Current clinical challenges in prostate cancer
Introduction
Prostate cancer is a highly heterogeneous disease, often with a long natural history. Nearly 240,000 men in the United States are newly diagnosed with prostate cancer annually, and more than 90% of these patients have local disease at diagnosis (1). Though statistics are variable, some autopsy reports indicate that the majority of men over age 50 harbor detectable prostate cancer after careful microscopic examination of the prostate (2). Although this data would suggest that prostate cancer follows an indolent course, it results in the death of nearly 30,000 American annually and approximately 2.7% of men in the United States are estimated to die from prostate cancer in their lifetime (3). The incidence/mortality ratio for prostate cancer is approximately 8, making it distinct from any other major cancer (Table 1) (1). This perplexing series of dichotomous facts were eloquently summarized by the late the late Dr. Whitmore, “when a cure is possible is it necessary? And when it’s necessary is it possible?” Reconciling this data involves stratifying patients by their risk of progression and offering appropriate therapy (or non-therapy) based on the risk of disease, comorbidities and life expectancy. After cancer progresses, additional challenges are encountered. Only radiation and surgery have been shown to reliably cure patients and when these modalities fail, additional management problems ensue within each disease state that follows. Much progress has been made in metastatic castrate-resistant disease of late and this progress is highlighted herein. This summary is an introduction to many of the pertinent clinical challenges that face clinicians in treating and managing this complex and multi-faceted disease.
Risk-classification and disease categorization
It is now customary to divide localize prostate cancer into low-, intermediate-, and high-risk categories (Table 2). These categories were initially proposed by D’Amico and colleagues and are now endorsed by the National Comprehensive Cancer Network (NCCN) and the American Urologic Association (AUA). Disease classification is based on the clinical stage, PSA, and digital rectal examination results. Despite the relatively simplistic nature of these categories, they have stood the test of time and continue to be relevant in therapeutic discussions. Low-risk prostate has a Gleason score of 6 on prostate biopsy, clinical stage of T1a, T1c, or T2a and a PSA <10 ng/mL. Intermediate risk prostate cancer can have a Gleason of 7, or a PSA of 10-20 ng/mL, or a clinical stage of T2b or T2c. High-risk localized cancer has a Gleason score between 8 and 10, or a PSA of >20 ng/mL, or a clinical stage of T3a. Patients with T3b or T4 disease are classified as locally advanced.

Full table
The D’Amico/NCCN risk (4) classification for categorical distinctions in risk stratification in those initially diagnosed with prostate cancer is one of many that now have been published. More sophisticated models evaluating similar variables in a continuous model such as the UCSF-CAPRA (5) score or Kattan nomograms (6) allow better discrimination of individual risk of progression but are more complex.
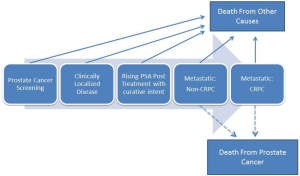
The clinical challenges in prostate cancer are many and depend on the disease category at presentation as well as a number of other factors including previously administered treatments. In order to best understand prostate cancer it can be viewed from a disease state model which was originally put forth by Scher and colleagues and subsequently modified many times (Figure 1) (7). It is helpful to view prostate cancer in a series of distinct clinical categories as these categories will define not only the appropriate treatments, but also the current clinical challenges.
PIVOT: critical review of treatment versus no treatment
There has only been one trial of PSA detected localized prostate cancer that has looked at a cohort of prostate cancer men that were treated with radical prostatectomy, or not treated, and followed for a minimum of 10 years. This trial termed PIVOT deserves special comment (8). The PIVOT trial was performed primarily in Veterans Administration centers in the United States along with some academic centers. Inclusion criteria required age less than 75 with a PSA ≤50 and the trial was initiated in 1994. Any Gleason score was allowed and a total of 731 patients were randomized with a mean age of 67. About 75% of men presented with a PSA elevation or rise as the primary indication for biopsy, making it distinct from other studies (i.e., Scandinavian Prostate Cancer Group Study-4) where PSA detection drove diagnosis only in a small minority.
In PIVOT, 40% of the men had low-risk, 34% intermediate-risk, and 21% high-risk prostate cancer (about 5% were missing data). After 10 years median follow-up, 77% of the men randomized to surgery underwent surgery and 20% of the men randomized to observation had definitive treatments with curative attempt. Over the course of the study 48.4% of the men died but only 7% died from prostate cancer. Given that it is generally accepted that men need to survive at least 10 years to benefit from surgery, this clearly indicates that the population was not ideal for this type of study.
There were no differences in prostate cancer specific mortality noted between the surgery and observation groups and a number of subsets were underpowered. Within the low-risk prostate cancer group, 62 deaths out of 148 were noted in the surgery arm and 54 out 148 men died in the observation arm. The hazard ratio (HR) for overall survival (OS) for low-risk disease was 1.15 (95% CI: 0.80-1.66). The intermittent- and high-risk diseases had favorable HRs for surgery with the HR for OS at 0.69 (95% CI: 0.49-0.98) and 0.74 (95% CI: 0.49-1.13), respectively, despite being underpowered with regard to subset analysis. Those with a PSA of >10 ng/mL also had as HR of 0.67 (95% CI: 0.48-0.94) favoring surgery. Thus some subsets favored surgery and some did not in the OS analysis.
