
Testis-preserving strategies in testicular germ cell tumors and germ cell neoplasia in situ
Introduction
Testicular germ cell tumors (TGCT) is the most common cancer in young men at the age of 15 and 40 years. Since several decades, radical orchiectomy is the standard treatment approach for testicular masses (1). Performing this treatment option, accurate diagnosis as well as local tumor control is provided, but potential hormonal disorders as well as an unnecessary organ loss in benign diseases are clear disadvantages of this treatment strategy. Consequently, less intensive systemic treatment regimens in the management of TGCT like testis-preserving strategies are evolving, thus reducing the need for an immediate radical orchidectomy. As TGCT show high cure rates and thus life expectancies, treatment-related toxicities as well as quality of life has become a more important concern, namely the protection of male body image, fertility and endocrine function (2). These endocrinological and psychological advantages are especially important for bilateral TGCT (3). In addition, the use of ultrasonography has led to an increasing incidental detection of small testicular masses, most of which have been shown to be benign with an almost linear correlation with the size of the tumor (4,5). Furthermore, frozen section examination (FSE) showed a higher diagnostic accuracy, favouring an organ-sparing approach especially in doubtful cases (2,6). According to the EAU guidelines, organ-sparing surgery including intraoperative FSE should be performed in metachronous TGCT in a solitary testicle, synchronous bilateral TGCT or TGCT in a solitary testis as well as small ultrasound-detected, nonpalpable intraparenchymal testicular masses (1). After testis-preserving surgery, adjuvant radiation therapy (20 Gy) should be performed. The rationale is that the remaining parenchyma might harbor germ cell neoplasia in situ (GCNIS) [old nomenclature: testicular intraepithelial neoplasia (TIN), carcinoma in situ (CIS) of the testis], potentially leading to locally recurrent TGCT (7). Therefore, radiation therapy should be delay in fertile patients wish to have children. These patients should furthermore be intensively monitored by regular testicular ultrasound examinations (1,8).
The objective of this review is to provide an overview on literature regarding testis-preserving strategies in TGCT as well as GCNIS, including indications, operative technique and treatment outcome.
Indications
Testis-preserving surgery with intraoperative FSE is indicated in the following situations (1): metachronous contralateral tumors, synchronous bilateral tumors and a tumor in a solitary testis. A retrospective analysis on ultrasound during follow-up showed, that a metachronous relapse in a solitary testis usually represents significantly smaller lesions compared to the primary TGCT (9). Consequently, an organ-preserving surgery is even more suitable in patients with a metachronous TGCT. However, testis-sparing surgery should only be performed in tumors less than one third of the testicular volume or tumors <2 cm. In addition, pre-operative LH and testosterone levels should be within normal range. The rationale is that elevated serum LH concentrations mostly display a compensated Leydig cell insufficiency, that might decompensate after surgery (10). Furthermore, organ-sparing surgery with intraoperative FSE should be performed in case of non-palpable small (<1 cm) testicular masses as these tumors are mostly of benign origin (1,11,12). Our working group has therefore introduced a stratification tool which help to preoperatively differentiate between potential malignant or benign tumors and thus guide the way to either radical orchiectomy or an organ-sparing approach in small masses of the testis (see chapter 4) (12).
Operative technique of testis-sparing surgery
The operative technique of a testis-preserving surgery was first described by Stoll in 1986, guiding the enucleation of a nonpalpable Leydig cell tumor with the help of ultrasound (13). The technique was that further developed by German researchers namely Weissbach and Heidenreich as well as Hopps and Goldstein (11,14,15).
