Patient-derived xenograft models to optimize kidney cancer therapies
Introduction
Kidney cancer is of the fifteen most common cancers in both men and women worldwide, with roughly 400,000 new diagnoses in 2018 (1-3). It also accounts for approximately 175,000 deaths per year worldwide (2,4). There are many types of kidney cancer, including renal cell carcinomas (RCC), urothelial carcinomas, sarcomas, and Wilms tumors, where RCC make up 85% of diagnoses (5). To enhance clinical tools and be predictive of patient responses to treatments, preclinical models are often used. Currently, human cancer cell lines are often used for cancer growth hypothesis development and testing and in vivo drug testing (6,7). However, the minimal predictive capacity relative to clinical trials makes them less effective than required for some studies (8). A more advanced and frequently used technique that overcomes this is to utilize patient-derived xenograft (PDX) models in animals.
The PDX model is not new but has consistently demonstrated correlation between responses found in the model system and the clinic (8). The first successful heterotransplantation of a human tumor sample into mice occurred in 1969, using colon cancer and athymic nude mice (9). However, this model has been expanded to include a variety of cancer types, such as colorectal, melanoma, pancreatic adenocarcinoma, lymphoma, sarcoma, gastric, RCC, and many more (10). The model offers many advantages, such as the ability to maintain histology, quick procedure time, and feasibility for researchers. The underlying hypothesis of the PDX model is that it will retain key characteristics of the donor tumor through successive mouse-to-mouse passages (8). PDX models better preserve the genomic integrity and tumor heterogeneity observed in patients better than cell line models (11).
Prevalence/importance
RCC account for 2–3% of all malignant diseases in adults. This disease is more prominent among men, particularly those in their sixties and seventies (12). The highest incidence rates are in Northern and Eastern Europe, North America, and Australia. Some of the most common risk factors include smoking, obesity, hypertension, and maintenance dialysis (13). RCC occurs predominantly in male patients with a median age of 64 years old at diagnosis. RCC is the most common solid neoplasm of the adult kidney and has a high potential for developing metastatic spread (14). Approximately 25–30% of RCC patients have metastatic disease at presentation, and 30–40% of patients develop metastases after the initial diagnosis. The 5-year survival rate of patients with metastatic disease is less than 10%, partly because RCC metastases become resistant to current therapies (15,16).
Among the five types of kidney cancers, the most prominent form, RCC, contain three main sub-types—clear cell RCC (ccRCC), papillary, and chromophobe, plus approximately 13 other subtypes (17). ccRCC are the most common sub-type, and account for roughly 70% of all kidney cancers (1,12,17-19). It can be caused by the loss or mutation of the von Hippel-Linadu (VHL) tumor suppressor gene (12,18,20-22). ccRCC is identified by a clear cytoplasm with nested clusters of cells, surrounded by a dense endothelial network (18). Papillary RCC (pRCC) are the second most common type of RCC (12,18,23). pRCC can present as either a sporadic or inherited disease due to a high incidence of chromosome 7 trisomy or tetrasomy (24). Histologically, pRCC display basophilic cellular cytoplasms and can have foamy histiocytes (12,18). The chromophobe RCC are rare and are due to complete chromosomal loss, such as of chromosomes 1, 2, 6, 10, 13, 17, and 21 (12,18). This carcinoma is characterized by cells with mostly empty cytoplasms, perinuclear clearing, low mitotic rate, and a low risk of developing metastatic tumors (12,18).
Recently, another type of RCC, clear cell papillary RCC, have been identified. These types of RCC have been shown to be associated with end-stage renal disease. They often present as a solitary mass and may exist with other renal tumors. The tumor is usually well circumscribed with variable architectural patterns. The tumor cells have clear cytoplasms and the nuclei are characteristically situated away from the basement membrane in a linear fashion (25).
Xenograft models
The development of small animal models that can mimic human RCC treatment responses is important in order to evaluate novel clinical drugs or test new therapeutic treatments (26). The following will discuss the various types of xenograft models.