In PIVOT, approximately 40% of the men had died by 10 years of followup indicating that either the age or comorbidity was suboptimal in this trial which has been characterized as being a trial of surgery in men appropriate for watchful waiting (instead of a trial of observation in men appropriate for surgery). It is clear that OS was suboptimal for a surgical-treated population and there was inadequate power to accurately assess various subsets. Regardless, the data indicate that patients with low-risk disease had no benefit from treatment. Of men in the low-risk category treated by surgery (N=148), 6 men died from prostate cancer, whereas in the observation group (N=148), 4 men died from prostate cancer. Taken together there was a strong trend toward benefit in men treated with surgery for those with intermediate and high risk disease but no trend toward benefit in low risk disease at 10 years of followup in a population which included many men who died less than 10 years after randomization. This trial points to the importance of risk stratification in decision making but also demonstrates that our current stratification schemes are imperfect. Better risk stratification is one of the key challenges for prostate cancer research going forward.
Life expectancy in prostate cancer management
The ability to predict an individual patient’s life expectancy is critical for screening, diagnosis, and/or treatment of localized prostate cancer (9). This is particularly important and difficult for prostate cancer patients due to the cancer’s variable and generally long natural history coupled with its prevalence in older men with competing comorbidities. Physicians are poor at predicting overall life expectancy. Several tools are available to assist in predicting life expectancy (10). The first are actuarial life tables, which represent an average number of remaining life years based on the age/sex of a group of individuals. While actuarial tables are easily accessible (11) and rapidly provide a reasonable estimation, they fail to account for individual medical comorbidities. The second tool available for life-expectancy calculations are comorbidity indices, perhaps the best known is the Charlson comorbidity index (12). This index assigns weights to 19 medical conditions and adjusts life expectancy based on those weights. The tool is limited in that patient’s comorbidities are dichotomized rather than considered in a continuous fashion and it may over emphasize the importance of some medical conditions. Nomograms for life expectancy that incorporate multiple variables are also available. Such nomograms, predict 10 years life expectancy following treatment for localized prostate cancer with a predictive accuracy in the range of 69-84% (13-15). Nomograms to predict life expectancy in patients electing active surveillance (AS) are currently under development.
Low-risk localized prostate cancer: concepts and challenges
Unfortunately there has largely been a failure of clinicians to meet the challenges of low risk prostate cancer with the great majority of patients receiving aggressive therapy (see Table 3) regardless of age or disease risk (16,17). Patients with low risk disease have a much greater probability of dying from causes other than prostate cancer, even 20 years after diagnosis (18). Clearly many patients with low-risk prostate cancer will not benefit from active treatment.

Full table
While there may be multiple reasons for the over treatment of low-risk disease, perhaps the most difficult to overcome is the fear, on the part of both the clinician and the patient, of missing the opportunity for high probability of cure with therapeutic intervention. Watchful waiting (WW) refers to conservative management of prostate cancer until the development of local or systemic progression at which point palliative measures are employed. A recognized alternative to WW or active treatment is AS; a therapeutic strategy that involves actively monitoring the patient’s disease with the expectation to intervene with intent to cure if the cancer progresses. AS is a recognized strategy that has emerged in the past decade and is endorsed by the NCCN, the American Urological Association (AUA), and the European Association of Urology (EAU) for select patients.
Although multiple ongoing clinical studies are evaluating the effectiveness of AS, existing data is largely from non-randomized, immature single institution with follow-up of less than 10 years. All agree that followup is suboptimal. Inclusion criteria are typically based on predictors of progression of disease and vary somewhat from study to study. Inclusion criteria include pathologic assessment of prostate biopsy with a particular emphasis of Gleason grading, clinical staging via digital rectal exam of the prostate, various measures of volume of cancer within the prostate (based on the number biopsy cores with cancer and the length of cancer in those cores), total PSA, and (to some extent) PSA adjusted for the size of the prostate (PSA density). More recently studies have assessed use of novel bio- and genetic-markers as part of AS cohorts, however determining which markers to use and how to best incorporate them is currently is investigational (19). Unfortunately all of these predictors of progression have significant limitations and better characterization of the extent and aggressiveness of disease at the time of diagnosis remains a challenge. Clinical staging with DRE is subjective and lacks precision. PSA or PSA density reflect not only the burden of cancer but the volume of benign prostatic hyperplasia and/or the presence of inflammation. PSA levels may fluctuate and a single test may be unreliable (20). Gleason score is subjective and dependent on the interpretation of individual pathologists. Biopsies fail to sample the entire gland and changes in Gleason grading from biopsy to radical prostatectomy have been demonstrated to be 36% at tertiary care centers with expert dedicated genitourinary pathologists examining both specimens (21). Perhaps most controversial of all is determining volume of disease; the number of biopsy cores containing cancer may depend in part on the total number of cores taken, and the length of the core containing cancer, but biopsy techniques are not standardized among urologists and methods of measurement not standardized amongst pathologists.
Several studies, despite nuanced differences in inclusion criteria, and intensity of follow up, have confirmed that, in well-selected patients with low-risk prostate cancer undergoing AS there is a low rate of cancer-specific death, but longer follow-up is needed before definitive conclusions can be reached (22-30). The randomized PIVOT trial is consistent with these observations as well as the SPCG-4 study (31). Both studies emphasized that long term followup is key to understanding cancer-specific survival (8,31). What is novel is that patients in these AS studies have undergone repeat evaluation including prostate biopsy and were offered curative treatment upon evidence of progression.
