noted that the largest or highest grade lesion (the index lesion) drives cancer progression in the majority of patients (9) and, after EBRT, the recurrence occurs at this site in 89–100% of patients, suggesting that a radioresistant clone from the original index lesion may be responsible (10). Salvage focal therapy may allow satisfactory oncological control whilst avoiding the morbidity of whole gland treatment (11).
In this article we discuss the key challenges in assessment and patient selection for salvage focal therapy before reporting our findings from a systematic review of partial gland treatment outcomes.
Methods
This study was prospectively registered on the PROSPERO International Prospective Register of Systematic Reviews (CRD42019138240). Reporting of this review follows recommendations defined in the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (12).
Eligibility criteria
English language empirical studies (randomised and non-randomised comparative and non-comparative studies) describing salvage focal (partial gland) treatment of localised radiorecurrent prostate cancer using brachytherapy, cryotherapy and HIFU were included. Studies describing whole-gland ablation were excluded. Review articles, unpublished studies, case reports, letters, bulletins, comments and conference abstracts were excluded.
Search strategy
Two authors (CC Khoo and TT Shah) performed a systematic review of the Medline and Embase databases for empirical studies (randomised and non-randomised comparative and non-comparative studies) describing salvage focal treatment of localised radiorecurrent prostate cancer up to 23rd April 2019. A hand-search of reference lists of relevant review articles was also undertaken. Search terms included combinations of “salvage”, “recurrent” or “radiorecurrent” with each of “focal brachytherapy”, “focal cryotherapy”, “focal cryoablation”, “focal high intensity focused ultrasound” or “focal HIFU” (for example, “salvage AND focal cryoablation”).
Study selection
Two authors (CC Khoo and TT Shah) reviewed potentially relevant articles for inclusion. The full text of remaining articles was obtained and further screened for inclusion. Small series (n<10), duplicates, studies with follow-up articles and articles not meeting eligibility criteria were excluded. Disparities were discussed to obtain consensus; in cases when agreement could not be reached, a third author (MJ Connor) arbitrated.
Data items
The primary outcome was BDFS [as per the “Phoenix” definition—prostate-specific antigen (PSA) ≥2 ng/mL above the nadir] (13). We also extracted data on rates of metastases, conversion to second-line therapies and adverse events (as per the Common Terminology Criteria for Adverse Events) (14).
Quality assessment
Each study was assessed using the Methodological Index for Non-Randomized Studies instrument, a validated tool designed to assess the quality of nonrandomized comparative and non-comparative surgical studies (15).
Statistical analysis
As no randomised controlled trials were identified in systematic searching of the literature, a narrative synthesis and not a meta-analysis was performed. All statistical data, including patient demographics and oncological outcomes (e.g., BDFS), are presented as reported directly by the authors of included studies.
Defining the population, interventions and endpoints
Defining biochemical recurrence (BCR) after radiotherapy
Surveillance after radiotherapy treatment is usually with serial PSA measurements. The American Society of Therapeutic Radiology and Oncology (ASTRO) initially defined BCR as three consecutive PSA rises after a nadir in the 1990s (16); this has been largely superseded by the joint Radiation Therapy Oncology Group/ASTRO “Phoenix” definition of a rise of 2 ng/mL or more above the PSA nadir level (13). It should be remembered that a “PSA bounce” may occur after EBRT due to remaining areas of viable glandular tissue producing PSA, with the time to first rise being the most useful distinguishing factor (17).
Assessing local disease recurrence
In patients with evidence of BCR, determining the site of recurrence allows planning of ongoing management. Digital rectal examination (DRE) and transrectal ultrasound (TRUS) are easy to perform and readily available but are not reliable (18). Multiparametric MRI (mpMRI) (which includes diffusion weighted and dynamic contrast enhanced phases) has good diagnostic accuracy and can be used to inform subsequent biopsy and local salvage treatments (19,20). mpMRI has been demonstrated to have significantly higher sensitivity and specificity than TRUS alone in the detection of recurrence (21).