The following surgical approach is adopted from a recent publication by our group (10). Organ-sparing surgery is done using an inguinal incision. Then, the surgeon should isolate and suspend the spermatic cord and the spermatic vessels. Clamping the vessels via a tourniquet or clamp should not necessarily be performed, as palpation of the testis during surgery does neither have a negative influence on testosterone production nor lead to metastasis (16). Then, the spermatic cord should be pulled in order to bring out the testis and it should be inspected after an incision of the tunica vaginalis. Most of the tumors can be palpated (Figure 1A). In case of an impalpable testicular mass, intraoperative ultrasound with a 7.5-MHz scanner should be used and the mass should be marked with a needle. The tunica albuginea should be incised and the tumor should be completely enucleated and sent for FSE (Figure 1B). Further biopsies should be taken from the margins as well as the tumor bed to disclose concomitant foci of GCNIS and should also be sent for FSE. If tumor margins are positive, additional biopsies should be taken until the margins are negative. Using FSE, histology can be identified and surgical margins can be intraoperatively assessed. In experienced centers, FSE has recently demonstrated to be highly sensitive regarding the differentiation of malignant and benign testicular tumors (17-20). However, an experienced uro-pathologist is warranted to safely accomplish FSE. If FSE is not available at the surgery-performing institution, organ preservation is not recommended. If histological findings are benign or in case of a solitary testis, metachronous or synchronous malignant testicular masses, orchiectomy should be avoided. Thus, after tumor enucleation, the tunica albuginea and vaginalis should be closed with a running suture and the testis should be replaced in the scrotum. If FSE reveals a malignant tumor in patients with a healthy contralateral testis, radical orchiectomy should be performed.
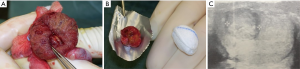
Small ultrasound-detected, nonpalpable testicular masses
Although most testicular masses are of malignant origin, 5–10% of these are postoperatively found to be benign (1). These small lesions include sex cord stromal tumors, consisting of Sertoli cell tumors, Leydig cell tumors, adenomatoid tumors of the scrotum as well as epidermoid cysts (1,21). The widespread use of ultrasound techniques, identified an increasing number of incidentally detected small testicular lesions, which are mostly of benign origin (Figure 1C) (4,22). This was confirmed in recent review on organ-sparing surgery, which identified that 75% of all resected testicular tumors would have been overtreated using immediate radical orchiectomy as they were of benign histology (2). However, in these small testicular masses, an organ-sparing approach including an intraoperative FSE should be performed, as testicular parenchyma can thus be preserved (2,6). Nevertheless, we need preoperative stratification tools in order to choose either radical orchiectomy or an organ-sparing approach. Previous studies have proposed that tumor size seems to predict malignancy (2,4,6,17,23,24), however, random cut-offs (from <2 to <2.5 cm) have been used (18,20,25-27). One recent study showed that a lesion size of 18.5 mm can predict histology with a specificity of 83% and a sensitivity of 87% (19). Our working group recently confirmed the role of tumor size in the prediction of histology, as benign findings had a significantly lower tumor volume and binary logistic regression identified tumor volume as a predictor for malignancy (12); 89% of all patients with a tumor <2.8 cm3 had a benign histology, which makes this cut-off a potential predictor of malignant and benign tumors. We furthermore proposed a preoperative stratification tools including detailed preoperative evaluation of the patients’ characteristics including serum tumor markers, symptoms, physical examination and fertility analysis (12). We found that a normal serum tumors marker, tumor volume less than 2.8 cm3, hormone disorders as well as long duration of symptoms seem to play the most important a role in the preoperative assessment to perform a testis-sparing surgery (12).
GCNIS
In case of a testis-preserving surgery, adjuvant radiation therapy (20 Gy) is recommended for the remaining testis. The rational for this approach is that the remaining parenchyma often harbors GCNIS (1). GCNIS will progress to invasive cancer in 50% of cases within 5 years if treatment is not performed (7).
