Androgen receptor genomic regulation
Introduction
The androgen receptor (AR) is a member of hormonal transcription factors. The expression of AR protein and its activation by male hormone androgen are fundamental to prostate development during pubertal and malignant transformation during later ages. These biological/pathological processes are determined by critical regulation of downstream molecules/pathways by the AR. AR is a DNA-binding protein that regulates a wide-range of target genes through directly binding to cis-regulatory elements. In the absence of androgen, the AR is sequestered in the cytoplasm by the chaperone super-complex including heat shock proteins (Hsp) 90, 70 and 56 (1). Once bound by androgen, AR undergoes conformational changes to dissociate from Hsp complex, becomes phosphorylated and translocates into the nucleus. For decades, understanding of AR-mediated transcriptional regulation was largely built upon the analysis of a handful of androgen-induced genes, one prototype of which is PSA. AR has been shown to form homodimers which preferentially bind DNA that contains androgen-responsive elements (AREs) (2,3). This binding activity and thus AR-mediated transcriptional regulation are tightly controlled by a large cohort of AR co-factors. Despite of these successes, very few AR target genes have been identified and characterized until recent advances in high-throughput genomic technologies. The advent of DNA microarrays at the beginning of this century and the emergence of massively parallel next-generation sequencing have rapidly transformed this field. Taking advantage of these approaches, a burst of studies have in recent years very carefully examined AR transcriptional regulation at the genome scale. Here we review these studies to provide up-to-date understanding of genome-wide androgen-regulated genes and the genomic landscapes of AR and its regulation during prostate cancer (PCa) progression.
Genome-wide analysis of androgen-responsive genes
Androgen-responsive genes or androgen-regulated genes are defined as genes whose expression levels are significantly altered by androgen treatment. The products of these genes are essential in the biological processes responsible for prostatic development, function and disease. Therefore, identification and characterization of androgen-responsive genes can potentially lead to the discovery of novel biomarkers and approaches for PCa diagnosis and treatment. For decades very few androgen-responsive genes have been identified, mostly limited to KLK3 (PSA), KLK2, and NKX3-1 (4). However, the completion of the human genome project and the advent of microarray technologies at the beginning of this century have enabled parallel analysis of the expression of thousands of genes at a time. Consequently, an unprecedented list of androgen-regulated genes have been recently identified and characterized through expression microarray analysis of PCa cells and tissues.
High-throughput profiling of androgen-regulated genes using PCa cell lines
Androgen-sensitive PCa cell lines such as LNCaP have been critically useful to identify the downstream genes/molecules that are regulated by androgen. Pioneered by Sanger sequencing of cDNA or EST libraries, researchers began the endeavors of genome-wide analysis of androgen-responsive genes approximately a decade ago (5). Through serial analysis of gene expression (SAGE), androgen-regulated genes were greatly expanded from a handful to approximately 100 of them (6-8). These EST-based assays, though have certainly made significant contributions to the field, were labor-intensive and cost-inefficient for the purpose of expression profiling. Rather, following the characterization of all human genes, EST clones were printed onto glass slides to generate cDNA microarray, which was then used for expression profiling in an efficient and affordable manner. The use of microarray has extensively facilitated the identification and the analysis of androgen-regulated gene expression under various biological/pathological conditions. For example, through microarray analysis of LNCaP cells before and after androgen stimulation, studies have revealed more than 500 genes that were differentially regulated by androgen (9,10). Further analysis revealed that approximately 300 genes were up-regulated while another 300 were down-regulated by androgen (11).
To pinpoint potential direct targets of AR, studies have examined global gene expression changes following androgen stimulation over a time-course. For example, Massie et al. identified more than 3,000 genes with altered expression in response to androgens by assessing 27 time points between 0 to 24 h following 1 nM R1881 stimulation in LNCaP cells. Out of these androgen-regulated genes, approximately 550 genes responded to R1881 within 5 hours and are likely to be directly regulated by AR (12). Despite some differences regarding androgen-regulated genes derived from various datasets, plausibly due to differences in array platforms and experimental conditions, a core set has been repeatedly identified. Based on a review from Dehm and Tindall, about 1.5-4.3% of genes expressed in LNCaP cells are androgen regulated (13). Through analysis of PubMed literatures, Androgen Responsive Gene Database (ARGDB) showed that at least 1,785 human, 583 rat, and 993 mouse genes have been reported as androgen-regulated (14). By comparing 9 representative studies of gene expression in androgen-treated LNCaP cells, we found that more than 1,000 genes have been reported in at least 2 independent studies, among which a core set of over 200 genes have been shown to be androgen-regulated in more than 4 independent studies (Tables 1,2).
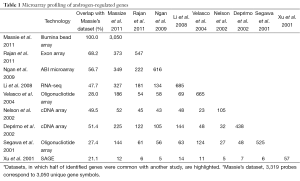
Full table
Genome-wide analysis of androgen-regulated genes in animal models
In addition to cell lines, animal models have been utilized to study androgen response in vivo. Although the structure of rodent prostate differs considerably to that of human, androgens are nonetheless critical for rodent prostate cell differentiation, proliferation, and overall prostate development (15). Human and rodent species are thought to share a variety of fundamental biochemical and functional pathways that are regulated by AR signaling. Using both subtractive hybridization and microarray approaches, Jiang et al. reported the identification of more than 100 androgen-responsive genes in the rat ventral prostate (16). Using the Dunning rat R3327 model system, Pfundt et al. identified several sets of prostatic androgen-responsive genes that are alternatively regulated in androgen-dependent and androgen-independent prostate tumors (17). Through microarray analysis of gene expression profile changes in the mouse prostate following castration and hormone replacement, Wang et al. identified a number of androgen-responsive genes, a majority of which are also regulated by androgen in cell line models. In particular, the authors reported sixty-five genes as androgen down-regulated (i.e., up-regulated upon castration and repressed by hormone replacement) (18), among which 72% have potential AREs, suggesting a direct transcriptional suppressor role of the AR on these genes. It was highlighted that these androgen-repressed genes may potentially inhibit prostate tumorigenesis. Furthermore, one of these androgen-repressed genes, H-Cad was experimentally evaluated and shown to function as a potential tumor suppressor in human PCa.