Two important and largely unresolved clinical challenges emerge from the AS studies; first is how do we define progression? Defining progression is challenging because the prostate is incompletely sampled on biopsy and it is unclear if increases in grade or volume on subsequent biopsies is a result of de-differentiation of the original tumor(s) or merely a result of more/better sampling (30). Most progression of tumors usually comes in the form of upgrading and occurs in first two years of enrollment in AS, supporting the theory of better sampling. One study demonstrated that immediate repeat biopsy prior to enrollment in AS resulted in upstaging or upgrading in 27% of patients (28). More follow will be needed to determine if rates of progression begin to rise as the cohorts are followed for longer periods. Better biopsy schemes (MRI-guided) have been proposed and this may help to answer some of the questions related to under-grading of biopsies (32). It is clear that conventional prostate biopsies are “blind” and that imaging plays little role in current standard of care.
The second major challenge with AS is to determine whether intervention for patients who experience progression (however it is defined) have outcomes that approximate their initial projected outcome? If patients who experience progression on AS protocols have worse prognosis, earlier intervention may be of benefit. Two randomized studies aimed to address these issues by randomly assigning men with low risk prostate cancer to AS or radical intervention; the ProtecT (Prostate testing for cancer and Treatment) has completed accrual at nine centers in the United Kingdom and the Surveillance Therapy Against Radical Treatment (START) which has recently been terminated due to poor accrual. Results for both are many years away.
High risk localized prostate cancer
High-risk clinical localized prostate cancer shares many of the same challenges with low risk prostate cancer; appropriate risk stratification based on an imprecise physical exam, limited random sampling of the prostate, and a variation in PSA. However, that is where the similarities end. While the primary challenge associated with low-risk prostate cancer is often an over treatment of disease, the primary challenge of high-risk prostate cancer is often under treatment. Many patients with high-risk disease who are likely to benefit from aggressive local therapy with curative intent only receive palliative treatment with androgen deprivation therapy (ADT). The CaPSURE database, a provider-based registry from a number of community based urology practices has demonstrated that 41% of high-risk patients receive ADT alone, compared with 24% and 28% that undergo RP and RT respectively (17,33).
The cause for this under treatment is not completely clear, but is likely based on the erroneous belief that treatment offers little benefit as these patients are likely to fail and die of disease. However, depending on the definition of high-risk disease, local treatment with either RP or RT results in progression free probability (PFP) of 49-80% (34,35). Perhaps even more convincing are several randomized trials which have demonstrated improved survival in men with high-risk prostate cancers who have received active treatment compared with observation or ADT alone. In PIVOT, men with intermediate- and high-risk prostate cancer had a strong trend toward improved OS with RP compared with observation (8). Similar findings were reported in the SPCG-4 study randomizing men with non-PSA detected prostate cancer to RP or observation (31). Finally, the randomized trial SPCG-7 for high-risk prostate cancer, demonstrated improvement in OS with external beam radiation plus ADT compared with ADT alone (36). Although no adequately powered randomized trial has determined the best active treatment for high-risk localized prostate cancer, monotherapy with ADT has the potential for significant harms, reduces QoL, and is not indicated for patients with asymptomatic localized prostate cancer. Its persistent use as monotherapy represents a challenge for the field (37).
Death from prostate cancer post-radical prostatectomy
A large data-base study consisting of over 11,000 patients (and confirmed in a data set of over 12,000 patients) with projected 15 years of followup from a series of excellent cancer centers around the country indicated that Gleason 8 or higher, seminal vesicle invasion, and lymph node positivity were particularly associated with a higher risk of prostate cancer death, regardless of the age group examined (37). The 15-year prostate cancer specific mortality risk was estimated as being 0.8% to 1.5%, 2.9% to 10%, 15% to 27% and 22% to 30% for organ confined cancer, extra-prostatic extension, seminal vesicle invasion, and lymph node metastasis, respectively. Nomograms have been developed to assess prostate cancer-specific mortality risks with long term follow-up (38). This study emphasizes the very low risk of death from prostate cancer in patients with low-risk disease, while demonstrating the potentially aggressive nature of other tumors in a manner that can be quantitated over time.
Adjuvant radiation therapy post-radical prostatectomy
With regards to the utilization of adjuvant radiation therapy, there is a randomized prospective Southwestern Oncology Group (SWOG) trial which supports the concept of OS benefit for adjuvant radiation therapy in individuals with pathologic T3a and T3b post-surgery (39). The data however are somewhat controversial in that there is a substantial proportion of these patients who will never recur post-operatively and the use of adjuvant radiation therapy may clearly be associated with over treatment. An important European trial (EORTC 22911) looked at adjuvant radiation therapy and demonstrated no survival benefit despite a PSA recurrence benefit (40). The clearest conclusions to be reached are that the PSA benefit was not translatable into a life expectancy benefit because so many of the patients who have a PSA recurrence post-prostatectomy are not destined to die from their disease. This emphasizes that PSA recurrence does not equate to death, a finding clearly demonstrated in careful analyses of the Johns Hopkins database (41).
Salvage radiation post-radical prostatectomy
One problematic area that has been not carefully examined in the context of the current clinical prostate cancer debate is the issue of salvage radiation and whether or not hormones may provide an additional positive benefit to external beam radiation (42). Although hormonal therapy in the context of radiation for localized intermediate or high-risk disease is certainly standard of care (43), the utility of hormonal therapy in combination with salvage radiation in the post-prostatectomy setting is not clear. The RTOG trial 0534 is addressing this issue in a prospective randomized manner with an accrual goal of nearly 1,700 patients (42). To date well over 1,000 patients have been accrued and this trial should be definitive in terms of answering the question of whether or not ADT adds value to salvage radiation for those with a PSA rise post-radical prostatectomy.