Traditional xenograft models
Traditional xenograft models are those that utilize established cell lines for implantation into animals (3). Cell lines are a cost-effective method of studying cancer, due to the indefinite lifespan, ease of maintenance, genetic manipulability, and similar gene expression patterns with primary human tumors (27). They are used to study the effects of genetic manipulations or of drug treatments on tumor development. They are also often transfected with fluorescent or bioluminescent expression proteins to continuously monitor growth with non-invasive imaging machines. Overall, they provide an effective method of studying tumor growth in vivo (8).
Some of the most commonly studied RCC cell lines include RP-R-01, RP-R-02, ACHN, Caki-1, Renca, and OS-RC-2 (28-33). Like other cell lines, these originated from different RCC samples, and therefore have various morphologies. While these models are useful for measuring responses to treatments, there are limitations. One main limitation is the ability to predict activity of specific cancer types in clinical trials (8). There is also an issue with the selection of cell lines available—most established cell lines are from aggressive tumors and are not as representative of the complex tumor heterogeneity seen in the clinic (8), and cell line modes are often unable to successfully capture tumor heterogeneity (34).
PDX models
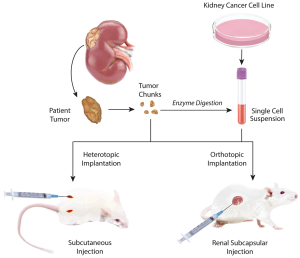
PDX models are created when a patient’s cancerous tumor is implanted into immunodeficient mice (Figure 1) (10). A critical requirement for any xenograft model to have clinical relevance is that it should faithfully replicate the original patient tumor for subsequent studies (35).
Methods
The type of mice that are used in PDX models are non-obese diabetic (NOD) severe combined immunodeficiency (scid) mice. The use of scid mice helped establish the PDX model, as they have a depressed innate immunity, including lack of T and B cells, reduced NK cell and macrophage activity, abnormal dendritic cell development, and lack of hemolytic complement (36). The immunocompromised characteristics are key, as they do not reject implanted human cells (37). NOD/scid mice are more receptive of primary solid tumors than nude or scid mice.
When establishing a new mouse model with a patient sample, pieces of primary or metastatic solid tumors, maintained as tissue structures, are collected by surgery or biopsy procedures. If solid tissue samples are unavailable, cell suspensions from blood or from digestion of tumors into single-cell suspensions can be used (Figure 1) (10). These samples are ready to be implanted but can often be co-injected with other cell types or enhancers. Some tumors are combined with basement membrane extracellular matrix proteins (such as Matrigel) which encourage the growth of tumors (38). Some studies have reported co-injecting tumors with fibroblasts, stromal cells, or endothelial cells (11,39,40).
There are two main methods of implantation used for the PDX model: heterotopic and orthotopic. Heterotopic implantation is a common and quick method and provides an easy way to track tumor development (10). The most common forms of heterotopic implantation are subcutaneous (usually on the dorsal region of mice) and intravenous (11,41,42). Orthotopic implantation into the organ of origin of the tumor is a slower process and may require ultrasound examinations or bioluminescent imaging to confirm tumor growth (37). Renal cancers, such as primary patient tumor samples, can be implanted into the kidney or under the renal capsule, to increase engraftment success rates (Figure 1) (21,34).
Advantages
The PDX model offers many advantages for translational oncological studies (Table 1). The process of implantation is minimally invasive for the animal and can be done directly from digested portions of real human samples. This allows for examination of the effects of various treatments and conditions from a single biopsy (43). Moreover, samples of the implanted tumor are viable for subsequent passages of the tumor to new mice (44).

Full table
One of the most easily identifiable advantages of the PDX model is the ability of tumors to display similar heterogeneity in mice as they do in humans (30). This allows for more specific predictions in clinical models, and for fidelity of the results. Moreover, cytogenetic analysis of the models demonstrates significant levels of conservation of the genetic composition of patient tumors, adding to the confidence of the model (45). The preservation of the histology, gene expression, DNA copy number alterations, mutations, and treatment responsiveness in trials establishes the PDX model as a good option for renal cancer mouse studies (34). Researchers can be confident in their models as tumor growth occurs in a physiological relevant setting of oxygen, nutrients, and hormones (11). Orthotopic implantation methods offer the most similar environments to mimic human conditions, which provides a translational advantage (8,10). Overall, this model offers potential for personalized precision medicine (36).