Prostate biopsy remains the only way to definitively confirm local relapse. However, distinguishing benign post-radiotherapy atypia from disease recurrence is challenging and biopsies should be examined by an experienced pathologist. Additionally, false negatives (due to sampling error) and false positives (due to delayed tumour regression) are not uncommon (22,23). Histologic resolution may take 24–36 months; for this reason, after EBRT, biopsies should not be taken before this unless there is a pressing clinical need (22).
Ruling out metastatic disease
Ruling out the presence of metastases is essential to establish treatment options. Bone is the sole site of spread in ≥80% of patients who develop metastases (24). Bone scans have traditionally formed part of assessment of patients with BCR; however, the utility of this modality for patients with PSA <10 ng/mL has been questioned (25,26). Bone scans can also give false positive results from trauma, inflammation, Paget’s disease and local inflammation (27). Positron emission tomography/computed tomography (PET/CT) with choline tracers has been shown to have a high diagnostic rate of bone metastases (28,29). Prostate-specific membrane antigen (PSMA) PET also provides excellent accuracy (and is especially useful when PSA is low) (30). Currently, high expense limits routine use. However, advancements in PET imaging technology and increasing demand for PET tracers (leading to more efficient production) may improve cost-efficiency and use of this modality in the future (28). Whole body MRI (WB-MRI) has good sensitivity and specificity, and allows for simultaneous assessment for metastasis and multiparametric imaging of the prostatic bed (31-33). However, accuracy compared to PET/CT has yet to be fully established (34). Additionally, scan duration is lengthy (although this has reduced to approximately an hour with modern 3T MRI machines) (27). Choline PET/MR may provide higher accuracy, but it is contested whether this is superior to PET/CT (35,36). Currently, the European Association of Urology acknowledges that Choline PET/CT, PSMA PET/CT and WB-MRI may provide greater sensitivity in the diagnosis of metastases than the traditional workup of bone scan plus abdominopelvic CT, but their added clinical utility remains unclear. They recommend staging using a cross-sectional imaging modality and a bone scan (37).
Results of systematic review
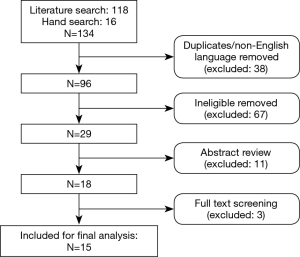
The search identified 134 relevant publications. After duplicates, non-English language and ineligible articles were removed, 29 were included for abstract review. Eighteen studies met eligibility criteria; after full text review, 3 were excluded (in all, n<10). Fifteen studies (14 case series and 1 comparative study) with a combined total of 628 patients were finally included (Figure 1).
With the exception of 1 study comparing salvage focal and whole-gland sCT (38), all eligible studies were single-arm case series. Only five studies were prospective. However, outcome data were complete and measured appropriately. The average Methodological Index for Non-Randomized Studies instrument score was 11.8/16 for non-comparative studies, with the single comparative study scoring 15/24 (Table 1).
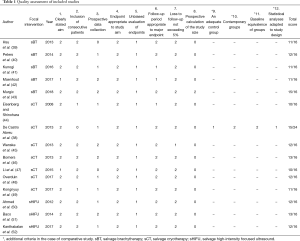
Full table
Primary and secondary outcomes were well reported by all studies. However, there was often heterogeneity in patient selection and treatment protocols (for example, regarding concurrent ADT use).
Salvage focal brachytherapy
Five case series met inclusion criteria (Table 2) (39-43). Although all studies reported outcomes of focal sBT for radiorecurrent disease, there was heterogeneity in radiation dose. Cohorts were small (n=12–20). Most studies reported short to mid-term follow-up, although one study approached 5-year outcomes (10–56 months).
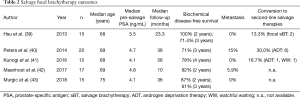
Full table
Two- (87–100%) and 3-year (61–71.4%) BDFS rates were reasonable. No study reported 5-year rates. Metastasis was uncommon (0–15%); Kunogi et al. reported no metastases after median follow-up of 56 months in 12 patients (41). Rate of conversion to second-line salvage treatment was similarly low (13.3–30.0%). The procedure was well tolerated; grade 3 toxicity adverse events were rare, and included urethral stricture (5–5.9%) (40,42) and self-resolving haematuria (6.7%) (43).