Timing of hormonal therapy
Another controversy in prostate cancer management is the timing of hormonal therapy for people who have failed primary treatment with curative intent and who have a rising PSA. To date there have been no trials that clearly indicate that earlier therapy is better for this particular patient population.
The data demonstrating that early ADT in combination with external beam radiation is superior to radiation alone, is plentiful and the original studies performed by the EORTC lead by Bolla and colleagues have stood the test of time (43). The use of hormonal therapy in the absence of radiation, as compared to hormonal therapy plus radiation, clearly leads to an inferior outcome (36).In one trial, important though very small, patients with lymph node metastases detected at the time of radical prostatectomy were randomized to receive ADT for life or observation. In this context the hormonal therapy was found to be better with regards to OS as well as other intermediate endpoints (44). Unfortunately, the small size of this trial, and the lack of additional prospective randomized trials supportive of these conclusions, are problematic.In a prospective study that utilized hormonal therapy early or later for those deemed to be unsuitable for definitive local therapy (EORTC 30891), there was slight improvement in OS for immediate androgen deprivation but quite oddly the prostate cancer specific mortality was not improved (45).A retrospective study performed in hospitals associated with the US Department of Defense, found overall that there was no difference in bone-scan radiographic progression-free survival for early as compared to later ADT for patients with a PSA rise post-radical prostatectomy (46). However, when considering those patients with a Gleason 8 or higher disease, or those patients with a pre-ADT PSA doubling time (PSADT) of <12 months, there was an improvement in bone scan progression-free survival for those with a PSA of <5 ng/mL as opposed to >5 ng/mL, or for those with a PSA of >10 ng/mL as opposed to those with a PSA of <10 ng/mL. It is possible that lead-time bias represents the explanation for this finding. Given the lack of randomization here, one cannot view these data as being definitive but the finding that men with a PSADT of more than one year and a Gleason of 7 or less did not benefit from early ADT may be important.Taken together, although ADT and radiation yields results that are superior to radiation alone in both intermediate and high risk disease, the use of early hormonal therapy for those with other disease states is considered controversial at best and no clear consensus can be drawn from the literature for those with a PSA rise after definitive therapy.
Intermittent versus continuous hormonal therapy
The use of hormonal therapy in an intermittent or continuous fashion is a current debate in our literature. For patients who have had a PSA recurrence after definitive radiation without evidence of metastatic disease, at 6.9 years of follow-up, both the intermittent and continuous therapeutic approach using ADT were not distinct when it comes to OS (47). However, there are improvements seen in the several quality of life parameters for patients treated with an intermittent approach, consequently many people now regard intermittent hormonal therapy as standard of care for individuals who have a non-metastatic PSA recurrence. Though this study convincingly shows that intermittent and continuous ADT showed no significant difference in OS for this population, the more important question regarding the timing of ADT (when should it begin) was not settled by this study (48).
A large SWOG trial addressed patients who were treated for initial metastatic disease with an intermittent versus continuous ADT regimen but unfortunately the conclusions were equivocal (49). In a non-inferiority analysis, the intermittent arm had a HR slightly worse (HR: 1.1; 95% CI: 0.99-1.23) but the confidence intervals overlapped both 1.0 and the pre-specified upper boundary of 1.2 thus the study concluded that intermittent ADT in this setting was not non-inferior. There was much about this trial that was suboptimal and notably there were little difference between the intermittent versus continuous regimens in terms of overall quality of life. While most individuals continue to regard continuous ADT as the standard of care for metastatic patients intermittent may be a reasonable alternative.
Non-metastatic CRPC (mCRPC)
No definitive studies demonstrate any agents offer survival advantage for patient with non- mCRPC. Modest improvements in bone-scan free survival were reported for denosumab therapy as compared to placebo but OS was not distinct and the incidence of osteonecrosis of the jaw was significantly higher in denosumab treated patients (50).
Overview of mCRPC
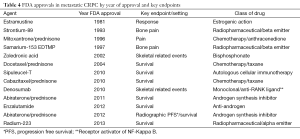
The summary and sequence of overall FDA approvals in mCRPC can be seen in Tables 4,5. The first drug to prolong survival in this setting was docetaxel in 2004. Prior to that, various FDA approvals involved pain or other non-OS endpoints. The progress in metastatic castrate resistant prostate cancer has been phenomenal since 2010 when two drugs, sipuleucel-T and cabazitaxel where both approved after demonstrating a prolongation of OS. Additional trials demonstrating prolongation of OS have subsequently been demonstrated for abiraterone, enzalutamide and radium. It is possible to classify these trials into different categories based on whether they were “front line” or post-docetaxel. The cabazitaxel approval in 2010 was in the post-docetaxel space, the first abiraterone approval in 2011 was in the post-docetaxel space, as was enzalutamide in 2012. Abiraterone was given a second approval for those individuals treated with for asymptomatic disease in the pre-docetaxel space in 2012. Sipuleucel-T in 2010 was approved in the asymptomatic or minimally symptomatic setting without regard for prior docetaxel treatment. The latest approval, radium-223 was approved in 2013 in symptomatic prostate cancer without visceral metastases. There was no mention of the docetaxel treatment in the radium-223 label as patients with or without docetaxel treatment both had a prolongation in OS in a pre-specified stratified analysis.
Full table
Full table
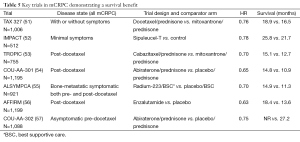
There are now a total of seven trials that have been pivotal for FDA approval in the mCRPC space as shown in Table 5. These trials all reported HRs for OS between 0.63 and 0.78 (51-58). The OS was quite variable from trial to trial but considering that some of these trials were conducted predominately in asymptomatic patients with no prior therapy for CRCP (52,57), whereas others were conducted in patients who had progressed post-docetaxel (53,54,56), a direct comparison of survival cannot be performed.