Androgen-regulated molecular pathways and cellular functions
Alongside the discovery of a comprehensive list of androgen-responsive genes, the downstream molecular pathways and cellular processes that are controlled by androgen became apparent. Initially, more than fifty androgen-regulated genes were identified by SAGE in LNCaP cells (6). Functional annotation revealed that a majority of them are involved in the regulation of transcription and energy metabolism. In addition, a significant number of genes regulating cell cycle, signal transduction and cellular protein trafficking were found to be induced by androgen, supporting the role of androgens in promoting survival and growth of prostatic epithelial cells. Subsequently, a microarray study by Deprimo et al. analyzed the gene expression program induced by R1881 in LNCaP cells and found that a significant portion of androgen-regulated genes are related to the production of seminal fluid (9). In accordance, Nelson et al. also found that androgen-responsive genes contribute to metabolism, protein trafficking, cell proliferation and differentiation (19). Study by Massie et al. highlighted that calcium/calmodulin-dependent protein kinase kinase 2 (CAMKK2) is overexpressed in PCa and acts as an important effector of AR signaling in controlling anabolic metabolism, cell proliferation and cell migration/invasion of PCa cells (12). In summary, a remarkable fraction of the genes induced by androgen appeared to be related to (I) production of modification of secretary proteins, protein folding, trafficking, and secretion (6,8,9); (II) cell-cycle, metabolism and biosynthetic pathways (6,12,20,21); (III) regulation of transcription factors such as GATA2, PDEF, ETV1, CREB3L4, HOXB13 and NKX3.1.
Androgen-regulated genes play important roles in PCa
Not only is androgen signaling indispensable to prostate development and function, it is also a key driver of PCa initiation and progression. Understanding of androgen-regulated genes/pathways is thus a first step towards the development of novel biomarkers and therapeutic targets of PCa. Accompanying the identification of a large set of androgen-regulated genes using cell line and animal models, studies have attempted to characterize the expression and function of some of these target genes in PCa. For example, Velasco et al. demonstrated that FKBP51, one of androgen-regulated genes identified by an oligonucleotide array study in LNCaP cells, was expressed significantly higher in prostate tumor samples relative to benign prostatic hyperplasia samples through tissue microarray immunohistochemistry (IHC) staining. FKBP51 may be as a potential diagnostic marker for PCa (10). Numerous studies have also comparatively analyzed the expression profile of androgen-regulated genes in model systems and in clinical specimens derived from PCa patients at different developmental stages or with varying treatment histories. For instance, Holzbeierlein et al. and Mostaghel et al. determined genes that were differentially expressed in human PCa specimens following androgen deprivation therapy (ADT) (22,23). More than 20% of these genes also showed significant expression changes in LNCaP cells after 36 h of hormone-deprivation (22). On the other hand, there were also many that were not down-regulated after short-term castration in LNCaP cells, indicating that androgen deprivation may be insufficient to completely suppress androgen activity, which may contribute to PCa cell survival in a low androgen environment (23). In addition, for the most part, these two human PCa tissue profiling studies revealed very few common genes (less than 5%), suggesting that androgen-responsive genes may vary considerably among individuals.
Besides analyzing androgen-responsive genes, a significant research area has been to determine genes that are differentially expressed in castration-resistant prostate cancer (CRPC) and thus may be responsible for the development of castration resistance. AR signaling pathways may differ between androgen-naïve and castration-resistant PCa. For example, Wang et al. reported that M-phase cell-cycle genes including UBE2C were upregulated by AR specifically in castration-resistant LNCaP-abl cells but not in androgen-sensitive LNCaP cells. Tissue microarray analysis further demonstrated that UBE2C protein overexpression correlates with the occurrence and progression of PCa (21). Interestingly, a recent study revealed that this increased expression of UBE2C may be due to induction by constitutively active AR splice variants, AR-V7 and ARV567ES, that were found in CRPC cells (24).
Besides regulation of downstream gene expression, groundbreaking studies have recently revealed an innovative mechanism of AR in promoting PCa through inducing chromosomal translocations. Back in 2005, Tomlins et al. made the seminal discovery of genomic translocations that juxtapose the androgen-responsive TMPRSS2 gene promoter with the oncogenic transcription factor ERG, leading to outlier profile of ERG expression in a subset of localized as well as metastatic human PCa (25). Further studies from this and other groups have in the subsequent years characterized a set of recurrent chromosomal translocations in PCa, a majority of which involve an androgen-responsive promoter and an oncogene (26-30). Among these, TMPRSS2-ERG gene fusions were the most frequently identified and have been shown to occur in 40-80% of PCa (31,32). Very interestingly, studies have later shown that these PCa-specific gene fusions were indeed generated by AR-induced chromosomal proximity, cellular stress, double-stand break, and erroneous DNA repair (33-35). Although the exact mechanisms and functions of these gene fusions in PCa are yet to be fully characterized, they undoubtedly represent an important pathway by which androgen regulates PCa.
New-generation targets of androgen
In addition to regulating conventional genes, AR’s transcriptional activities may also be manifested by mRNA alternative splicing events. In a study using exon array analyses of the LNCaP transcriptome, Rajan et al. were able to simultaneously identify global androgen-dependent gene expression and alternative mRNA isoform expression (36). Among more than 1,000 putative androgen-regulated alternative events, several validated events were derived from alternative promoter selection and direct binding by the AR, while others may be due to indirect effects of androgen. In addition, the AR itself may be alternatively spliced to expressed AR variants (37,38). Some of these AR variants have been shown up-regulated in aggressive PCa and may be associated with castration resistance (24,39,40). It is believed that these AR variants are able to turn on an alternative AR program thereby regulating distinct set of genes, which are important research areas currently under intensive investigation. Recent development of RNA-seq technology has further revolutionized the studies of whole transcriptomes, providing potentially unlimited measure of all transcripts and splicing variants that are expressed in a cell type. The Fu’s group pioneered in using RNA-seq to screen androgen-responsive transcripts in PCa cells. With a sequencing depth of ~10 million of 36-nt sequence tags per sample, they identified ~700 androgen-regulated genes in LNCaP cells and a large fraction of tags corresponding to alternative exons (41).
In the past few years, evidence has accumulated that non-coding RNAs (ncRNAs), like coding genes, may play similarly important roles in regulating cellular functions. The ncRNAs have been shown highly abundant and functionally important in a number of essential cellular processes (42-44). Abnormal expression patterns of ncRNAs have been detected in a variety of human diseases including PCa. In addition, some ncRNAs have been shown to have prostatic-specific expression pattern. These includes microRNAs (miRNAs) (45), small nucleolar RNA (snoRNA) (46), and long non-coding RNAs (lncRNA) (47). For instance, Ribas et al. reported 16 miRNAs that were induced by androgen in both LNCaP and LAPC4 cells, and provided evidence that elevated expression of miR-21 promotes enhanced androgen-dependent tumor growth and castration resistance in vivo (48). Most recently, by genome-wide RNA profiling of LNCaP cells across 9 time points from 0 to 48 h following 10 nM DHT treatment, more than a hundred miRNAs were found to respond to androgen, among which 3 miRNAs (miR-19a, miR-27a and miR-133b) were found to be induced by androgen. These miRNAs were found to be directly regulated by AR and play essential roles in regulating cell viability (49). Further, these miRNAs may regulate the expression of a large set of mRNAs, which were previously found to respond to androgen.