Salvage focal cryotherapy
Seven single-arm and one comparative trial examining outcomes post focal sCT were included (Table 3) (38,44-49). There was homogeneity in treatment methodology, with the majority of studies using a hemiablative strategy. Although most cohorts were small, three studies reported outcomes for >50 patients (n=10–91) (45,47,49). Follow-up was short to mid-term (12–37 months).
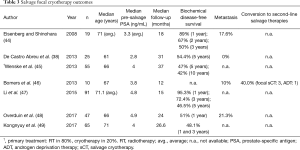
Full table
There was significant variability in reported BDFS rates. At 1 year, BDFS ranged from 48.1% to 95.3%. Three-year (48.1–72.4%) and 5-year (46.5–54.4%) rates were more consistent. In their comparative study, De Castro Abreu et al. report 5-year BDFS to be 54.4% in the focal sCT (n=25) and 86.5% in the whole-gland sCT treatment group (n=25) (although no statistical comparison was made due to underlying selection bias and differences in treatment protocols) (38). Rates of metastasis (10–21.3%) and conversion to second-line therapies (40.0%) were poorly reported, with only Bomers et al. describing patients undergoing further treatment with focal sCT (three patients) or ADT (one patient) (n=10) (46). Complications were uncommon; grade 3 adverse events included rectourethral fistula (3.3–5.5%) (45,47) and urethral stricture (5.3–10%) (44,46). Minor complications included transient haematuria, temporary incontinence and erectile dysfunction (49).
Salvage focal high-intensity focused ultrasound
Three studies met eligibility criteria (Table 4) (50-52). Treatment strategies varied, and included quadrant-, hemi- and index lesion ablation (with residual cancer left untreated). The largest cohort (n=150) also had the longest follow-up (35 months) (52).

Full table
There was heterogeneity in reporting of rates of BDFS. Ahmed et al. split their cohort into patients who achieved a PSA nadir <0.5 ng/mL and those who did not; in the former group BDFS was 86% at 1 year, 75% at 2 years and 63% at 3 years, and in the latter, 55% at 1 year, 24% at 2 years and 0% at 3 years (50). Baco et al. reported BDFS to be 67% at the end of median follow-up of 16.3 months (51). Kanthabalan et al. estimate 3-year BDFS to be 48% (52). Rates of metastasis were comparable (5–12.5%). Conversion rate to second-line treatment was extractable from only one study and was 8.0% (52). Reported complications included rectourethral fistula (2–3.6%) (50,52), bladder neck stenosis (8.0%) (52), and pubic bone osteitis (0.7–4.2%) (51,52).
Functional outcomes were well described by two studies. Ahmed et al. report a pad-free, leak-free continence rate at 64% and pad-free rate was 87% at last follow-up. International Index of Erectile Function (IIEF5) scores worsened from a pre-procedure median of 18 to 13 at 6 months (50). Concordantly, Kanthabalan et al. note that, at 2 years, 87.5% of those pad-free at baseline remained pad-free (42/48 patients), and 67.6% of patients drip-free continent at baseline remained drip-free (23/34 patients). There was a minor decline in median IIEF5 scores from 15 to 13 (52).
Discussion
In summary, our systematic review shows that, in the treatment of radiorecurrent prostate cancer, focal sBT, sCT and sHIFU all provide acceptable oncological control and have low rates of complications. Salvage focal treatment may represent a viable and less invasive strategy for select patients with localised radiorecurrent disease. Unfortunately, most of the available evidence is level 3 only, and long-term follow-up is lacking. Additionally, more robust evaluation of urinary and sexual functional outcomes with validated questionnaires is needed to help inform patient choice.