Pivotal docetaxel trials
In 2004, the FDA approved docetaxel/prednisone for mCRPC. Two trials examined the efficacy of docetaxel in patients with metastatic castrate resistant prostate cancer and served as the basis for the FDA approval. The TAX 327 trial randomized 1,006 men with metastatic castrate resistant prostate cancer to either 12 mg/m2 mitoxantrone every three weeks, 30 mg/m2 of docetaxel weekly for 5 out of 6 weeks or to 75 mg/m2 of docetaxel every three weeks (51). The every 3 weeks schedule of docetaxel demonstrated a survival advantage with a median survival of 18.9 months compared to 16.5 months in the mitoxantrone group and 17.4 months in the weekly docetaxel group. The SWOG 9916 trial randomized 674 men to either docetaxel at 60 mg/m2 with estramustine every three weeks or to 12 mg/m2 of mitoxantrone every three weeks (58). Docetaxel demonstrated a survival advantage with a median survival of 17.5 compared to 15.6 months for mitoxantrone. Progression of prostate cancer on docetaxel is an inevitability and presents one of the challenges for the clinician that has been more recently addressed by a series of trials and FDA approvals in the post-docetaxel space (53,54,56).
Immunology therapy: sipuleucel-T
Immunology therapy has been a debatable topic in all of cancer with considerable discussion and little promise until recent years. After initial submission of limited data, and a convoluted review process that did not involve the usual divisions at the FDA, sipuleucel-T was initially not approved. The trials initially submitted included two relatively small randomized trials which were considerably smaller than typical for FDA approvals. The sponsors then designed and implemented a much larger trial called D9902B or the IMPACT study which was conducted in patients with asymptomatic or minimally symptomatic mCRPC. There was no benefit in terms of progression free survival or radiographic response, but the group randomized to initial treatment with sipuleucel-T had better OS compared to the placebo group (52). It has been questioned whether the control group did worse than might have been anticipated however our review of the data do not support this concept and the control group in this study did no worse than patients in other analogous trials.
Abiraterone and enzalutamide
The approvals of abiraterone and enzalutamide challenged commonly held beliefs in metastatic prostate cancer—specifically, both are hormonal therapies that have shown activity in what has been termed castration resistant disease. Abiraterone works through selective inhibition of CYP17 lyase, and a phase I/II study of the agent highlighted significant activity of the drug in both the pre- and post-docetaxel setting. Two phase III studies of abiraterone ensued, encompassing both of these disease spaces. In the COU-AA-301 trial, a total of 1,195 patients with mCRPC and prior docetaxel therapy were randomized in a 2:1 fashion to receive abiraterone or placebo (both with prednisone) (54). The trial met its primary endpoint, demonstrating an improvement in OS with abiraterone therapy (14.8 vs. 10.9 months; P<0.001). Secondary endpoints, including time to PSA progression and PSA response, were also improved with abiraterone. In contrast to COU-AA-301, COU-AA-302 examined a cohort of patients with mCPRC who were docetaxel naïve (57). In this study, patients were randomized in a 1:1 fashion to either abiraterone or placebo (again with prednisone). The study had a co-primary endpoint of improvement in radiographic PFS (rPFS) and OS. Ultimately, PFS was improved with abiraterone (16.5 vs. 8.3 months; P<0.0001). Although OS was improved with abiraterone (35.3 vs. 30.1 months; P=0.0151), the difference did not meet the threshold established by the O’Brien-Fleming method (P=0.0035). Nonetheless, on the basis of the two studies noted herein, abiraterone has garnered FDA approval in both the pre-docetaxel and post-docetaxel setting.
The mechanism of enzalutamide differs significantly from abiraterone. Specifically, enzalutamide is a potent antiandrogen that inhibits nuclear translocation of the androgen receptor. With phase I/II data showing compelling activity in mCRPC, two phase III programs were launched. In the AFFIRM trial, 1,199 patients with mCPRC and prior docetaxel therapy were randomized in a 2:1 fashion to receive enzalutamide or placebo (55). The study was stopped after a planned interim analysis, where it was determined that enzalutamide was associated with an improvement in OS (18.4 vs. 13.6 months; P<0.001). Secondary endpoints such as PSA response and soft tissue response were also improved with enzalutamide. Results from the second phase III study of enzalutamide are highly anticipated—in the phase III PREVAIL study, docetaxel-naïve patients with mCRPC were randomized to enzalutamide or placebo.
The clinical trajectories of abiraterone and enzalutamide have moved in parallel, creating a quandary for investigators. Given the results from COU-AA-301 and AFFIRM, would it be preferable to use abiraterone/prednisone or enzalutamide in the docetaxel refractory patients? Notably, radium-223 and cabazitaxel (discussed elsewhere in this manuscript) are also options in this setting. Furthermore, if the noted PREVAIL (pre-docetaxel) enzalutamide study is positive, the oncologist is left with additional choices five valid options for first line therapy in mCRPC—sipuleucel-T, docetaxel, enzalutamide, radium-223, and abiraterone.