RCC are especially well suited for PDX models (41). The tumors are generally large, and rarely treated with chemotherapy, therefore the behavior and genetics of the mass are similar to the original composition, and unaffected by exposure to DNA damaging agents (41,45). Previous examples of implanted RCC have demonstrated preserved histology and karyotypes of the patient tumors (41).
Disadvantages
While PDX models have been effective models for many studies, there are associated disadvantages. The ability to overcome these challenges could provide even more advanced preclinical models (Table 1).
One challenge is in the nature of NOD/scid mice. Due to their suppressed immune system, they are susceptible to thymic lymphomas, which can develop as early as three to four months of age. This can be problematic considering their already short lifespan of roughly eight to nine months. It should be noted, however, that males develop these tumors at lower rates than females, and can be preferable for longitudinal studies (36). Also, these mice cannot be used in immunotherapy experiments due to the fact that they lack an intact immune system.
Another issue of the PDX model is the low engraftment rate. Roughly 20% of localized primary tumors are successfully grafted subcutaneously. When metastatic tissue is grafted in immunodeficient mice, there is about an 80% success rate for RCC (46). Similarly, the tumors might not metastasize the same way in mouse models as they did in patients, meaning there are some lost developmental patterns of the disease.
A third concern with this model is the loss of the primary tumor’s original characteristics. Heterotopic implantation poses challenges, such as the potential for the primary tumor’s biology to be affected (41). Similarly, this model can experience the replacement of human tumor stroma in the primary grafted tumor by murine stroma after successive passages (46). While PDX orthotopic models may better mimic metastatic patterns, they present their own challenges. Generally, it is the more expensive and more difficult procedure (37).
Examples of established PDX models
Many researchers use PDX models to study the effects of drugs and tumor sensitivities to treatments. Alongside surgery, radiation therapy and immunotherapy, molecular-targeted therapy is one of the principal treatment options for patients with kidney cancer (47). There are currently 7 drugs that have been approved for the treatment of all types of RCC (14). Some of the most common include: sunitinib, everolimus, cabozantinib, and temsirolimus. These have all been studied using PDX models.
Sunitinib
Sunitinib, or Sutent, is a multikinase inhibitor that has shown success in patients with metastatic kidney cancer. Various tyrosine kinase receptors within the cancer cells, such as vascular endothelial growth factor receptors (VEGFRs) 1, 2, and 3, and platelet derived growth factor receptor (PDGFR) alpha and beta, become inhibited, limiting growth and subsequent cell divisions (48,49). Sunitinib is also unique, as it can minimize intracellular blood vessel growth, reducing oxygen supply to the cancer cells (50-52). Worldwide, this drug is an approved first line treatment for metastatic RCC in patients with an intermediate to good prognosis (53).
Researchers at the University of Texas Southwestern Medical Center and Illumina Cambridge Ltd., studied the drug responsiveness of RCC in a PDX model. To begin, 94 tumor samples were implanted orthotopically, to eventually form 16 stable patient-derived tumor-graft lines, each of which was passaged at least twice. The histology and gene expression of these tumors were maintained throughout the experiment, as determined by both unsupervised hierarchical clustering analysis and bidirectional Sanger sequencing. The study began by implanting 20 mice with a 64 mm3 sized fragment of tumor for each of 8 tumor-graft lines. Drug administration began when the tumor sizes averaged 250–300 mm3. Three to five mice were allocated to one of four treatment groups: sunitinib, sirolimus, erlotinib, or a vehicle control. For 28 days, tumors were measured twice weekly. The amount of drug administered was adjusted based on weekly mouse weight variations. Overall, 122 mice were studied in the drug trials. It was observed that the ccRCC PDX model growth was substantially inhibited by sunitinib and sirolimus. This study also tested an investigational agent in clinical development, dovitinib, which may have shown more potent inhibition of tumor-graft growth than sunitinib and sirolimus (41). This finding is consistent with clinical responses of sunitinib (6).