How do these outcomes compare to salvage surgery? Traditional surgical treatment of radiorecurrent prostate cancer is with sRP. A retrospective multi-institutional cohort analysis of 404 men who underwent sRP for localised radiorecurrent prostate cancer demonstrated reasonable survival outcomes; at 5 years, rates of being free from metastasis and cancer-specific death were 83% and 92% respectively; these decreased to 77% and 83% at 10 years. However, the BDFS rate was 48% (5 years) and 37% (10 years), and complications after sRP are common [e.g., urinary incontinence (20–78.1%) and erectile dysfunction (29–100%)] (5,6). Consequently, there has been increased interest in salvage treatments using other modalities. A meta-regression analysis of predominately whole-gland series comparing sRP with sBT, sCT and sHIFU demonstrated no difference in oncological outcomes, but significantly increased rate of urinary incontinence with sRP, supporting the role of sBT, sCT and sHIFU for patients with localised recurrence (53).
Our systematic search did not return any randomised controlled studies comparing sRP with focal sBT, sCT and sHIFU. The limited available evidence compares sRP with whole-gland sCT and sHIFU treatment (no studies comparing sRP and sBT are available). A retrospective study of 440 men found significantly higher overall mortality rates with sRP (n=99) than whole-gland sCT (n=341) (21.57 vs. 6.14 deaths/100 person years) (54). Conversely, other smaller series have come to different conclusions. Pisters et al. found that sRP (n=42) resulted in a significantly superior 5-year BDFS (66% vs. 42%) and overall survival (95% vs. 85%) than whole-gland sCT (n=56) (55), and Vora et al. report similar BCR rates for sRP (16.7%; n=6) and whole-gland sCT (23.5%; n=17) (although median follow-up times differed; 7.2 vs. 14.1 months) (56). Of note, this latter study reported rates of severe urinary incontinence of 16.7% after sRP and 5.9% after whole-gland sCT (56). Devos et al. retrospectively compared outcomes of sRP (n=25) with whole-gland sHIFU (n=27). Median follow-up was similar (43 vs. 45 months). There were no significant differences in estimated 5-year overall survival, cancer-specific survival or metastasis-free survival. However, patients who had undergone sHIFU were more continent at 12 months and had experienced fewer Clavien-Dindo ≥3 complications (57). These whole-gland comparisons suggest that sCT and sHIFU may provide similar oncological and functional outcomes to sRP. Formal comparative studies are needed prior to making firm conclusions regarding the oncological outcomes from salvage focal series; however, it appears that functional outcomes are superior when compared to the either sRP or whole-gland ablative modalities.
After salvage focal therapy, how do we define BCR? Most of the included studies used the “Phoenix” threshold (13). However, this definition was designed for use post RT in a primary setting; this strategy has not been validated for use in primary focal therapy, let alone in a salvage setting. The use of PSA surveillance after focal therapy is controversial as the untreated tissue may continue to secrete PSA, resulting in a “false positive” biochemical failure (not representative of oncological failure) (58). If the initial PSA reading post focal therapy is high, does this represent recurrence or undertreatment? Ahmed et al. analysed their cohort by “responders” (those who achieved a PSA nadir <0.5 ng/mL) and “non-responders” (those who did not) (50). It has been suggested that, post focal therapy, PSA should reduce by 50% within 3 months of treatment and remain stable, with recurrence defined as any deviation from this protocol (59). Validation of post salvage focal therapy surveillance strategies is needed.
As ablative technologies develop, it seems inevitable that their use in a salvage focal setting will be explored further. Our results suggest that salvage focal treatment provides acceptable oncological outcomes with low rates of adverse events; however, how do we decide between modalities? Unfortunately, to date, there have been no studies comparing focal sBT, sCT and sHIFU or comparisons to sRP or ADT. Choice is likely to depend on both patient and tumour characteristics (such as size and anatomical location).