Cabazitaxel
Cabazitaxel represents the only cytotoxic therapy to demonstrate an OS advantage post-doctaxel (46). The TROPIC trial randomized 755 men who had progressed post-docetaxel were randomized to either 12 mg/m2 of mitoxantrone every three weeks or to the novel taxane cabazitaxel at 25 mg/m2 every three weeks (53). Median OS was 15.1 months in the cabazitaxel group and 12.7 months in the mitoxantrone group. The use of cabazitaxel represented the first therapy FDA approved for patients whose prostate cancer has progressed post-docetaxel. Febrile neutropenia was 7.5% and caution with regard to treatments in patients with borderline counts or performance status is advised. Given that cabazitaxel was approved in the post-docetaxel space, as was enzalutamide and abiraterone, one might question which drug is best for which patient in this setting. Thus far, we have no comparative trials so conclusions are limited.
Radium-223
The radium-223 approval was based on the ALSYMPCA trial which randomized 921 patients with an OS primary endpoint (55). Inclusion criteria specified at least 2 bone metastatic lesions on bone scan and the presence of some symptoms. Those with visceral disease were excluded. Patients were required to be post-docetaxel, have refused docetaxel, unfit to receive docetaxel, or did not have docetaxel available. Randomization was to intravenous radium at 50 kBq/kg or placebo for six doses with a 2 to 1 randomization. All patients received “best standard of care”. The “best standard of care” consists of whatever hormonal treatments might be appropriate in the mind of the investigator (ketoconazole, estrogens, dexamethasone, etc.) but no concomitant chemotherapy, experimental agents, or other radiopharmaceuticals were allowed.
The pre-specified interim analysis was positive for OS and the placebo group patients were subsequently allowed to cross over to radium-223. An updated OS analysis was presented to the FDA, with median OS at 14.9 months in the radium treated group and 11.3 months in the placebo treated group (49). The HR was 0.695 and the P value was 0.00007. There was also a reduction in symptomatic skeletal events which consisted of radiation to bone, surgery to bone, pathologic fracture, or spinal cord compression. Overall the treatment was well tolerated with a 6% incidence of grade 3/4 thrombocytopenia being the most significant finding; 2% of the patients had grade 3/4 neutropenia.
One of the many challenges regarding radium-223 is an understanding of how best to optimize and integrate this novel therapy into the overall treatment paradigm. The initial clinical trial was conducted prior to the approval of enzalutamide or abiraterone and whether or not combinations of these novel hormonal agents would have provided additive value to radium-223 is untested. Phase I trials with radium-223 and docetaxel have been conducted (59) and phase II trials are now underway utilizing the 50 kB/kg radium dose q six weeks in combination with 60 mg/m2 of docetaxel q three weeks. Looking at combination therapies with radium-223 may be quite interesting. It is also unclear whether or not the optimal dose and schedule of radium-223 was utilized in ALSYMCA and trials will examine various alternative doses and durations of radium therapy in hopes of defining what may or may not be more optimal doses and schedules.
Selecting appropriate therapies in the mCRPC patient
Front line therapies include docetaxel, sipuleucel-T, abiraterone/prednisone, and radium-223. Therapies available in the post-docetaxel space are abiraterone, enzalutamide, cabazitaxel, and radium-223. The sequence of therapies remains an area of debate but given there are no direct comparisons in clinical trials, the debate is more conjectural than data driven. Some agents are only currently approved post-docetaxel, such as enzalutamide and carbazitaxel—so those agents have a quite defined space. Given that many patients do not receive docetaxel, the issue of how to address these non-docetaxel patients in terms of second-line therapy is not at all clear. The radium-223 trials were the only trials with eligibility criteria that included those who were unfit for docetaxel or for those that refused docetaxel.
There are several tremendous challenges with regard to making appropriate choices as to which drug we should administer to each patient. We currently have very little data with regards to making appropriate drug choices guided by anything but clinical parameters. Our much studied biomarkers have yet to adequately inform clinicians regarding appropriate steps to take in individual patients. This is a major challenge in our field.
The presence or absence of prior docetaxel treatment is important to consider given some FDA approvals are specifically in this space. Performance status is always critical, as is the location of the metastatic lesions. Poor performance status patients should not receive cytotoxic chemotherapy as a rule. Are the metastatic lesions in the bone, viscera, both, or neither? Taking into account the pace of the disease progression influences clinical thinking. In addition the presence or absence of focal pains (which may be amenable to palliative external beam radiation therapy) is important to assess. Tolerance or intolerance of prior therapies, hematopoeitic function, and the availability of clinical trials are also important to consider (as are various laboratory parameters). Patients’ preferences as always are part of the issue, as are out of pocket costs. Many therapies are not administered because out of pocket costs are prohibitive. Cytotoxics such as docetaxel and cabazitaxel required good performance status/blood counts/liver functions. Sipuleucel-T should be restricted to good performance patients with minimal pain and preferably a relatively low burden/pace of the disease. Radium is for patients with bone-metastatic disease and neither radium nor sipuleucel-T are suitable for patients with extensive visceral disease. Out of pocket costs drive many decisions for oral drugs particularly in the United States.
The post-abiraterone/post-enzalutamide space
The question of what to do with patients who have failed abiraterone for mCRPC is currently subject to debate. Utilization of docetaxel has been viewed by many as being standard for patients who have not previously received any chemotherapy but results are mixed at best. The de Bono group has published data to indicate that docetaxel activity is diminished in patients’ post-abiraterone (60). There are no large trials in this setting so conclusions must be tempered until more data are available.
Fizazi and colleagues studied cabazitaxel/prednisone in patients who had received abiraterone and reported relatively high PSA response rates (61). These data have only been published in abstract form so there is much we more to learn about response durability and characteristics of the treated patients.