Sunitinib was initially approved by the Food and Drug Administration because of a high response rate (roughly 40% partial response) but it is susceptible to resistance (46,50,51). Researchers in France aimed to develop a strategy to overcome resistance by testing if the resistance was transient and if it could be overcome by increasing the dose (31). Currently, the recommend dose of sunitinib for patients with advanced RCC is 50 mg orally, once a day, for four weeks (50,51). Two patient-derived ccRCC tumor-graft lines were used for this experiment. RP-R-01 and RP-R-02 were patient tumors that originally responded to sunitinib treatment, but eventually developed drug resistance. One mm3 samples of these tumors, from mice that had previously had the tumor passaged to them, were subcutaneously implanted into control and treatment groups for this study (n=20). Treatment groups received 40 mg/kg sunitinib for five days on, two days off, by oral gavage. Once tumors established resistance, the dose was increased to 60 mg/kg, and then 80 mg/kg upon secondary resistance. Despite dose escalation, mice did not show signs of drug toxicity. There were no vascular changes or incidence of necrosis upon histopathological assessment of liver tissues (31). One important finding from this study is the changes in expression of methyltransferase EZH2 at the time of resistance, which is often modified in various cancers (31,54). Moreover, when EZH2 was inhibited, there was an increase in the anti-tumor effect of sunitinib in vitro (31). A major challenge to improving treatment and management of ccRCC is resistance to receptor tyrosine kinase inhibitors (RTKi). However, EZH2 can be a target for therapeutic intervention in sunitinib-resistance ccRCC, as well as a predictive marker for RTKi response in this disease (54).
Systemic sunitinib treatment has also been shown to reduce tumor dendritic cell Stat3 activity. This transcription factor was studied in mice injected with the cell lines, 786-O, RCC4, and Renca. This study found that sunitinib inhibits tumor Stat3 and that it induces tumor cell death as early as one day post treatment. Thus, the effectiveness of sunitinib in kidney cancers was validated in this study (55).
Everolimus
When a patient has previously undergone treatment for advanced kidney cancer, and has failed to respond to sunitinib, a second line of treatment is everolimus (56). Everolimus, marketed as Afinitor, is a serine-threonine kinase inhibitor of mTOR, mammalian target of rapamycin kinase that controls cell growth, division, and cell metabolism (57,58). This drug was approved within the past ten years and is often used in conjunction with lenvatinib, a multiple kinase inhibitor that works against VEGFRs 1, 2, and 3 (57,59).
Larkin et al. studied the use of this drug in a PDX model. Murine RCC, which expressed luciferase, were orthotopically implanted into female BALB/c mice. Tumor-bearing mice were placed into various treatment groups: PBS (vehicle control), sunitinib, sunitinib then sorafenib, or sunitinib then everolimus. In vivo bioluminescence signal tracking occurred weekly, to assess tumor-derived luciferase activity and to determine when to switch to the second drug, as appropriate. Mice were treated with the second drug when they reached a bioluminescence signal level that was equivalent to the half maximal signal of the control group (60). As expected, the animals treated with sunitinib had an increasing bioluminescence signal that occurred at a slower rate than that of the PBS group. However, when animals were switched to sorafenib, the luciferase activity stabilized. There was a notable decrease in in vivo luciferase signaling upon the switch to everolimus, which suggested a reduction in tumor volume. Another important finding was that the group that later received everolimus had a reduced primary tumor and metastatic tumor incidence rate (60).
Cabozantinib
Cabozantinib, marketed as Cabometyx, is a small-molecule tyrosine kinase inhibitor and an inhibitor of MET, and was approved for advanced RCC treatment within the past three years. MET, also known as the N-methyl-N'-nitroso-guanidine human osteosarcoma transforming gene, is a proto-oncogene encoding a receptor tyrosine kinase c-MET for hepatocyte growth factor (HGF) (61). Similar to everolimus, this treatment is best suited for patients with advanced RCC who have previously had anti-angiogenic therapy (23,62). While it can be effective, it does have significant side effects that require a reduction in dose for more than half of patients (62).
Researchers at Stanford University School of Medicine were interested in the drug’s ability to inhibit tumor growth and metastasis in PDX models. This study utilized a new PDX model with a unique pRCC sample, which had a specific activating mutation of MET. The tissue used for this study was implanted under the renal capsule of immunodeficient mice and was passed through seven generations of mice. One unique finding from this study was that some mice had metastasis to the lung, which mimics the patient’s tumor exactly. Eventually, mice with sufficiently sized tumors were divided into control and treatment groups, where the treatment groups received 30 mg/kg of cabozantinib per day by oral gavage for 21 days. Overall, tumor volume decreased four-fold, compared to the control group, and MET activity was reduced (23).