This systematic review has a number of possible limitations. Despite a comprehensive search strategy, it is possible we missed some relevant articles. Fourteen of the 15 included studies were single-arm case series without comparator, and there was lack of standardisation in patient selection (e.g., concurrent ADT use), treatment protocols (e.g., both high and low dose brachytherapy were used) and outcome reporting. Long-term follow-up was lacking. Study quality was moderate only. It is not possible to make strong recommendations based on available evidence. Although we did not formally extract urinary and sexual functional outcomes these were poorly reported by studies evaluating sBT and sCT; the patient’s post-treatment quality of life is undoubtedly a key factor in decision-making. Finally, cost-effectiveness was not evaluated.
Conclusions
Salvage therapies are underutilised in men with recurrent prostate cancer. Focal sBT, sCT and sHIFU may provide at least comparable oncological control to whole-gland treatment with fewer operative complications. Currently, although patients must be carefully selected and counselled on an individual basis, salvage focal treatment has become a realistic management option. Future research comparing salvage focal with existing whole-gland treatments with long-term follow-up is required to refine indications, inform choice of modality and ensure outcomes endure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin J. Connor, Saiful Miah, Taimur T. Shah, Hashim U. Ahmed) for the series “Prostate Imaging and Focal Therapy” published in Translational Andrology and Urology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tau.2019.08.21). The series “Prostate Imaging and Focal Therapy” was commissioned by the editorial office without any funding or sponsorship. MJC, SM, TTS and HUA served as the unpaid Guest Editors of the series. MW receives a travel grant and a loan of device from Zicom Biobot. MJC is funded by the Wellcome Trust. TTS would like to acknowledge funding from Prostate Cancer UK and the St Peters Trust for clinical research and has received funding for conference attendance from Astellis, Ferring and Galil Medical. HUA’s research is supported by core funding from the United Kingdom’s National Institute of Health Research (NIHR) Imperial Biomedical Research Centre. HUA currently receives funding from the Wellcome Trust (grant 204998/Z/16/Z), Prostate Cancer UK, Sonacare Inc., MRC, Cancer Research UK, Imperial Health Charity, BMA Foundation, The Urology Foundation, Trod Medical and Sophiris Biocorp for trials in prostate cancer. Ahmed is a paid medical consultant for Sophiris Biocorp, BTG/Galil and Sonacare Inc. Ahmed is a paid proctor for HIFU and cryotherapy and Rezum water therapy and is paid for training other surgeons in these procedures. The other authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclaimer: None of the funding sources had any role or input into the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cancer Research UK. Prostate Cancer Statistics [Online]. Accessed: April 2019. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/prostate-cancer
- Stewart AJ, Drinkwater KJ, Laing RW, et al. The Royal College of Radiologists' audit of prostate brachytherapy in the year 2012. Clin Oncol (R Coll Radiol) 2015;27:330-6. [Crossref] [PubMed]
- Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047-60. [Crossref] [PubMed]
- Patil T, Bernard B. Complications of Androgen Deprivation Therapy in Men With Prostate Cancer. Oncology (Williston Park) 2018;32:470-4, CV3.