Microscopically, GCNIS cells are large with distinct nucleoli and positioned in a single row at the basement membrane of seminiferous tubules (7). In these tubules that harbor GCNIS cells, active spermatogenesis is not found. Detection of GCNIS is accomplished by standardized immunohistochemical staining methods (28). Placental-like alkaline phosphatase (PLAP) is the most common marker to identify GCNIS (29). The malignant transformation from a precursor cell to the GCNIS cell initiates during the early development of germline stem cells. The target cell seems to be a gonocyte, as it shows morphological resemblance to GCNIS cells, as they partly express the same proteins (AP-2γ, OCT3/4, KIT) and can thus be used as markers in immunohistochemical staining methods (7,30-32). Furthermore, GCNIS are the precursors of all TGCT and differentiate to either the germ-cell determined lineage (seminoma) or teratoma, embryonal carcinoma, or extra-embryonic elements (choriocarcinoma, yolk sac tumor).
Local radiotherapy (16–20 Gy) should be performed in GCNIS and can thus eradicate all GCNIS cells (1). The German Testicular Cancer Study Group analysed the outcome of adjuvant radiotherapy after testis-sparing surgery in 101 men with bilateral TGCT or TGCT in a solitary testis (11). Eighty-five patients had concomitant GCNIS in the surrounding parenchyma and adjuvant radiotherapy was offered, but only 80 patients received local radiotherapy. After a mean follow-up of 80 months, the local recurrence rate was only 6% and the cancer-specific survival was 99%. Of the 6 relapsing patients, 4 patients had refused adjuvant radiotherapy (11). Another study analysing 11 patients with adjuvant radiotherapy after testis-sparing surgery for TGCT confirmed these results, as 10 patients had GCNIS and the only relapsing patient rejected adjuvant radiotherapy (25).
If metastatic disease of the primary tumor requires chemotherapy, the treatment of GCNIS should be delayed, as 30% of all GCNIS cases will persist and 42% will recur after chemotherapy (1). A repeat biopsy of the remaining testicle should be done one year after completion of chemotherapy (1). Additional radiotherapy is recommended in case of persistent GCNIS (1).
In contrast to TGCTs, benign testicular tumors like epidermoid cysts are never accompanied by GCNIS. To corroborate with this finding, we reported about our experience on testis-sparing surgery, which included 18 patients that suffered from a epidermoid cysts and found that no patient suffered from local or distant recurrence after a follow-up of 37 years (5). Consequently, adjuvant radiotherapy after testis-sparing surgery is not needed in benign testicular lesions.
Fertility
Treatment-related toxicities as well as quality of life, like the endocrine function, male body image and the preservation of fertility have become more important for treatment decision in TGCT (2). As TGCT have per se a reduced spermiogenesis, testicular masses are often incidentally found during infertility evaluation (1,33). In these patients, a testis-sparing approach is even more important, as a further loss of testicular parenchyma might affect the patient’s psychological well-being, exocrine and endocrine function (2). Consequently, fertility aspects have to be considered before any kind of treatment of suspected TGCT, but also potential benign testicular tumors.
Several studies have analysed the benefit of organ-sparing surgery regarding the maintenance of a physiological testosterone levels. We recently analysed 73 patients with a solitary testis after organ-sparing surgery regarding their endocrine function and found that physiological testosterone levels were preserved in 85% of the patients, and only 15% of all patients had a secondary hypogonadism (11). Further other studies confirmed the high rate of preservation of postoperative testosterone levels and fertility in most cases after an organ-sparing surgery (2,20,34). Hypogonadism with the need of testosterone replacement was not observed in patients with benign lesions, as shown in another study (27). This is especially for the coincidence of unilateral TGCT with a contralateral benign non-GCT (35). In these cases, the histology of a contralateral tumor should be assured by FSE. An organ-sparing approach should not only perform in benign histology, but could even be considered in bilateral TGCT, if strict conditions for resection and follow-up are respected (35). Our working group furthermore reported on paternity in one patient with a metachronous bilateral TGCT and in another patient with a unilateral TGCT with contralateral GCNIS. We thus demonstrated that patients with bilateral TGCT or GCNIS in their solitary testicle are not necessarily infertile and should undergo either a testis-sparing strategy or a close follow-up program (36).
As local radiotherapy will have influence on the endocrine and exocrine testicular function, the treatment of GCNIS should be adapted to the particular situation of each patient (8).