Some efforts have also been made recently to identify AR-regulated small ncRNAs in PCa cells. Louro et al. detected approximately 40 intronic antisense ncRNAs that were up-regulated by androgen in LNCaP cells (50). Functional ARE motif has been reported at the upstream region of some of these ncRNA such as that mapped to MYO5 locus and Combining Chromatin Immunoprecipitation (ChIP)-PCR confirmed androgen-activated AR binding to this loci. These intronic transcripts may be involved in transcriptional regulation similar to miRNAs, but their biological functions remain undetermined.
Through transcriptomic analysis of PCa specimens, several prostate-specific lncRNA have been reported, including PCA3 (51), PCGEM1 (52), ANRIL (53) and PCAT-1 (54). Although an increasing number of new lncRNA has been identified in the last few years, a majority of these lncRNA has not been functionally characterized, which will be important lines for future investigation. Interestingly, an androgen-responsive lncRNA CTBP1-AS, located in the AS region of C-terminal binding protein 1 (CTBP1), was recently reported. It was shown promote AR transcriptional activity through suppression of CTBP1 (55). This provocative study suggests an innovative basic regulatory pathway involving an antisense lncRNA counteracting the activity of its corresponding coding gene.
Genome-wide location analysis of AR in PCa
AR regulates target genes through binding to cis-regulatory elements
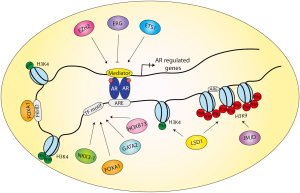
Androgen regulates downstream genes by acting as a ligand of the hormonal transcription factor, the AR. Once liganded, AR translocates into the nucleus, where it homodimerizes and binds directly to DNA through the ARE. The consensus AR-binding motif (i.e., canonical AREs, AGAACAnnnTGTTCT) consists of two hexameric half-sites (5'-AGAACA-3') often arranged as inverted repeats with 3bp of separating nucleotide. AR recognizes and interacts with AREs through its DNA-binding domain (DBD). In addition to ARE, the selective binding of AR across the genome is tightly regulated by a collection of transcription co-factors and/or pioneering factors. Accumulating evidence suggests that AR primarily binds distal enhancers that can be several kb to over 100 kb away from the promoter regions of coding genes. These AR-bound enhancers have been shown to interact with the promoters through chromatin looping (56,57) (Figure 1). AR recruits the translation initiation complex and regulates transcription through interaction with as many as over 150 co-regulators, some of which are co-activators while others are co-repressors. In order to fully understand AR-mediated transcriptional regulation, in the past decade researchers have put forth significant efforts to determine the DNA binding patterns and the genomic landscapes of AR and its cofactors, taking advantage of modern technologies.
DNA microarray analysis of AR binding sites
Immediately following the completion of the human genome project, DNA microarrays were invented to contain oligonucleotides complementing to selected regions of the genome or tiling through the entire genome. For example, promoter microarrays contain oligonulceotides that span through the promoters of all annotated genes. ChIP with DNA microarrays, termed ChIP-on-chip, researchers begun to determine whether AR binds to any of the regions represented on the DNA microarrays. Using ENCODE tiling arrays Takayama et al. identified 10 AR binding sites and subsequently validated AR recruitment to and regulation of these genes (58). In a separate study, Massie et al. used proximal promoter microarrays to identify 1,532 genomic sites that were occupied by AR. Motif analysis revealed that half ARE and ETS motifs were enriched within these regions, which led to the discovery of cooperated regulation of target genes by AR and ETS-family transcription factor ETS1 (59). Using custom oligonucleotide tiling arrays covering approximately 104 kb genomic regions centered on the TSS of 548 pre-selected potential androgen-regulated genes, another report demonstrated that a large proportion of AR binding sites are not within the proximal promoter regions, rather they are often more than 10 kb away from the TSS of androgen-responsive genes (60). In addition, many androgen-responsive genes were found to harbor two or more AR binding events within their regulatory regions. Using Affimetrix microarrays tiling through the chromosomes 21 and 22, Wang et al. identified 90 ARBS in the LNCaP cells, most of which were also far away from the promoter of androgen-responsive genes (61). Similarly using tiling arrays, another group examined AR binding to chromosomes 19 and 20 in C4-2B cells (62). They reported that there were no androgen-responsive genes at the vicinity of a majority of these ARBS, indicating that they may not be functional in terms of transcriptional regulation. However, H3 acetylation at these loci could be used to define the subset of functional ARBS associated with androgen-responsive genes (62).
Although above studies have analyzed AR binding events at selected regions, it was not only very recently has genome-wide mapping of ARBS become possible. Using whole-genome tiling arrays, Wang et al. pioneered in mapping global ARBS in PCa cells (21). They identified approximately 8,000 and 6,000 ARBS genome-wide in LNCaP and LNCaP abl cells, respectively. Comparatively analysis revealed a set of abl-specific AR binding events that lead to upregulated expression of genes involved in cell cycle, one of which is UBE2C. This study revealed that a distinct AR transcriptional program is associated with CRPC. This rapid expansion of ARBS from tens to thousands has also greatly advanced the understanding of AR-chromatin interaction. Although AR is thought to have high affinity binding with canonical full AREs, a majority of the ARBS were found to bind primarily half AREs (59-61). For example, while 78% of ARBS identified in LNCaP cells were found to harbor at least one half ARE, only 10% contained a full ARE (61). Similar results were observed in an independent study which showed that 79% and 27% of ARBS respectively contained half ARE and full ARE (59). Likewise, Chen et al. reported that 4% and 46% of the ARBS had the canonical ARE and half ARE, respectively, in an independent PCa cell line 22Rv1 (63). Moreover, the majority of these non-canonical half ARE ARBS may be functional. In vitro oligonucleotide pull-down assay and ChIP analysis confirmed that AR is able to bind to these ‘half-sites’ (59), while reporter assays verified that they mediate significant androgen-induced luciferase activity (61). Therefore, like full AREs half AREs are able to recruit AR to regulate transcription. However, it remains to be determined what fraction of these AR binding events is functional and what the determinants are.
High-resolution mapping of AR genomic landscape
Although ChIP-on-chip assays are potentially able to provide genome-wide protein location provided genome-wide tiling arrays were utilized, such approaches are usually very costly and time consuming. It was not until very recently that genome-wide mapping of protein-DNA interaction became easily feasible for regular labs due to the development of ChIP-seq, which couples ChIP with massively parallel next-generation sequencing (64,65). Unlike ChIP-on-chip, ChIP-seq is in the most part able to provide protein occupancy across unlimited genome with much increased sensitivity and accuracy. ChIP-seq of AR in PCa cells was first carried out in the PC3 cell line that expresses ectopic AR (66). This study suggested that as many as 800 genes may contain an AR binding site within their cis-regulatory elements and are responsive to androgen treatment. Unlike in LNCaP cells, ectopic AR appears to directly induce genes of the growth-inhibition response program in PC3 cells, being consistent with its reported role in this particular condition.