- Chade DC, Shariat SF, Cronin AM, et al. Salvage radical prostatectomy for radiation-recurrent prostate cancer: A multi-institutional collaboration. Eur Urol 2011;60:205-10. [Crossref] [PubMed]
- Golbari NM, Katz AE. Salvage Therapy Options for Local Prostate Cancer Recurrence After Primary Radiotherapy: a Literature Review. Curr Urol Rep 2017;18:63. [Crossref] [PubMed]
- Nguyen PL, D’Amico AV, Lee AK, et al. Patient selection, cancer control, and complications after salvage local therapy for postradiation prostate-specific antigen failure: A systematic review of the literature. Cancer 2007;110:1417-28. [Crossref] [PubMed]
- Alongi F, De Bari B, Campostrini F, et al. Salvage therapy of intraprostatic failure after radical external-beam radiotherapy for prostate cancer: A review. Crit Rev Oncol Hematol 2013;88:550-63. [Crossref] [PubMed]
- Ahmed HU. The Index Lesion and the Origin of Prostate Cancer. N Engl J Med 2009;361:1704-6. [Crossref] [PubMed]
- van Son M, Peters M, Moerland M, et al. Focal Salvage Treatment of Radiorecurrent Prostate Cancer: A Narrative Review of Current Strategies and Future Perspectives. Cancers (Basel) 2018. [Crossref] [PubMed]
- Kanthabalan A, Shah T, Arya M, et al. The FORECAST study - Focal recurrent assessment and salvage treatment for radiorecurrent prostate cancer. Contemp Clin Trials 2015;44:175-86. [Crossref] [PubMed]
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1-34. [Crossref] [PubMed]
- Abramowitz MC, Li T, Buyyounouski MK, et al. The phoenix definition of biochemical failure predicts for overall survival in patients with prostate cancer. Cancer 2008;112:55-60. [Crossref] [PubMed]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0 [Online]. 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- Slim K, Nini E, Forestier D, et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003;73:712-6. [Crossref] [PubMed]
- Cox JD, Gallagher MJ, Hammond EH, et al. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol 1999;17:1155. [Crossref] [PubMed]
- Caloglu M, Ciezki JP, Reddy CA, et al. PSA Bounce and Biochemical Failure after Brachytherapy for Prostate Cancer: A Study of 820 Patients with a Minimum of 3 Years of Follow-Up. Int J Radiat Oncol Biol Phys 2011;80:735-41. [Crossref] [PubMed]
- Zdrojowy R, Dembowski J, Małkiewicz B, et al. Salvage local therapy for radiation-recurrent prostate cancer - where are we? Cent European J Urol 2016;69:264-70. [PubMed]
- Rouvière O, Vitry T, Lyonnet D. Imaging of prostate cancer local recurrences: why and how? Eur Radiol 2010;20:1254-66. [Crossref] [PubMed]
- Wu LM, Xu JR, Gu HY, et al. Role of magnetic resonance imaging in the detection of local prostate cancer recurrence after external beam radiotherapy and radical prostatectomy. Clin Oncol (R Coll Radiol) 2013;25:252-64. [Crossref] [PubMed]
- Kara T, Akata D, Akyol F, et al. The value of dynamic contrast-enhanced MRI in the detection of recurrent prostate cancer after external beam radiotherapy: correlation with transrectal ultrasound and pathological findings. Diagn Interv Radiol 2011;17:38-43. [PubMed]
- Crook J, Malone S, Perry G, et al. Postradiotherapy prostate biopsies: What do they really mean? Results for 498 patients. Int J Radiat Oncol Biol Phys 2000;48:355-67. [Crossref] [PubMed]
- Miller EB, Ladaga LE, el-Mahdi AM, et al. Reevaluation of prostate biopsy after definitive radiation therapy. Urology 1993;41:311-6. [Crossref] [PubMed]
- Scher HI. Prostate carcinoma: defining therapeutic objectives and improving overall outcomes. Cancer 2003;97:758-71. [Crossref] [PubMed]
- Kane CJ, Amling CL, Johnstone PAS, et al. Limited value of bone scintigraphy and computed tomography in assessing biochemical failure after radical prostatectomy. Urology 2003;61:607-11. [Crossref] [PubMed]
- Dotan ZA, Bianco FJ, Rabbani F, et al. Pattern of prostate-specific antigen (PSA) failure dictates the probability of a positive bone scan in patients with an increasing PSA after radical prostatectomy. J Clin Oncol 2005;23:1962-8. [Crossref] [PubMed]
- Kanthabalan A, Arya M, Punwani S, et al. Role of focal salvage ablative therapy in localised radiorecurrent prostate cancer. World J Urol 2013;31:1361-8. [Crossref] [PubMed]
- Wondergem M, Van Der Zant FM, Van Der Ploeg T, et al. A literature review of 18F-fluoride PET/CT and 18F-choline or 11C-choline PET/CT for detection of bone metastases in patients with prostate cancer. Nucl Med Commun 2013;34:935-45. [Crossref] [PubMed]
- Piccardo A, Paparo F, Piccazzo R, et al. Value of fused 18F-Choline-PET/MRI to evaluate prostate cancer relapse in patients showing biochemical recurrence after EBRT: preliminary results. Biomed Res Int 2014;2014:103718. Erratum in: Biomed Res Int 2016;2016:5047948. Picazzo, Riccardo [corrected to Piccazzo, Riccardo]. Corrigendum to "Value of Fused (18)F-Choline-PET/MRI to Evaluate Prostate Cancer Relapse in Patients Showing Biochemical Recurrence after EBRT: Preliminary Results". [Biomed Res Int 2016].