First, radiotherapy lead to the disappearance of all germ cells, thus leading to an irreversible infertility. In this case, histopathology shows a Sertoli-cell only syndrome in a biopsy after radiotherapy (37). Nevertheless, supporters of radiotherapy propose that the semen quality is low anyway as GCNIS is significantly associated with poor spermatogenesis and with testicular atrophy, and consequently radiotherapy is not the only parameter responsible for infertility (8,38). However, poor sperm quality at the time of orchidectomy can improve during follow-up time thus resulting in conception, which have been shown in various cases (36,37,39,40). Consequently, semen analysis can be performed when TGCT is found and local radiation can be deferred in patients with a desire for fatherhood (37). Furthermore, cryopreservation of testicular tissue or sperms should be offered to highly oligospermic patients who wish to have children.
Second, endocrine testicular function might be impaired after radiotherapy. In prior studies, Leydig cell function was impaired in 20–30% of all cases and patients needed androgen substitution in 25% of all cases after radiation (20 Gy) due to GCNIS in a solitary testis (41). However, these patients might already have a compensated Leydig cell insufficiency before treatment, being more susceptible to an additional gonadotoxic treatment (37). Testosterone levels should therefore be analysed every 6 months during follow-up in these patients (1).
Treatment outcome
Regarding treatment outcome, several analyses on organ-sparing surgery in benign testis tumors as well as in small testicular lesions reported excellent treatment outcomes (5,6,12,17,42,43). In line, the German Testicular Cancer Study Group showed a high disease-free survival rate (99%) and a low local recurrence rate (5.5%) in patients with TGCT who underwent a testis-sparing surgery (11). In this study, 85% of all patients showed normal postoperative testosterone serum levels (11). As a result, testis-preserving strategies seem to be reasonable in testicular masses of benign and malignant origin.
Conclusions
Taken together, testis-preserving surgery including intraoperative frozen section analysis should be offered to patients with a suspicious lesion in a solitary testicle, potential TGCT in both testicles as well as small testicular lesions. Afterwards, adjuvant local radiotherapy should be applied to TGCT due to a high risk of GCNIS. However, radiotherapy might lead to Leydig cell insufficiency with the need of androgen substitution as well as a destruction of germ cells leading to infertility. Consequently, radiation therapy should be delayed or sperm banking should be performed in fertile patients who desire for fatherhood.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Albers P, Albrecht W, Algaba F, et al. Guidelines on Testicular Cancer: 2015 Update. Eur Urol 2015;68:1054-68. [Crossref] [PubMed]
- Giannarini G, Dieckmann KP, Albers P, et al. Organ-sparing surgery for adult testicular tumours: a systematic review of the literature. Eur Urol 2010;57:780-90. [Crossref] [PubMed]
- Heidenreich A, Bonfig R, Derschum W, et al. A conservative approach to bilateral testicular germ cell tumors. J Urol 1995;153:10-3. [Crossref] [PubMed]
- Carmignani L, Gadda F, Gazzano G, et al. High Incidence of Benign Testicular Neoplasms Diagnosed by Ultrasound. J Urol 2003;170:1783-6. [Crossref] [PubMed]
- Heidenreich A, Engelmann UH, Vietsch HV, et al. Organ preserving surgery in testicular epidermoid cysts. J Urol 1995;153:1147-50. [Crossref] [PubMed]