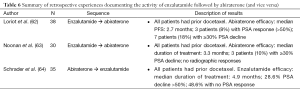
Minimal data are available for enzalutamide post-abiraterone (Table 6). One series, recently published retrospective analysis indicates that the response to enzalutamide post-abiraterone/post-docetaxel is blunted relative to those patients treated post-docetaxel alone (64). One study noted that 28.6% of men had a PSA decline of >50%. Further, 48.6% of men had no PSA response at all. This is much lower than expected. In the phase I/II trials, 56% of post-docetaxel patients had a PSA decline of >50% and only 17% had no PSA response (65). This German series did not assess PFS in a traditional sense so PFS data are limited.
Full table
The finding of any responses to enzalutamide post-abiraterone is of interest and implications of this observation are several. It should be clearly noted that post-abiraterone patients are a major challenge in our field. It may be that more androgens are present in the post-abiraterone state than appreciated and this concept is supported by finding that some urinary androgens can be still be detected despite abiraterone use (66). It is also possible that some non-androgenic steroids can engage the androgen receptor (AR) and that enzalutamide can block this interaction. After CYP17 inhibition, progesterone and its metabolites are increased (58). Given that synthetic progestin withdrawal can be associated with PSA declines (67), we suggest that progestin/AR interactions might be relevant. It is possible that enzalutamide blockade of the putative progestin/AR interactions could be growth-inhibitory. It is known that selected AR mutations can recognize progesterone as an agonist (68) lending plausibility to this hypothesis. Alternatively, it may be that simply post-abiraterone withdrawal, that androgen-synthesis resumes and that simply that intratumoral androgens are effectively blocked by enzalutamide.
Two studies have examined abiraterone effects post-enzalutamide (and also post-docetaxel). Both of these small case series indicated a high degree of cross-resistance between enzalutamide and abiraterone with PSA responses (>50% declines) being less than 10% and the median PFS being less than 4 months (62,63).
Taken together, it is clear that cross-resistance between abiraterone and other agents is an issue and understanding this cross-resistance and devising methods to over-come it, is a top priority in the field of CRPC research. Space limitations preclude the complete discussion on this topic but AR splice variants may also be partially responsible for cross resistance in some instances (69). Devising methods to block ligand-independent AR signaling is a key challenge for progress in CRPC.
Limitations of sequencing therapies in CRPC
We are currently in the “sequencing era” where we administer drug A then drug B and then drug C for patients with mCRPC. It is unusual in other cancers to choose this strategy. In Hodgkin’s disease, at curable malignancy, we utilize four drug regimens to cure. In prostate cancer we have only begun to explore combination therapy and this will be a tremendous challenge going forward, particularly given the cost of the various therapies involved. Regardless, combination therapies will likely be necessary to continue to improve patient outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
The study was conceived by OS.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Sakr WA, Grignon DJ, Crissman JD, et al. High grade prostatic intraepithelial neoplasia (HGPIN) and prostatic adenocarcinoma between the ages of 20-69: an autopsy study of 249 cases. In Vivo 1994;8:439-43. [PubMed]
- Available online: , accessed Aug 9, 2013.http://seer.cancer.gov/csr/1975_2010/results_merged/topic_lifetime_risk.pdf
- National Comprehensive Cancer Network Prostate Cancer Treatment Guidelines. Available online: , accessed July 2, 2013.http://www.nccn.org/professionals/physician_gls/f_guidelines_nojava.asp#site
- Cooperberg MR, Pasta DJ, Elkin EP, et al. The University of California, San Francisco Cancer of the Prostate Risk Assessment score: a straightforward and reliable preoperative predictor of disease recurrence after radical prostatectomy. J Urol 2005;173:1938-42. [PubMed]
- Kattan MW, Eastham JA, Stapleton AM, et al. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 1998;90:766-71. [PubMed]
- Scher HI. Prostate Carcinoma: Defining Therapeutic Objectives and Improving Overall Outcomes. Cancer 2003;97:758-71. [PubMed]
- Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [PubMed]
- Albertsen PC, Fryback DG, Storer BE, et al. The impact of co-morbidity on life expectancy among men with localized prostate cancer. J Urol 1996;156:127-32. [PubMed]
- Leung KM, Hopman WM, Kawakami J. Challenging the 10-year rule: The accuracy of patient life expectancy predictions by physicians in relation to prostate cancer management. Can Urol Assoc J 2012;6:367-73. [PubMed]
- Available online: , accessed August 10, 2013.http://www.ssa.gov/OACT/STATS/table4c6.html
- Kastner C, Armitage J, Kimble A, et al. The Charlson comorbidity score: a superior comorbidity assessment tool for the prostate cancer multidisciplinary meeting. Prostate Cancer Prostatic Dis 2006;9:270-74. [PubMed]
- Cowen ME, Halasyamani LK, Kattan MW. Predicting life expectancy in men with clinically localized prostate cancer. J Urol 2006;175:99-103. [PubMed]
- Tewari A, Johnson CC, Divine G, et al. Long-term survival probability in men with clinically localized prostate cancer: a case-control, propensity modeling study stratified by race, age, treatment and comorbidities. J Urol 2004;171:1513-9. [PubMed]
- Walz J, Gallina A, Saad F, et al. A nomogram predicting 10-year life expectancy in candidates for radical prostatectomy or radiotherapy for prostate cancer. J Clin Oncol 2007;25:3576-81. [PubMed]
- Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol 2010;28:1117-23. [PubMed]
- Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA 2013;309:2587-95. [PubMed]
- Albertsen PC, Hanley JA, Fine J. 20-Year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. [PubMed]