- Perera M, Papa N, Roberts M, et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer-Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur Urol 2020;77:403-17. [Crossref] [PubMed]
- Robertson NL, Sala E, Benz M, et al. Combined Whole Body and Multiparametric Prostate Magnetic Resonance Imaging as a 1-Step Approach to the Simultaneous Assessment of Local Recurrence and Metastatic Disease after Radical Prostatectomy. J Urol 2017;198:65-70. [Crossref] [PubMed]
- Venkitaraman R, Cook G, Dearnaley DP, et al. Whole-body magnetic resonance imaging in the detection of skeletal metastases in patients with prostate cancer. J Med Imaging Radiat Oncol 2009;53:241-7. [Crossref] [PubMed]
- Lecouvet FE, El Mouedden J, Collette L, et al. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol 2012;62:68-75. [Crossref] [PubMed]
- Wieder H, Beer AJ, Holzapfel K, et al. 11C-choline PET/CT and whole-body MRI including diffusion-weighted imaging for patients with recurrent prostate cancer. Oncotarget 2017;8:66516-27. [Crossref] [PubMed]
- Freitag MT, Kesch C, Cardinale J, et al. Simultaneous whole-body 18F–PSMA-1007-PET/MRI with integrated high-resolution multiparametric imaging of the prostatic fossa for comprehensive oncological staging of patients with prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging 2018;45:340-7. [Crossref] [PubMed]
- Eiber M, Rauscher I, Souvatzoglou M, et al. Prospective head-to-head comparison of 11C-choline-PET/MR and 11C-choline-PET/CT for restaging of biochemical recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2017;44:2179-88. [Crossref] [PubMed]
- Mottet N, Van den Bergh RCN, Briers E, et al. Prostate Cancer. European Association of Urology. [Online]. 2019. Available online: https://uroweb.org/guideline/prostate-cancer/
- de Castro Abreu AL, Bahn D, Leslie S, et al. Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int 2013;112:298-307. [Crossref] [PubMed]
- Hsu CC, Hsu H, Pickett B, et al. Feasibility of MR imaging/MR spectroscopy-planned focal partial salvage permanent prostate implant (PPI) for localized recurrence after initial PPI for prostate cancer. Int J Radiat Oncol Biol Phys 2013;85:370-7. [Crossref] [PubMed]
- Peters M, Maenhout M, Van Der Voort Van Zyp JRN, et al. Focal salvage iodine-125 brachytherapy for prostate cancer recurrences after primary radiotherapy: A retrospective study regarding toxicity biochemical outcome and quality of life. Radiother Oncol 2014;112:77-82. [Crossref] [PubMed]
- Kunogi H, Wakumoto Y, Yamaguchi N, et al. Focal partial salvage low-dose-rate brachytherapy for local recurrent prostate cancer after permanent prostate brachytherapy with a review of the literature. J Contemp Brachytherapy 2016;8:165-72. [Crossref] [PubMed]
- Maenhout M, Peters M, van Vulpen M, et al. Focal MRI-Guided Salvage High-Dose-Rate Brachytherapy in Patients With Radiorecurrent Prostate Cancer. Technol Cancer Res Treat 2017;16:1194-201. [Crossref] [PubMed]
- Murgic J, Morton G, Loblaw A, et al. Focal Salvage High Dose-Rate Brachytherapy for Locally Recurrent Prostate Cancer After Primary Radiation Therapy Failure: Results From a Prospective Clinical Trial. Int J Radiat Oncol Biol Phys 2018;102:561-7. [Crossref] [PubMed]
- Eisenberg ML, Shinohara K. Partial Salvage Cryoablation of the Prostate for Recurrent Prostate Cancer After Radiotherapy Failure. Urology 2008;72:1315-8. [Crossref] [PubMed]