At the mean time, we become the first to map genomic landscape of endogenous AR in PCa cells using ChIP-seq (67). We identified more than 37,000 and 13,000 ARBS respectively in the androgen-sensitive LNCaP and VCaP cells, covering nearly all ARBS that have been identified previously using various technologies. Approximately 60% of the ARBS found in the VCaP cells overlapped with those identified in LNCaP. The fewer number of ARBS identified in VCaP cells is likely due to technical issues as ChIP-PCR was able to detect a number of ARBS that were only detected in LNCaP by ChIP-seq. Being consistence with previous results, ChIP-seq data also demonstrated that a majority of AR binds distal enhancers and intragenic regions, with less than 10% binding to promoters. De novo motif search of all ChIP-seq ARBS revealed a consensus sequence highly resembling the canonical ARE.
Recently, multiple groups have reported thousands of AR binding events in PCa cells such as LNCaP and VCaP (12,68-72). Significant overlap of ARBS between these cell lines have been observed and AR was consistently found to primarily bind distal enhancers that can be more than 50 kb away of any coding genes. However, Massie et al. reported that genes located within 25 kb of an AR binding event were the most significantly enriched for androgen-regulated expression (12). Moreover, AR ChIP-seq has also begun to be carried out in breast cancer cell lines such as MDA-MB-453 cells with the intention to examine the role of AR in breast cancer (68,72).
In summary, combinatorial efforts using ChIP-on-chip and ChIP-seq have resulted in high-resolution mapping of the genomic landscape of AR in a variety of AR binding sites (Table 3). It will be of great interest for future studies to determine how AR binding profile changes during development and diseases. In addition, future technological advances, such as ChIP-exo, may be able to determine base-pair level mapping of AR binding sites, which may further enhance our understanding of AR transcriptional regulation (73,74). Most studies have thus far unanimously found that AR binds to distal enhancers, suggesting a model wherein chromatin-looping brings the AR-bound enhancers to the proximity of a target promoter, thereby regulating transcription (21,56,61) (Figure 1). Recently, Hi-C and chromatin interaction analysis with paired-end tag sequencing (ChIA-PET) have been successfully used to uncover three-dimensional organization of the genome and global long-range chromatin interactions (75,76). Yet, it remains largely undetermined where these chromatin loopings occur. In addition, as AR primarily binds to enhancers which can be tens or hundreds of kb away from a target gene, it has been challenging to pinpoint the target gene of a particular binding event. Although 3C-based assays are useful in demonstrating chromatin looping, functional assays are missing to decisively show the regulation in vivo. This, however, has become increasingly plausible with the development of genome editing tools including transcription activator-like effector nucleases (TALENs) and clustered regulatory interspaced short palindromic repeat (CRISPR)/Cas-based RNA-guided DNA endonucleases (77-79). We thus believe that genome-wide mapping of AR binding events is just a first step towards the elucidation of AR transcriptional regulation and intriguing results will soon emerging in the near future.

Full table
Genomic regulation of AR binding profile
Pioneering factors define AR binding profile and prostate gene expression
Functional AR binding sites were not only determined by sequence motifs but also chromatin accessibility. Single-gene based approaches have already demonstrated the importance of chromatin-opening transcription factors such as FOXA1 in regulating AR binding to target genes such as PSA (80). FOXA1 is able to directly bind to the chromatin to open up the local nucleosomal domain (81). In prostate cells, FOXA1 protein has been shown physically interact with the AR protein and plays critical roles in regulating the transcription of prostate genes such as PSA (80). Following recent mapping of genome-wide ARBS, the mechanisms underlying AR recruitment to genomic loci have also become increasingly explicit. Studies from the laboratories of Dr. Myles Brown and others have established a model wherein the interactions between epigenetic modifications, pioneering factors, and AR define prostate-specific AR binding profile (61,82-84). Histone modifications such as histone H3 Lys4 mono- and di-methylation (H3K4me1 and H3K4me2) exhibit distinct genomic landscapes between prostate and breast cells and are thought to guide cell type-specific recruitment of FOXA1. The binding of FOXA1 induces chromatin accessibility, which subsequently enables AR binding to these pre-selected sites. Other active histone marks such as histone acetylation have also been associated with AR binding and androgen-induced expression (62). By contrast, AR is much less likely to bind chromatin regions marked by repressive histone modifications, such as H3K9me1 and H3K9me2 (84). Once AR binds target promoters and enhancers, they form DNA loops that coordinately assemble a multi-protein transcription complex (Figure 1) (85-87).
Counter-intuitively, recent studies showed that FOXA1 knockdown in PCa cells did not result in global inhibition of AR binding (70,71). Rather it resulted in an overall increase in AR binding events accompanied by a significant redistribution. This suggests that while FOXA1 mediates AR-binding to some genomic regions, it primarily exhibits an inhibitory role on a majority of other potential AR binding sites. It appears that FOXA1 defines a prostate-specific AR binding profile by restricting/facilitating AR binding to specific sites (e.g., those containing both ARE and FKHD motifs), while inhibiting/reducing its binding to other sites (e.g., those containing only ARE motifs) (Figure 1). The detailed mechanism as to how FOXA1 regulates AR binding profile, however, remains largely unclear and entails careful investigation.
Besides FOXA1, several other transcription factors such as GATA2 and HOXB13 may have similar pioneering cofactor effects on AR-chromatin binding and transcriptional regulation. Similar as FOXA1, GATA family transcription factors have also been shown to open compact chromatin through their conserved zinc finger domains (81,88). In addition, GATA2 expression is also essential for AR-mediated transcription of prostate genes such as PSA and TMPRSS2 (61,89). Likewise, HOXB13 is a member of the homeodomain family of sequence-specific transcription factors. Mouse studies have shown that HOXB13 play an essential role in prostate development (90). Through analysis of several candidate genes, HOXB13 has been shown a multifaceted regulator of AR-chromatin interaction, similar as FOXA1 (91). Specifically, HOXB13 was found to inhibit transcription of genes regulated by AR binding to ARE, but enhances AR binding to cis-regulatory regions containing HOX elements juxtaposed to AREs, thereby inducing corresponding gene expression. How GATA2 and HOXB13 regulate genome-wide AR binding profile, however, are yet to be carefully examined.