- Lin DW, Newcomb LF, Brown EC, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res 2013;19:2442-50. [PubMed]
- Eastham JA, Riedel E, Scardino PT, et al. Variation of serum prostate-specific antigen levels: an evaluation of year-to-year fluctuations. JAMA 2003;289:2695-700. [PubMed]
- Epstein JI, Feng Z, Trock BJ, et al. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol 2012;61:1019-24. [PubMed]
- Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol 2011;29:2185-90. [PubMed]
- Soloway MS, Soloway CT, Eldefrawy A, et al. Careful selection and close monitoring of low-risk prostate cancer patients on active surveillance minimizes the need for treatment. Eur Urol 2010;58:831-5. [PubMed]
- Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer 2008;112:2664-70. [PubMed]
- Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol 2011;29:2795-800. [PubMed]
- van den Bergh RC, Roemeling S, Roobol MJ, et al. Outcomes of men with screen-detected prostate cancer eligible for active surveillance who were managed expectantly. Eur Urol 2009;55:1-8. [PubMed]
- van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol 2008;54:1297-305. [PubMed]
- Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol 2011;185:477-82. [PubMed]
- Klotz L, Zhang L, Lam A, et al. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol 2010;28:126-31. [PubMed]
- Sheridan TB, Carter HB, Wang W, et al. Change in prostate cancer grade over time in men followed expectantly for stage T1c disease. J Urol 2008;179:901-4. [PubMed]
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. [PubMed]
- Siddiqui MM, Rais-Bahrami S, Truong H, et al. Magnetic Resonance Imaging/Ultrasound-Fusion Biopsy Significantly Upgrades Prostate Cancer Versus Systematic 12-core Transrectal Ultrasound Biopsy. Eur Urol 2013;64:713-9. [PubMed]
- Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU international 2011;107:576-84. [PubMed]
- Yossepowitch O, Eggener SE, Bianco FJ, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol 2007;178:493-9. [PubMed]
- Walz J, Joniau S, Chun FK, et al. Pathological results and rates of treatment failure in high-risk prostate cancer patients after radical prostatectomy. BJU Int 2011;107:765-70. [PubMed]
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet 2009;373:301-8. [PubMed]
- Liu J, Shi L, Sartor O, et al. Androgen-deprivation therapy versus radical prostatectomy as monotherapy among clinically localized prostate cancer patients. Onco Targets Ther 2013;6:725-32. [PubMed]
- Eggener SE, Scardino PT, Walsh PC, et al. Predicting 15-year prostate cancer specific mortality after radical prostatectomy. J Urol 2011;185:869-75. [PubMed]
- Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol 2009;181:956-62. [PubMed]
- Bolla M, van Poppel H, Tombal B., et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomized controlled trial (EORTC trial 22911). Lancet 2012;380:2018-27. [PubMed]
- Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999;281:1591-7. [PubMed]
- Available online: , accessed Aug 9, 2013.http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0534
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiation and goserelin. N Engl J Med 1997;337:295-300. [PubMed]
- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol 2006;7:472-9. [PubMed]
- Studer UE, Whelan P, Albrecht W, et al. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol 2006;24:1868-76. [PubMed]
- Moul JW, Wu H, Sun L, et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol 2004;171:1141-7. [PubMed]
- Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med 2012;367:895-903. [PubMed]
- Sartor O. Androgen deprivation--continuous, intermittent, or none at all? N Engl J Med 2012;367:945-6. [PubMed]
- Hussain M, Tangen CM, Berry DL, et al. Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368:1314-25. [PubMed]
- Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39-46. [PubMed]
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351:1502-12. [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [PubMed]
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomized open-label trial. Lancet 2010;376:1147-54. [PubMed]
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995-2005. [PubMed]
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369:213-23. [PubMed]
- Scher HI, Fizazi K, Saad F, et al. Increase survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187-97. [PubMed]
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138-48. [PubMed]
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004;351:1513-20. [PubMed]
- Available online: , accessed Aug 9, 2013.http://www.mskcc.org/cancer-care/trial/10-086
- Mezynski J, Pezaro C, Bianchini D, et al. Antitumour activity of docetaxel following treatment with the CYP17A1 inhibitor abiraterone: clinical evidence for cross-resistance? Ann Oncol 2012;23:2943-7. [PubMed]
- Albiges L, Le Moulec S, Loriot Y, et al. Response to cabazitaxel in the post-chemotherapy setting in CRPC patients previously treated with docetaxel and abiraterone acetate. ESMO 2012:3061.
- Loriot Y, Bianchini D, Ileana E, et al. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 2013;24:1807-12. [PubMed]
- Noonan KL, North S, Bitting RL, et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann Oncol 2013;24:1802-7. [PubMed]
- Schrader AJ, Boegemann M, Ohlmann CH, et al. Enzalutamide in Castration-resistant Prostate Cancer Patients Progressing After Docetaxel and Abiraterone. Eur Urol 2014;65:30-6. [PubMed]
- Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet 2010;375:1437-46. [PubMed]
- Attard G, Reid AH, Auchus RJ, et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 2012;97:507-16. [PubMed]
- Sartor O, Eastham JA. Progressive prostate cancer associated with use of megesterol acetate administered for control of hot flashes. South Med J 1999;92:415-6. [PubMed]
- Culig Z, Hobisch A, Cronauer MV, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol 1993;7:1541-50. [PubMed]
- Li Y, Chan SC, Brand LJ, et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res 2013;73:483-9. [PubMed]