Temsirolimus and Sapanisertib
Temsirolimus, a drug that is marketed as Torisel, is also an inhibitor of mTOR. One of the significant pathogenetic features of kidney cancers is the upregulation of hypoxia genes by mTOR (52,63). Temsirolimus arrests kidney cancer cells in G1 phase and inhibits tumor angiogenesis through the reduction in synthesis of vascular endothelial growth factor (64).This treatment has been on the market for roughly a decade, and was the first mTOR inhibitor approved for cancer therapy (63,65). Temsirolimus is indicated for the first-line treatment for poor prognosis metastatic kidney cancer (66).
MLN0128, or Sapanisertib, is a second-generation, ATP-competitive, mTOR inhibitor that has been shown to have stronger effects on tumor suppression than temsirolimus (67,68). mTOR is composed of two complexes, TORC1 and TORC2, which are both inhibited by Sapanisertib (69). This presents the possibility of tumor cell apoptosis and minimizing cell proliferation. While this drug is still currently in phase I and II clinical trials, it promises to be a more potent alternative to temsirolimus. So far, this drug has shown success in antitumor activity for breast cancer, prostate cancer, B-cell leukemia, and RCCs (68). Ingels et al. developed a patient-derived tissue slice graft (TSG) model from three fresh primary RCC specimens to study the effects of MLN0128. The samples were implanted under the renal capsule into immunodeficient mice. Randomly, mice were treated with a placebo, temsirolimus, or, MLN0128. Temsirolimus was administered once a week at 10 mg/kg by intraperitoneal injection, while MLN0128 was administered daily by oral gavage at 1 mg/kg. MLN0128 consistently suppressed primary RCC growth in three TSG cohorts for up to 2 months, whereas temsirolimus only inhibited growth of TSGs in one of two cohorts tested before resistance developed (67).
Conclusion and future directions
Among various pre-clinical models for RCC, PDX models are a better choice for testing therapeutic approaches for patients. PDX models have been used to study different treatment modalities in kidney cancers. The main treatment that is currently used is sunitinib. There has been extensive use of PDX models to study sunitinib which has allowed researchers to understand the mechanism by which sunitinib works. There have also been advances in understanding the effects of sunitinib on the genetic level. The second line treatment for RCC is everolimus. Everolimus has been studied in murine models and has been shown to effectively reduce tumor size. Other drugs that have been studied using PDX models include cabozantinib, temsirolimus and Sapanisertib. The use of PDX models allows for quick stratification of various treatments. Another reason why PDX models are used is that they are able to retain tumor heterogeneity in mice. Despite this advantage, current PDX models cannot be used to test therapies such as immune checkpoint inhibitor therapy.
Future models, including humanized mouse models for immunotherapies (70), and mouse avatar models that use patient-derived tumor carry mice, will identify the best chemotherapeutic choice for a particular cancer patient (71). In a recent study, RCC mouse models were first generated orthotopically then added allogeneic human peripheral blood mononuclear cells to evaluate the efficacy of antibody targeting the carbonic anhydrate IX protein in RCC. This study demonstrated that the antibody inhibited cancer growth by priming T-cell activity (72,73). Avatar models can be used to generate personalized treatment for cancer. They allow for the generation of individualized mouse xenografts and a platform for therapeutic decision making (71,73).
Acknowledgments
Authors would like to thank Barbara Siede for assistance with the excellent medical illustration.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Dalgliesh GL, Furge K, Greenman C, et al. Systematic sequencing of renal carcinoma reveals inactivation of histone modifying genes. Nature 2010;463:360-3. [Crossref] [PubMed]
- Kidney Cancer. Cancer.net. Available online: https://www.cancer.net/cancer-types/kidney-cancer/introduction. Accessed 10/10/2018.