Co-factor regulation of AR transcriptional activity
AR transcriptional activity is tightly controlled. Over the years, a significant number of cofactor proteins have been identified that regulate AR signaling by remodeling the chromatin structure or affecting the recruitment of RNA polymerase II to the promoters of target genes. These include but are not limited to p160 family of transcription factors, CBP/P300, HDAC, CARM1 and LSD1 (92). In addition, PDEF, a prostate epithelial-specific ETS transcription factor, was reported as a co-regulator of AR, leading to enhanced transcription of PSA gene (93). Using ChIP-Seq, genome-wide location analyses have recently enabled more accurate characterization of cofactor co-occupancy at subset of target genomes (59,61,67,83,91,94). For instance, motif analysis revealed that ETS motif and AR half-sites co-occur in approximately 70% of AR-bound promoter regions. This led to further experimentation showing that ETS1 protein physically interacts with AR and enhances AR transactivation (59). Similarly, through motif analyses of AR binding sites found in VCaP cells we reported ETS motif as the 2nd most enriched motif, only ranked after the AREs (67). ChIP-seq analysis of ERG showed that it indeed co-occupied a majority of the ARBS in VCaP cells. Mechanistic studies revealed that ERG protein physically interacts with AR but surprisingly inhibits AR transcriptional activity, thus acting as a co-repressor. This role of ERG in inhibiting AR-mediated prostate gene expression has later been independently validated by multiple studies (95,96).
In addition to ETS family cofactors, genome-wide location studies have yielded the discovery or validation of a number of other AR cofactors at the genome scale. For instance, OCT and GATA motifs were found most enriched in AR binding sites found in chromosome 21 and 22 through ChIP-on-chip assay (61). GATA2 and OCT1, together with AR, form a regulatory hierarchy that governs androgen-dependent gene expression. In a separate study, analysis of ChIP-seq data discovered that 92% of the NKX3-1 binding sites overlapped with the ARBS across the PCa genome (94). NKX3-1 is a homeobox transcription factor that contributes to prostate tumor progression. This study showed that NKX3-1 and AR directly regulate each other through a feed-forward loop. Moreover, NKX3-1 collaborates with AR and FOXA1 to mediate gene expression in advanced and recurrent prostate carcinomas.
Collectively, these ChIP-seq studies have been valuable in identifying novel AR cofactors and in revealing the cooperative regulatory network that controls AR chromatin binding and prostate gene expression (Figure 1). These AR collaborating cofactors often exhibit some common characteristics such as: (I) they often physically interact with AR; (II) they frequently co-occupy the genome with the AR; (III) they regulate the transcription of AR target genes; (IV) they might directly regulate the expression of AR itself (e.g., ERG, GATA2) or might themselves be a target of androgen or AR (e.g., GATA2, NKX3-1); (V) they usually play critical roles in prostate development (e.g., ERG, FOXA1, HOXB13, and NKX3-1). These results suggest a complex network of transcription cofactors that altogether tightly controls AR-chromatin interaction and androgen-mediated gene expression. Alterations to this regulatory network might result in the disruption of AR signaling and consequently lead to PCa.
AR as a transcriptional repressor
Although a majority of studies of AR signaling and genomic regulation have focused on androgen-induced genes, microarray analyses of androgen response have consistently revealed genes that are down-regulated by androgen. In fact, AR itself was found to be repressed by androgen in VCaP cells (97). Very few studies, however, have examined androgen-repressed genes such as c-MET (98), SOX2 (99), and DDC (100). Androgen-repressed genes as a while have not been carefully investigated in the past likely due to the difficulty in determining distal AR-binding enhancers on these genes. With the use of ChIP-seq technology to map AR binding across the entire genome, AR-repressed genes have lately become the focus of multiple studies. For example, an AR binding site was found within the intragenic region of the AR gene itself (97). AR binding to this site represses AR gene expression via recruitment of LSD1 and demethylation of H3K4me1 and me2.
Recently, we have systematically examined AR binding on the regulatory elements of androgen-induced and—repressed genes in LNCP cells (101). We report that AR can act as a transcriptional repressor to directly inhibit gene expression. This repression is mediated by AR binding to AREs and facilitated by EZH2-mediated repressive chromatin remodeling. EZH2 thus cooperates with AR in transcriptional repression of target genes. Through meta-analysis of microarray datasets profiling androgen-treated PCa cells, we have nominated a number of robust AR-repressed genes (57). These genes may have important cellular functions and their repression by androgen may be critical for prostate physiology and disease. They are important genes for further examination which may lead to the development of novel biomarkers and therapeutic targets of PCa.
AR genomic regulation in CRPC
Altered AR transcription program in CRPC
AR is a key driver of PCa progression. The expression and transcriptional activity of AR remain required and sufficient to CRPC growth. Numerous studies have attempted to understand the mechanisms underlying AR activity in an androgen-depleted milieu. Through ChIP-on-chip comparison of AR binding in LNCaP and abl cells, Wang et al. has demonstrated that AR acquired new binding sites and regulates a distinct transcriptional program that is responsible for CRPC cell growth (21). Similarly, Decker et al. investigated genome-wide AR binding in LNCaP and C4-2B cells under the androgen-deprived conditions to understand how AR functions in CRPC (102). Like Wang et al. study, this study also revealed that AR persistently occupied a set of genomic regions in the absence of androgen in CRPC cells that were void of AR binding in LNCaP cells. Interestingly, these androgen-independent ARBS have constitutively open chromatin structure, often locate at promoter regions, lack ARE motif, and are independent of FOXA1. These data suggested that androgen deprivation may result in a dramatic alteration of genome-wide AR binding profiling and that nonconventional AR binding sites may be acquired (21). It will be very interesting to determine in future studies how this oncogenic AR program is regulated and may be targeted for therapy.
In addition to androgen deprivation, recent studies show that FOXA1 knockdown may also trigger a distinct AR binding profile resulting in dramatic alteration of the androgen response pathway (71). AR was again shown to bind new genomic loci, which contribute to gene expression that enhanced cell growth and established an appropriate microenvironment in CRPC. Interestingly, transcriptomic studies have recently discovered recurrent FOXA1 gene mutations in PCa, suggesting that the wildtype FOXA1 may be beneficial whilst the mutants are more tumorigenic and thus colonially selected (103,104). Being consistent with this perception, we have recently reported that FOXA1 possesses an AR-independent and even-opposing role in inhibiting cell motility and tumor metastasis, a functionality that was significantly impaired by the FOXA1 (105). However, it remains a challenge to dissect out the potentially tumor suppressive role of FOXA1 in the context of altering AR binding profile and its downstream transcriptional activity.
To determine AR binding profile in human prostate tissues and during PCa progression, Sharma et al. mapped the genomic landscape of AR in 12 human PCa tissue samples (2 benign, 3 untreated localized tumors, 2 treatment-responding cancer, 5 CRPC), and 3 cell lines (LNCaP, VCaP and 22RV1) (106). Thousands of ARBS were identified in CRPC tissues, which, surprisingly, have little overlap with the ARBS identified in PCa cell line. ARBS identified in CRPC tissue significantly overlapped with E2F, MYC and STAT binding sites, while ARBS found in PCa cell lines showed no such enrichment. In addition, many genes adjacent to the ARBS found in CRPC showed androgen regulation in xenografts, but, surprisingly, not in cultured LNCaP cells. This study suggests again that AR may be reprogrammed during PCa progression and that the AR transcriptional programs may differ significantly not only between disease stages but also among CRPC tissues. It will be critical for future studies to investigate the regulation or evolution of oncogenic AR transcriptional programs in human PCa between or within individual patients.