- Wenske S, Quarrier S, Katz AE. Salvage cryosurgery of the prostate for failure after primary radiotherapy or cryosurgery: Long-term clinical, functional, and oncologic outcomes in a large cohort at a tertiary referral centre. Eur Urol 2013;64:1-7. [Crossref] [PubMed]
- Bomers JG, Yakar D, Overduin CG, et al. MR imaging-guided focal cryoablation in patients with recurrent prostate cancer. Radiology 2013;268:451-60. [Crossref] [PubMed]
- Li YH, Elshafei A, Agarwal G, et al. Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: Initial results from the cryo on-line data registry. Prostate 2015;75:1-7. [Crossref] [PubMed]
- Overduin CG, Jenniskens SFM, Sedelaar JPM, et al. Percutaneous MR-guided focal cryoablation for recurrent prostate cancer following radiation therapy: retrospective analysis of iceball margins and outcomes. Eur Radiol 2017;27:4828-36. [Crossref] [PubMed]
- Kongnyuy M, Berg CJ, Kosinski KE, et al. Salvage focal cryosurgery may delay use of androgen deprivation therapy in cryotherapy and radiation recurrent prostate cancer patients. Int J Hyperthermia 2017;33:810-3. [PubMed]
- Ahmed HU, Cathcart P, McCartan N, et al. Focal salvage therapy for localized prostate cancer recurrence after external beam radiotherapy. Cancer 2012;118:4148-55. [Crossref] [PubMed]
- Baco E, Gelet A, Crouzet S, et al. Hemi salvage high-intensity focused ultrasound (HIFU) in unilateral radiorecurrent prostate cancer: A prospective two-centre study. BJU Int 2014;114:532-40. [Crossref] [PubMed]
- Kanthabalan A, Peters M, Van Vulpen M, et al. Focal salvage high-intensity focused ultrasound in radiorecurrent prostate cancer. BJU Int 2017;120:246-56. [Crossref] [PubMed]
- Philippou Y, Parker RA, Volanis D, et al. Comparative Oncologic and Toxicity Outcomes of Salvage Radical Prostatectomy Versus Nonsurgical Therapies for Radiorecurrent Prostate Cancer: A Meta-Regression Analysis. Eur Urol Focus 2016;2:158-71. [Crossref] [PubMed]
- Friedlander DF, Gu X, Prasad SM, et al. Population-based comparative effectiveness of salvage radical prostatectomy vs cryotherapy. Urology 2014;83:653-7. [Crossref] [PubMed]
- Pisters LL, Leibovici D, Blute M, et al. Locally recurrent prostate cancer after initial radiation therapy: a comparison of salvage radical prostatectomy versus cryotherapy. J Urol 2009;182:517-25; discussion 525-7. [Crossref] [PubMed]
- Vora A, Agarwal V, Singh P, et al. Single-institution comparative study on the outcomes of salvage cryotherapy versus salvage robotic prostatectomy for radio-resistant prostate cancer. Prostate Int 2016;4:7-10. [Crossref] [PubMed]
- Devos B, Al Hajj Obeid W, Andrianne C, et al. Salvage high-intensity focused ultrasound versus salvage radical prostatectomy for radiation-recurrent prostate cancer: a comparative study of oncological, functional, and toxicity outcomes. World J Urol 2019;37:1507-15. [Crossref] [PubMed]
- Huber PM, Afzal N, Arya M, et al. Pd34-07 Psa Fails To Predict Treatment Failure in Focal High-Intensity Focused Ultrasound Therapy in Prostate Cancer. J Urol 2018;199:e658. [Crossref]
- Barret E, Harvey-Bryan KA, Sanchez-Salas R, et al. How to diagnose and treat focal therapy failure and recurrence? Curr Opin Urol 2014;24:241-6. [Crossref] [PubMed]