- Jang J, Rath O, Schueler J, et al. Development of novel patient-derived preclinical models from malignant effusions in patients with tyrosine kinase inhibitor-resistant clear cell renal cell carcinoma. Transl Oncol 2017;10:304-10. [Crossref] [PubMed]
- Abaan OD, Polley EC, Davis SR, et al. The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology. Cancer Res 2013;73:4372-82. [Crossref] [PubMed]
- Hidalgo M, Amant F, Biankin AV, et al. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov 2014;4:998-1013. [Crossref] [PubMed]
- Rygaard J, Povlsen CO. Heterotransplantation of a human malignant tumour to "Nude" mice. Acta Pathol Microbiol Scand 1969;77:758-60. [Crossref] [PubMed]
- Lai Y, Wei X, Lin S, et al. Current status and perspectives of patient-derived xenograft models in cancer research. J Hematol Oncol 2017;10:106. [Crossref] [PubMed]
- Sanmamed MF, Chester C, Melero I, et al. Defining the optimal murine models to investigate immune checkpoint blockers and their combination with other immunotherapies. Ann Oncol 2016;27:1190-8. [Crossref] [PubMed]
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119-32. [Crossref] [PubMed]
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-30. [Crossref] [PubMed]
- Volpe A, Patard JJ. Prognostic factors in renal cell carcinoma. World J Urol 2010;28:319-27. [Crossref] [PubMed]
- Takyar S, Diaz J, Sehgal M, et al. First-line therapy for treatment-naive patients with advanced/metastatic renal cell carcinoma: a systematic review of published randomized controlled trials. Anticancer Drugs 2016;27:383-97. [Crossref] [PubMed]
- Kanesvaran R, Tan MH. Targeted therapy for renal cell carcinoma: The next lap. J Carcinog 2014;13:3. [Crossref] [PubMed]
- Grisanzio C, Seeley A, Chang M, et al. Orthotopic xenografts of RCC retain histological, immunophenotypic and genetic features of tumours in patients. J Pathol 2011;225:212-21. [Crossref] [PubMed]
- Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ 2014;349:g4797. [Crossref] [PubMed]
- Wallace EM, Rizzi JP, Han G, et al. A small-molecule antagonist of HIF2alpha is efficacious in preclinical models of renal cell carcinoma. Cancer Res 2016;76:5491-500. [Crossref] [PubMed]
- Chen W, Hill H, Christie A, et al. Targeting renal cell carcinoma with a HIF-2 antagonist. Nature 2016;539:112-7. [Crossref] [PubMed]
- Jiménez-Valerio G, Martinez-Lozano M, Bassani N, et al. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Rep 2016;15:1134-43. [Crossref] [PubMed]
- Kim KT, Lee HW, Lee HO, et al. Application of single-cell RNA sequencing in optimizing a combinatorial therapeutic strategy in metastatic renal cell carcinoma. Genome Biol 2016;17:80. [Crossref] [PubMed]
- Zhao H, Nolley R, Chan AMW, et al. Cabozantinib inhibits tumor growth and metastasis of a patient-derived xenograft model of papillary renal cell carcinoma with MET mutation. Cancer Biol Ther 2017;18:863-71. [Crossref] [PubMed]
- Schuller AG, Barry ER, Jones RD, et al. The MET inhibitor AZD6094 (Savolitinib, HMPL-504) induces regression in papillary renal cell carcinoma patient-derived xenograft models. Clin Cancer Res 2015;21:2811-9. [Crossref] [PubMed]
- Zhou H, Zheng S, Truong LD, et al. Clear cell papillary renal cell carcinoma is the fourth most common histologic type of renal cell carcinoma in 290 consecutive nephrectomies for renal cell carcinoma. Hum Pathol 2014;45:59-64. [Crossref] [PubMed]
- Zeng Y, Liu B, Rubio MT, et al. Creation of an immunodeficient HLA-transgenic mouse (HUMAMICE) and functional validation of human immunity after transfer of HLA-matched human cells. PLoS One 2017;12:e0173754. [Crossref] [PubMed]
- Lum DH, Matsen C, Welm AL, et al. Overview of human primary tumorgraft models: comparisons with traditional oncology preclinical models and the clinical relevance and utility of primary tumorgrafts in basic and translational oncology research. Curr Protoc Pharmacol 2012;Chapter 14:Unit 14 22.