AR reprogramming by oncogenic transcription factors
It remains puzzling how AR transcriptional activity became reprogrammed in CRPC. Several studies have recently begun to address this important research paradigm. AR overexpression, which occurs in about 30% of CRPC, may allow AR to acquire new binding sites (107). FOXA1 knockdown has been shown to reprogram AR to activate an oncogenic transcriptional program (71). LSD1, in addition to being recruited to AR intragenic region to suppress AR expression, has also been shown to globally inhibit many other androgen-repressed genes through similar mechanism (97). Androgen deprivation, conversely, decreased AR and LSD1 recruitment to target genes, thereby restoring the expression of a subset of androgen-repressed genes that contribute to increased androgen synthesis, DNA replication, and proliferation in CRPC.
Recently, we have reported that the polycomb group protein EZH2 cooperates with AR to mediate its transcriptional repression of target genes (101). For example, we showed that AR occupies the distal enhancer of NOV, an AR-repressed gene, and communicates with the NOV promoter through DNA looping (57). This AR activation recruits EZH2, which subsequently catalyzes histone H3 lysine 27 tri-methylation around the NOV promoter, resulting in the suppression of NOV gene transcription. Very interestingly, another study has demonstrated similar AR and EZH2 cooperation, however, on androgen-induced genes (108). Xu et al. found that, in CRPC cells, EZH2 acts as a coactivator for AR through phosphorylation at Ser21, which is mediated by the PI3K-Akt pathway. EZH2 thus cooperates with AR to induce a set of genes, which are significantly overexpressed in CRPC cells. It is important to note that EZH2 is among the most highly expressed gene in metastatic PCa (109). Therefore, AR transcriptional program may be altered by oncogenic transcription factors that become abundantly expressed in CRPC. Many of these regulations are yet to be identified and such studies may lead to important discovery with significant clinical impacts.
Future directions
In summary, significant advances have been made in the last decade regarding genomic regulation of AR. Global androgen-responsive genes have been carefully examined in various cell line systems, animal models, and clinical specimens. Genome-wide AR binding profile in PCa cells have been comprehensively mapped by several independent research labs in various systems. These successes will form a solid foundation for potentially ground-breaking discoveries in the years to come. The identification and characterization of non-conventional targets of androgen, such as miRNAs and lncRNAs, are still in their infancy. Although studies to date have mapped the basal AR binding profiles in PCa cells, a lot remain to be learned regarding the transcriptional regulatory network that determines precise AR binding events at each developmental and disease stages. The precise mechanisms by which AR pioneering factors and coregulators control AR transcriptional program are yet to be delineated. It will be exceedingly important for future studies to determine AR transcriptional reprogramming in CRPC and how this is regulated by various oncogenic factors. Such mechanistic studies will be essential for strategic disruption of AR signaling in CRPC and may dramatically improve patient care.
Acknowledgements
We thank Angela Yang for helpful discussion.
Funding: This work was supported in part by the NIH P50CA090386 pilot project (to J.Y.), U54CA143869 pilot project (to J.Y.), K99/R00CA129565 (to J.Y.), R01CA172384 (to J.Y.), the U.S. Department of Defense PC080665 (to J.Y.), and the Research Scholar Award RSG-12-085-01 (to J.Y.) from the American Cancer Society.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev 2004;25:276-308. [PubMed]
- van Royen ME, van Cappellen WA, de Vos C, et al. Stepwise androgen receptor dimerization. J Cell Sci 2012;125:1970-9. [PubMed]
- Claessens F, Alen P, Devos A, et al. The androgen-specific probasin response element 2 interacts differentially with androgen and glucocorticoid receptors. J Biol Chem 1996;271:19013-6. [PubMed]
- Prescott JL, Blok L, Tindall DJ. Isolation and androgen regulation of the human homeobox cDNA, NKX3.1. Prostate 1998;35:71-80. [PubMed]
- Clegg N, Eroglu B, Ferguson C, et al. Digital expression profiles of the prostate androgen-response program. J Steroid Biochem Mol Biol 2002;80:13-23. [PubMed]
- Xu LL, Su YP, Labiche R, et al. Quantitative expression profile of androgen-regulated genes in prostate cancer cells and identification of prostate-specific genes. Int J Cancer 2001;92:322-8. [PubMed]
- Waghray A, Feroze F, Schober MS, et al. Identification of androgen-regulated genes in the prostate cancer cell line LNCaP by serial analysis of gene expression and proteomic analysis. Proteomics 2001;1:1327-38. [PubMed]
- Romanuik TL, Wang G, Holt RA, et al. Identification of novel androgen-responsive genes by sequencing of LongSAGE libraries. BMC Genomics 2009;10:476. [PubMed]
- DePrimo SE, Diehn M, Nelson JB, et al. Transcriptional programs activated by exposure of human prostate cancer cells to androgen. Genome Biol 2002;3:RESEARCH0032.
- Velasco AM, Gillis KA, Li Y, et al. Identification and validation of novel androgen-regulated genes in prostate cancer. Endocrinology 2004;145:3913-24. [PubMed]
- Ngan S, Stronach EA, Photiou A, et al. Microarray coupled to quantitative RT-PCR analysis of androgen-regulated genes in human LNCaP prostate cancer cells. Oncogene 2009;28:2051-63. [PubMed]
- Massie CE, Lynch A, Ramos-Montoya A, et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J 2011;30:2719-33. [PubMed]
- Dehm SM, Tindall DJ. Ligand-independent androgen receptor activity is activation function-2-independent and resistant to antiandrogens in androgen refractory prostate cancer cells. J Biol Chem 2006;281:27882-93. [PubMed]
- Jiang M, Ma Y, Chen C, et al. Androgen-responsive gene database: integrated knowledge on androgen-responsive genes. Mol Endocrinol 2009;23:1927-33. [PubMed]
- Clegg N, Nelson PS. Androgen-regulated genes in the prostate. Androgen Action in Prostate Cancer 2009;631-61.