- Elert A, Olbert P, Hegele A, et al. Accuracy of frozen section examination of testicular tumors of uncertain origin. Eur Urol 2002;41:290-3. [Crossref] [PubMed]
- Hoei-Hansen CE, Rajpert-De Meyts E, Daugaard G, et al. Carcinoma in situ testis, the progenitor of testicular germ cell tumours: a clinical review. Ann Oncol 2005;16:863-8. [Crossref] [PubMed]
- Dieckmann KP, Kulejewski M, Pichlmeier U, et al. Diagnosis of contralateral testicular intraepithelial neoplasia (TIN) in patients with testicular germ cell cancer: systematic two-site biopsies are more sensitive than a single random biopsy. Eur Urol 2007;51:175-83; discussion 183-5. [Crossref] [PubMed]
- Stoehr B, Zangerl F, Steiner E, et al. Routine scrotal ultrasonography during the follow-up of patients with testicular cancer leads to earlier detection of asynchronous tumours and a high rate of organ preservation. BJU Int 2010;105:1118-20. [Crossref] [PubMed]
- Heidenreich A, Angerer-Shpilenya M. Organ-preserving surgery for testicular tumours. BJU Int 2012;109:474-90. [Crossref] [PubMed]
- Heidenreich A, Weissbach L, Höltl W, et al. Organ sparing surgery for malignant germ cell tumor of the testis. J Urol 2001;166:2161-5. [Crossref] [PubMed]
- Paffenholz P, Held L, Loosen SH, et al. Testis Sparing Surgery for Benign Testicular Masses: Diagnostics and Therapeutic Approaches. J Urol 2018;200:353-60. [Crossref] [PubMed]
- Stoll S, Goldfinger M, Rothberg R, et al. Incidental detection of impalpable testicular neoplasm by sonography. AJR Am J Roentgenol 1986;146:349-50. [Crossref] [PubMed]
- Weissbach L. Organ Preserving Surgery of Malignant Germ Cell Tumors. J Urol 1995;153:90-3. [Crossref] [PubMed]
- Hopps CV, Goldstein M. Ultrasound guided needle localization and microsurgical exploration for incidental nonpalpable testicular tumors. J Urol 2002;168:1084-7. [Crossref] [PubMed]
- Pfister D, Paffenholz P, Haidl F. Testis-Sparing Surgery in Patients with Germ Cell Cancer: Indications and Clinical Outcome. Oncol Res Treat 2018;41:356-8. [Crossref] [PubMed]
- Ates F, Malkoc E, Zor M, et al. Testis-Sparing Surgery in Small Testicular Masses Not Suspected to Be Malignant. Clin Genitourin Cancer 2016;14:e49-53. [Crossref] [PubMed]
- Gentile G, Brunocilla E, Franceschelli A, et al. Can testis-sparing surgery for small testicular masses be considered a valid alternative to radical orchiectomy? A prospective single-center study. Clin Genitourin Cancer 2013;11:522-6. [Crossref] [PubMed]
- Shilo Y, Zisman A, Lindner A, et al. The predominance of benign histology in small testicular masses. Urol Oncol 2012;30:719-22. [Crossref] [PubMed]
- Matei DV, Vartolomei MD, Renne G, et al. Reliability of Frozen Section Examination in a Large Cohort of Testicular Masses: What Did We Learn? Clin Genitourin Cancer 2017;15:e689-96. [Crossref] [PubMed]
- Horstman WG, Sands JP, Hooper DG. Adenomatoid tumor of testicle. Urology 1992;40:359-61. [Crossref] [PubMed]
- Shtricker A, Silver D, Sorin E, et al. The value of testicular ultrasound in the prediction of the type and size of testicular tumors. Int Braz j Urol 2015;41:655-60. [Crossref] [PubMed]
- Müller T, Gozzi C, Akkad T, et al. Management of incidental impalpable intratesticular masses of < or = 5 mm in diameter. BJU Int 2006;98:1001-4. [Crossref] [PubMed]
- Steiner H, Höltl L, Maneschg C, et al. Frozen section analysis-guided organ-sparing approach in testicular tumors: technique, feasibility, and long-term results. Urology 2003;62:508-13. [Crossref] [PubMed]