- Fu X, Nakamori M, Tao L, et al. Antitumor effects of two newly constructed oncolytic herpes simplex viruses against renal cell carcinoma. Int J Oncol 2007;30:1561-7. [PubMed]
- Miyake M, Anai S, Fujimoto K, et al. 5-fluorouracil enhances the antitumor effect of sorafenib and sunitinib in a xenograft model of human renal cell carcinoma. Oncol Lett 2012;3:1195-202. [Crossref] [PubMed]
- Miles KM, Seshadri M, Ciamporcero E, et al. Dll4 blockade potentiates the anti-tumor effects of VEGF inhibition in renal cell carcinoma patient-derived xenografts. PLoS One 2014;9:e112371. [Crossref] [PubMed]
- Adelaiye R, Ciamporcero E, Miles KM, et al. Sunitinib dose escalation overcomes transient resistance in clear cell renal cell carcinoma and is associated with epigenetic modifications. Mol Cancer Ther 2015;14:513-22. [Crossref] [PubMed]
- Ciamporcero E, Miles KM, Adelaiye R, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther 2015;14:101-10. [Crossref] [PubMed]
- Sun J, Wang X, Tang B, et al. A tightly controlled Src-YAP signaling axis determines therapeutic response to dasatinib in renal cell carcinoma. Theranostics 2018;8:3256-67. [Crossref] [PubMed]
- Pavía-Jiménez A, Tcheuyap VT, Brugarolas J. Establishing a human renal cell carcinoma tumorgraft platform for preclinical drug testing. Nat Protoc 2014;9:1848-59. [Crossref] [PubMed]
- Karam JA, Zhang XY, Tamboli P, et al. Development and characterization of clinically relevant tumor models from patients with renal cell carcinoma. Eur Urol 2011;59:619-28. [Crossref] [PubMed]
- Inoue T, Terada N, Kobayashi T, et al. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat Rev Urol 2017;14:267-83. [Crossref] [PubMed]
- Richmond A, Su Y. Mouse xenograft models vs GEM models for human cancer therapeutics. Dis Model Mech 2008;1:78-82. [Crossref] [PubMed]
- Gock M, Kuhn F, Mullins CS, et al. Tumor take rate optimization for colorectal carcinoma patient-derived xenograft models. Biomed Res Int 2016;2016:1715053. [Crossref] [PubMed]
- Gills J, Moret R, Zhang X, et al. A patient-derived orthotopic xenograft model enabling human high-grade urothelial cell carcinoma of the bladder tumor implantation, growth, angiogenesis, and metastasis. Oncotarget 2018;9:32718-29. [Crossref] [PubMed]
- Margolin DA, Myers T, Zhang X, et al. The critical roles of tumor-initiating cells and the lymph node stromal microenvironment in human colorectal cancer extranodal metastasis using a unique humanized orthotopic mouse model. FASEB J 2015;29:3571-81. [Crossref] [PubMed]
- Sivanand S, Pena-Llopis S, Zhao H, et al. A validated tumorgraft model reveals activity of dovitinib against renal cell carcinoma. Sci Transl Med 2012;4:137ra75. [Crossref] [PubMed]
- Diaz-Montero CM, Mao FJ, Barnard J, et al. MEK inhibition abrogates sunitinib resistance in a renal cell carcinoma patient-derived xenograft model. Br J Cancer 2016;115:920-8. [Crossref] [PubMed]
- Frank I, Blute ML, Leibovich BC, et al. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol 2005;173:1889-92. [Crossref] [PubMed]
- Lin D, Wyatt AW, Xue H, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res 2014;74:1272-83. [Crossref] [PubMed]
- Hong B, Yang Y, Guo S, et al. Intra-tumour molecular heterogeneity of clear cell renal cell carcinoma reveals the diversity of the response to targeted therapies using patient-derived xenograft models. Oncotarget 2017;8:49839-50. [Crossref] [PubMed]
- Bousquet G, Janin A. Patient-derived xenograft: an adjuvant technology for the treatment of metastatic disease. Pathobiology 2016;83:170-6. [Crossref] [PubMed]
- Renal Cell Carcinoma Treatment & Management. Medscape. Available online: https://emedicine.medscape.com/article/281340-treatment#d1. Accessed 10/10/2018.