- Jiang F, Wang Z. Identification of androgen-responsive genes in the rat ventral prostate by complementary deoxyribonucleic acid subtraction and microarray. Endocrinology 2003;144:1257-65. [PubMed]
- Pfundt R, Smit F, Jansen C, et al. Identification of androgen-responsive genes that are alternatively regulated in androgen-dependent and androgen-independent rat prostate tumors. Genes Chromosomes Cancer 2005;43:273-83. [PubMed]
- Wang XD, Wang BE, Soriano R, et al. Expression profiling of the mouse prostate after castration and hormone replacement: implication of H-cadherin in prostate tumorigenesis. Differentiation 2007;75:219-34. [PubMed]
- Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A 2002;99:11890-5. [PubMed]
- Frigo DE, Howe MK, Wittmann BM, et al. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res 2011;71:528-37. [PubMed]
- Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell 2009;138:245-56. [PubMed]
- Holzbeierlein J, Lal P, LaTulippe E, et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am J Pathol 2004;164:217-27. [PubMed]
- Mostaghel EA, Page ST, Lin DW, et al. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: therapeutic implications for castration-resistant prostate cancer. Cancer Res 2007;67:5033-41. [PubMed]
- Hu R, Lu C, Mostaghel EA, et al. Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res 2012;72:3457-62. [PubMed]
- Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005;310:644-8. [PubMed]
- Tomlins SA, Bjartell A, Chinnaiyan AM, et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 2009;56:275-86. [PubMed]
- Tomlins SA, Laxman B, Dhanasekaran SM, et al. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature 2007;448:595-9. [PubMed]
- Chinnaiyan AM, Palanisamy N. Chromosomal aberrations in solid tumors. Prog Mol Biol Transl Sci 2010;95:55-94. [PubMed]
- Rubin MA. ETS rearrangements in prostate cancer. Asian J Androl 2012;14:393-9. [PubMed]
- Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol 2011;29:3659-68. [PubMed]
- Demichelis F, Rubin MA. TMPRSS2-ETS fusion prostate cancer: biological and clinical implications. J Clin Pathol 2007;60:1185-6. [PubMed]
- Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer 2008;8:497-511. [PubMed]
- Lin C, Yang L, Tanasa B, et al. Nuclear receptor-induced chromosomal proximity and DNA breaks underlie specific translocations in cancer. Cell 2009;139:1069-83. [PubMed]
- Mani RS, Tomlins SA, Callahan K, et al. Induced chromosomal proximity and gene fusions in prostate cancer. Science 2009;326:1230. [PubMed]
- Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet 2010;42:668-75. [PubMed]
- Rajan P, Dalgliesh C, Carling PJ, et al. Identification of novel androgen-regulated pathways and mRNA isoforms through genome-wide exon-specific profiling of the LNCaP transcriptome. PLoS One 2011;6:e29088. [PubMed]
- Dehm SM, Schmidt LJ, Heemers HV, et al. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res 2008;68:5469-77. [PubMed]
- Hu R, Dunn TA, Wei S, et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res 2009;69:16-22. [PubMed]
- Sun S, Sprenger CC, Vessella RL, et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest 2010;120:2715-30. [PubMed]
- Guo Z, Yang X, Sun F, et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res 2009;69:2305-13. [PubMed]
- Li H, Lovci MT, Kwon YS, et al. Determination of tag density required for digital transcriptome analysis: application to an androgen-sensitive prostate cancer model. Proc Natl Acad Sci U S A 2008;105:20179-84. [PubMed]
- Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet 2009;10:704-14. [PubMed]
- Esteller M. Non-coding RNAs in human disease. Nat Rev Genet 2011;12:861-74. [PubMed]
- Eddy SR. Non-coding RNA genes and the modern RNA world. Nat Rev Genet 2001;2:919-29. [PubMed]
- Ambs S, Prueitt RL, Yi M, et al. Genomic profiling of microRNA and messenger RNA reveals deregulated microRNA expression in prostate cancer. Cancer Res 2008;68:6162-70. [PubMed]
- Martens-Uzunova ES, Jalava SE, Dits NF, et al. Diagnostic and prognostic signatures from the small non-coding RNA transcriptome in prostate cancer. Oncogene 2012;31:978-91. [PubMed]
- Du Z, Fei T, Verhaak RG, et al. Integrative genomic analyses reveal clinically relevant long noncoding RNAs in human cancer. Nat Struct Mol Biol 2013;20:908-13. [PubMed]
- Ribas J, Ni X, Haffner M, et al. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 2009;69:7165-9. [PubMed]
- Mo W, Zhang J, Li X, et al. Identification of novel AR-targeted microRNAs mediating androgen signalling through critical pathways to regulate cell viability in prostate cancer. PLoS One 2013;8:e56592. [PubMed]
- Louro R, Nakaya HI, Amaral PP, et al. Androgen responsive intronic non-coding RNAs. BMC Biol 2007;5:4. [PubMed]
- Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 1999;59:5975-9. [PubMed]
- Srikantan V, Zou Z, Petrovics G, et al. PCGEM1, a prostate-specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci U S A 2000;97:12216-21. [PubMed]
- Yap KL, Li S, Muñoz-Cabello AM, et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Mol Cell 2010;38:662-74. [PubMed]
- Prensner JR, Iyer MK, Balbin OA, et al. Transcriptome sequencing across a prostate cancer cohort identifies PCAT-1, an unannotated lincRNA implicated in disease progression. Nat Biotechnol 2011;29:742-9. [PubMed]
- Takayama K, Horie-Inoue K, Katayama S, et al. Androgen-responsive long noncoding RNA CTBP1-AS promotes prostate cancer. EMBO J 2013;32:1665-80. [PubMed]
- Wang Q, Carroll JS, Brown M. Spatial and temporal recruitment of androgen receptor and its coactivators involves chromosomal looping and polymerase tracking. Mol Cell 2005;19:631-42. [PubMed]
- Wu L, Runkle C, Jin HJ, et al. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene 2014;33:504-13. [PubMed]
- Takayama K, Kaneshiro K, Tsutsumi S, et al. Identification of novel androgen response genes in prostate cancer cells by coupling chromatin immunoprecipitation and genomic microarray analysis. Oncogene 2007;26:4453-63. [PubMed]
- Massie CE, Adryan B, Barbosa-Morais NL, et al. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep 2007;8:871-8. [PubMed]
- Bolton EC, So AY, Chaivorapol C, et al. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev 2007;21:2005-17. [PubMed]
- Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell 2007;27:380-92. [PubMed]
- Jia L, Berman BP, Jariwala U, et al. Genomic androgen receptor-occupied regions with different functions, defined by histone acetylation, coregulators and transcriptional capacity. PLoS One 2008;3:e3645. [PubMed]
- Chen H, Libertini SJ, George M, et al. Genome-wide analysis of androgen receptor binding and gene regulation in two CWR22-derived prostate cancer cell lines. Endocr Relat Cancer 2010;17:857-73. [PubMed]
- Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell 2007;129:823-37. [PubMed]
- Robertson G, Hirst M, Bainbridge M, et al. Genome-wide profiles of STAT1 DNA association using chromatin immunoprecipitation and massively parallel sequencing. Nat Methods 2007;4:651-7. [PubMed]
- Lin B, Wang J, Hong X, et al. Integrated expression profiling and ChIP-seq analyses of the growth inhibition response program of the androgen receptor. PLoS One 2009;4:e6589. [PubMed]
- Yu J, Yu J, Mani RS, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell 2010;17:443-54. [PubMed]
- Robinson JL, Macarthur S, Ross-Innes CS, et al. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J 2011;30:3019-27. [PubMed]
- Andreu-Vieyra C, Lai J, Berman BP, et al. Dynamic nucleosome-depleted regions at androgen receptor enhancers in the absence of ligand in prostate cancer cells. Mol Cell Biol 2011;31:4648-62. [PubMed]
- Sahu B, Laakso M, Ovaska K, et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J 2011;30:3962-76. [PubMed]
- Wang D, Garcia-Bassets I, Benner C, et al. Reprogramming transcription by distinct classes of enhancers functionally defined by eRNA. Nature 2011;474:390-4. [PubMed]
- Ni M, Chen Y, Lim E, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell 2011;20:119-31. [PubMed]
- Rhee HS, Pugh BF. Comprehensive genome-wide protein-DNA interactions detected at single-nucleotide resolution. Cell 2011;147:1408-19. [PubMed]
- Rhee HS, Pugh BF. Genome-wide structure and organization of eukaryotic pre-initiation complexes. Nature 2012;483:295-301. [PubMed]
- Zhang Y, McCord RP, Ho YJ, et al. Spatial organization of the mouse genome and its role in recurrent chromosomal translocations. Cell 2012;148:908-21. [PubMed]
- Li G, Ruan X, Auerbach RK, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell 2012;148:84-98. [PubMed]
- Gaj T, Gersbach CA, Barbas CF 3rd. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol 2013;31:397-405. [PubMed]
- Bedell VM, Wang Y, Campbell JM, et al. In vivo genome editing using a high-efficiency TALEN system. Nature 2012;491:114-8. [PubMed]
- Cong L, Ran FA, Cox D, et al. Multiplex genome engineering using CRISPR/Cas systems. Science 2013;339:819-23. [PubMed]
- Gao N, Zhang J, Rao MA, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol 2003;17:1484-507. [PubMed]
- Cirillo LA, Lin FR, Cuesta I, et al. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell 2002;9:279-89. [PubMed]
- Wang Y, Klijn JG, Zhang Y, et al. Gene-expression profiles to predict distant metastasis of lymph-node-negative primary breast cancer. Lancet 2005;365:671-9. [PubMed]
- Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell 2008;132:958-70. [PubMed]
- Eeckhoute J, Lupien M, Meyer CA, et al. Cell-type selective chromatin remodeling defines the active subset of FOXA1-bound enhancers. Genome Res 2009;19:372-80. [PubMed]
- Cleutjens KB, van Eekelen CC, van der Korput HA, et al. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem 1996;271:6379-88. [PubMed]
- Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell 2002;9:601-10. [PubMed]
- Kang Z, Jänne OA, Palvimo JJ. Coregulator recruitment and histone modifications in transcriptional regulation by the androgen receptor. Mol Endocrinol 2004;18:2633-48. [PubMed]
- Boyes J, Omichinski J, Clark D, et al. Perturbation of nucleosome structure by the erythroid transcription factor GATA-1. J Mol Biol 1998;279:529-44. [PubMed]
- Perez-Stable CM, Pozas A, Roos BA. A role for GATA transcription factors in the androgen regulation of the prostate-specific antigen gene enhancer. Mol Cell Endocrinol 2000;167:43-53. [PubMed]
- Huang L, Pu Y, Hepps D, et al. Posterior Hox gene expression and differential androgen regulation in the developing and adult rat prostate lobes. Endocrinology 2007;148:1235-45. [PubMed]
- Norris JD, Chang CY, Wittmann BM, et al. The homeodomain protein HOXB13 regulates the cellular response to androgens. Mol Cell 2009;36:405-16. [PubMed]
- Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: a diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev 2007;28:778-808. [PubMed]
- Oettgen P, Finger E, Sun Z, et al. PDEF, a novel prostate epithelium-specific ets transcription factor, interacts with the androgen receptor and activates prostate-specific antigen gene expression. J Biol Chem 2000;275:1216-25. [PubMed]
- Tan PY, Chang CW, Chng KR, et al. Integration of regulatory networks by NKX3-1 promotes androgen-dependent prostate cancer survival. Mol Cell Biol 2012;32:399-414. [PubMed]
- Kunderfranco P, Mello-Grand M, Cangemi R, et al. ETS transcription factors control transcription of EZH2 and epigenetic silencing of the tumor suppressor gene Nkx3.1 in prostate cancer. PLoS One 2010;5:e10547. [PubMed]
- Chng KR, Chang CW, Tan SK, et al. A transcriptional repressor co-regulatory network governing androgen response in prostate cancers. EMBO J 2012;31:2810-23. [PubMed]
- Cai C, He HH, Chen S, et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 2011;20:457-71. [PubMed]
- Verras M, Lee J, Xue H, et al. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res 2007;67:967-75. [PubMed]
- Kregel S, Kiriluk KJ, Rosen AM, et al. Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer. PLoS One 2013;8:e53701. [PubMed]
- Margiotti K, Wafa LA, Cheng H, et al. Androgen-regulated genes differentially modulated by the androgen receptor coactivator L-dopa decarboxylase in human prostate cancer cells. Mol Cancer 2007;6:38. [PubMed]
- Zhao JC, Yu J, Runkle C, et al. Cooperation between Polycomb and androgen receptor during oncogenic transformation. Genome Res 2012;22:322-31. [PubMed]
- Decker KF, Zheng D, He Y, et al. Persistent androgen receptor-mediated transcription in castration-resistant prostate cancer under androgen-deprived conditions. Nucleic Acids Res 2012;40:10765-79. [PubMed]
- Grasso CS, Wu YM, Robinson DR, et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012;487:239-43. [PubMed]
- Barbieri CE, Baca SC, Lawrence MS, et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet 2012;44:685-9. [PubMed]
- Jin HJ, Zhao JC, Ogden I, et al. Androgen receptor-independent function of FoxA1 in prostate cancer metastasis. Cancer Res 2013;73:3725-36. [PubMed]
- Sharma NL, Massie CE, Ramos-Montoya A, et al. The androgen receptor induces a distinct transcriptional program in castration-resistant prostate cancer in man. Cancer Cell 2013;23:35-47. [PubMed]
- Linja MJ, Savinainen KJ, Saramäki OR, et al. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res 2001;61:3550-5. [PubMed]
- Xu K, Wu ZJ, Groner AC, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science 2012;338:1465-9. [PubMed]
- Varambally S, Dhanasekaran SM, Zhou M, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002;419:624-9. [PubMed]