- Carmignani L, Morabito A, Gadda F, et al. Prognostic parameters in adult impalpable ultrasonographic lesions of the testicle. J Urol 2005;174:1035-8. [Crossref] [PubMed]
- De Stefani S, Isgrò G, Varca V, et al. Microsurgical testis-sparing surgery in small testicular masses: seven years retrospective management and results. Urology 2012;79:858-62. [Crossref] [PubMed]
- Borghesi M, Brunocilla E, Schiavina R, et al. Role of testis sparing surgery in the conservative management of small testicular masses: oncological and functional perspectives. Actas Urol Esp 2015;39:57-62. [Crossref] [PubMed]
- Berney DM, Looijenga LHJ, Idrees M, et al. Germ cell neoplasia in situ (GCNIS): evolution of the current nomenclature for testicular pre-invasive germ cell malignancy. Histopathology 2016;69:7-10. [Crossref] [PubMed]
- Manivel JC, Jessurun J, Wick MR, et al. Placental alkaline phosphatase immunoreactivity in testicular germ-cell neoplasms. Am J Surg Pathol 1987;11:21-9. [Crossref] [PubMed]
- Hoei-Hansen CE, Nielsen JE, Almstrup K, et al. Transcription factor AP-2gamma is a developmentally regulated marker of testicular carcinoma in situ and germ cell tumors. Clin Cancer Res 2004;10:8521-30. [Crossref] [PubMed]
- Rajpert-De Meyts E, Nielsen JE, Skakkebaek NE, et al. Diagnostic markers for germ cell neoplasms: from placental-like alkaline phosphatase to micro-RNAs. Folia Histochem Cytobiol 2015;53:177-88. [Crossref] [PubMed]
- Jørgensen N, Rajpert-De Meyts E, Graem N, et al. Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest 1995;72:223-31. [PubMed]
- Suardi N, Strada E, Colombo R, et al. Leydig cell tumour of the testis: presentation, therapy, long-term follow-up and the role of organ-sparing surgery in a single-institution experience. BJU Int 2009;103:197-200. [Crossref] [PubMed]
- Giannarini G, Mogorovich A, Bardelli I, et al. Testis-sparing surgery for benign and malignant tumors: A critical analysis of the literature. Indian J Urol 2008;24:467. [Crossref] [PubMed]
- Neubauer S, Heidenreich A. Bilateral testicular tumors. Contralateral benign lesions in germ cell tumors of the testis. Urologe A 1999;38:282-4. [Crossref] [PubMed]
- Heidenreich A, Vorreuther R, Neubauer S, et al. Paternity in patients with bilateral testicular germ cell tumors. Eur Urol 1997;31:246-8. [Crossref] [PubMed]
- Heidenreich A. Contralateral testicular biopsy in testis cancer: current concepts and controversies. BJU Int 2009;104:1346-50. [Crossref] [PubMed]
- Petersen PM, Giwercman A, Hansen SW, et al. Impaired Testicular Function in Patients With Carcinoma-In-Situ of the Testis. J Clin Oncol 1999;17:173-9. [Crossref] [PubMed]
- Dieckmann KP, Loy V. Paternity in a patient with testicular seminoma and contralateral testicular intraepithelial neoplasia. Int J Androl 1993;16:143-6. [Crossref] [PubMed]
- Kliesch S, Bergmann M, Hertle L, et al. Semen parameters and testicular pathology in men with testicular cancer and contralateral carcinoma in situ or bilateral testicular malignancies. Hum Reprod 1997;12:2830-5. [Crossref] [PubMed]
- Giwercman A, von der Maase H, Berthelsen JG, et al. Localized irradiation of testes with carcinoma in situ: effects on Leydig cell function and eradication of malignant germ cells in 20 patients. J Clin Endocrinol Metab 1991;73:596-603. [Crossref] [PubMed]
- Nicolai N, Necchi A, Raggi D, et al. Clinical Outcome in Testicular Sex Cord Stromal Tumors: Testis Sparing vs Radical Orchiectomy and Management of Advanced Disease. Urology 2015;85:402-6. [Crossref] [PubMed]
- Carmignani L, Colombo R, Gadda F, et al. Conservative Surgical Therapy for Leydig Cell Tumor. J Urol 2007;178:507-11. [Crossref] [PubMed]