- Dong Y, Manley BJ, Becerra MF, et al. Tumor xenografts of human clear cell renal cell carcinoma but not corresponding cell lines recapitulate clinical response to sunitinib: feasibility of using biopsy samples. Eur Urol Focus 2017;3:590-8. [Crossref] [PubMed]
- Motzer RJ, Michaelson MD, Redman BG, et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 2006;24:16-24. [Crossref] [PubMed]
- Chow LQM, Eckhardt SG. Sunitinib: From Rational Design to Clinical Efficacy. J Clin Oncol 2007;25:884-96. [Crossref] [PubMed]
- Approved Drugs. U.S. Food and Drug Administration. Available online: https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm585686.htm. Accessed 10/10/2018.
- Find your targeted (biological) therapy. MacMillan Cancer Support. Available online: https://www.macmillan.org.uk/information-and-support/treating/targeted-biological-therapies/find-your-therapy/sunitinib.html. Accessed 10/10/2018.
- Kim KH, Kim HY, Kim HR, et al. Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with renal insufficiency. Eur J Cancer 2014;50:746-52. [Crossref] [PubMed]
- Adelaiye-Ogala R, Budka J, Damayanti NP, et al. EZH2 modifies sunitinib resistance in renal cell carcinoma by kinome reprogramming. Cancer Res 2017;77:6651-66. [Crossref] [PubMed]
- Xin H, Zhang C, Herrmann A, et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res 2009;69:2506-13. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 2008;372:449-56. [Crossref] [PubMed]
- Hall MN. mTOR-what does it do? Transplant Proc 2008;40:S5-8. [Crossref] [PubMed]
- Matsui J, Funahashi Y, Uenaka T, et al. Multi-kinase inhibitor E7080 suppresses lymph node and lung metastases of human mammary breast tumor MDA-MB-231 via inhibition of vascular endothelial growth factor-receptor (VEGF-R) 2 and VEGF-R3 kinase. Clin Cancer Res 2008;14:5459-65. [Crossref] [PubMed]
- Larkin J, Esser N, Calvo E, et al. Efficacy of sequential treatment with sunitinib-everolimus in an orthotopic mouse model of renal cell carcinoma. Anticancer Res 2012;32:2399-406. [PubMed]
- Sattler M, Salgia R. c-Met and hepatocyte growth factor: potential as novel targets in cancer therapy. Curr Oncol Rep 2007;9:102-8. [Crossref] [PubMed]
- Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814-23. [Crossref] [PubMed]
- Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007;356:2271-81. [Crossref] [PubMed]
- Wan X, Shen N, Mendoza A, et al. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia 2006;8:394-401. [Crossref] [PubMed]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell 2012;149:274-93. [Crossref] [PubMed]
- Barthélémy P, Hoch B, Chevreau C, et al. mTOR inhibitors in advanced renal cell carcinomas: from biology to clinical practice. Crit Rev Oncol Hematol 2013;88:42-56. [Crossref] [PubMed]
- Ingels A, Zhao H, Thong AE, et al. Preclinical trial of a new dual mTOR inhibitor, MLN0128, using renal cell carcinoma tumorgrafts. Int J Cancer 2014;134:2322-9. [Crossref] [PubMed]
- Slotkin EK, Patwardhan PP, Vasudeva SD, et al. MLN0128, an ATP-competitive mTOR kinase inhibitor with potent in vitro and in vivo antitumor activity, as potential therapy for bone and soft-tissue sarcoma. Mol Cancer Ther 2015;14:395-406. [Crossref] [PubMed]
- NCI Drug Dictionary. National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/sapanisertib. Accessed 10/10/2018.
- Suarez ER, Chang DK, Sun J, et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget 2016;7:34341-55. [Crossref] [PubMed]
- Garralda E, Paz K, Lopez-Casas PP, et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin Cancer Res 2014;20:2476-84. [Crossref] [PubMed]
- Chang DK, Moniz RJ, Xu Z, et al. Human anti-CAIX antibodies mediate immune cell inhibition of renal cell carcinoma in vitro and in a humanized mouse model in vivo. Mol Cancer 2015;14:119. [Crossref] [PubMed]
- Choi Y, Lee S, Kim K, et al. Studying cancer immunotherapy using patient-derived xenografts (PDXs) in humanized mice. Exp Mol Med 2018;50:99. [Crossref] [PubMed]