
Chronic kidney disease after nephrectomy: a clinically-significant entity?
Introduction
Worldwide, the kidney is the 9th and 14th most common primary site of cancer in men and women respectively (1). Either radical (total) or partial (nephron-sparing) nephrectomy is the most common approach to management of patients with kidney cancer.
There is a large volume of literature evaluating postoperative kidney function following nephrectomy. In a recent systematic review, we identified 312 studies published after the year 2000 which considered kidney function as an outcome after nephrectomy (2). Interestingly, less than 5% of these studies followed patients for longer than an average of five years, and less than 2% considered an average follow-up time beyond 7.5 years (2). Many studies have aimed to describe the benefits of partial nephrectomy compared with radical nephrectomy for improving both postoperative kidney function and overall survival (3,4). While partial nephrectomy undoubtedly leads to nephron mass preservation and higher postoperative kidney function on average, there is still controversy as to benefits in terms of survival outcomes. Indeed, many well-powered observational studies have noted survival benefits for partial nephrectomy compared with radical nephrectomy (3), whereas others, including the only randomised clinical trial comparing the two procedures (5), have reported that this not the case. This apparent incongruity has perpetuated the argument that reductions in kidney function secondary to nephrectomy are not as clinically-significant as chronic kidney disease (CKD) due to a medical aetiology.
It will be argued here that surgical loss of kidney parenchyma should be viewed as a risk factor for worsening kidney function over time; and, that incident CKD [defined as an estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 persisting for a duration of three months or greater] is of clinical significance, regardless of the underlying cause. This will be achieved by evaluating studies which report survival and kidney functional outcomes in: (I) otherwise healthy adults who have donated a kidney; (II) patients with kidney tumours who have undergone partial or radical nephrectomy; and (III) patients who have undergone surgical management of kidney cancer, with or without comparisons made with the general population.
Living kidney donors
Perhaps the strongest evidence for the view that surgical removal of nephrons should be considered as a risk factor for future CKD comes from population studies of living kidney donors. Historically, it was understood that living kidney donors were unlikely to experience adverse consequences, and this belief was preserved through studies showing better health outcomes for donors compared with non-donors, which either failed to consider baseline health status as a potential confounding factor, or did not consider an adequate follow-up time to observe adverse events related to unilateral nephrectomy (see Table 1 for a summary of selected studies).
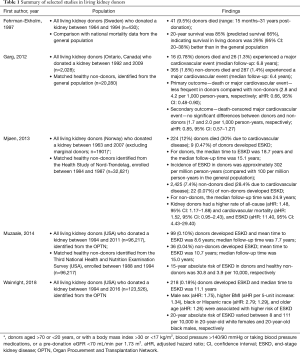
Full table
A well-cited example of the former is a population-based study published in 1997 by Fehrman-Ekholm and colleagues, which suggested that Swedish living kidney donors had longer survival times compared with the general population. They reported that 20-year survival was 85% for living donors (compared with 66% predicted survival, based on data from the general population) (6), a result which was most likely a consequence of not adjusting for baseline health status, as suggested by the study’s authors. An example of the latter is a population-based study from Ontario, Canada, published by Garg et al. in 2012 (7). This study reported that kidney donors were less likely to experience death or a major cardiovascular event compared with matched non-donors, with a median follow-up of 6.5 years (the rate of death or major cardiovascular event per 1,000 person-years was 4.1 and 2.8 for non-donors and donors, respectively) (7). This study adequately accounted for baseline health status by matching donors to members of the general population, restricting to only the healthiest, by excluding from the analysis anyone with a medical comorbidity which may preclude donation; however, it is possible that the follow-up time was not adequate to discern the adverse effects of unilateral nephrectomy.
In more recent studies with longer follow-up periods, a higher likelihood of death or end-stage kidney disease (ESKD) has been reported following kidney donation (Table 1). In a study which evaluated 1,901 Norwegian kidney donors, Mjøen and colleagues reported higher rates of cardiovascular and all-cause mortality, and ESKD [adjusted hazard ratio (aHR): 1.52, 1.48, and 11.40, respectively] compared with matched non-donors, with a median time to ESKD of 18.7 years (8). Similarly, in the United States, using data from the Organ Procurement and Transplantation Network, Muzaale and colleagues found that the 15-year absolute risk of ESKD in donors was 30.8 per 10,000, compared with only 3.9 per 10,000 in matched non-donors (9). Using data from the same source, Wainright and colleagues recently reported that potential risk factors for ESKD in kidney donors included male sex, higher body mass index, black or Hispanic race, and older age; however, they also reported interactions between race and age (10).
These data all seem to suggest that, when considering a long-enough follow-up time and adequately accounting for baseline health status, kidney donors generally have a small but significantly higher absolute risk of developing ESKD than people who do not undergo unilateral nephrectomy. This inference is supported further by data indicating that patients who have undergone nephrectomy in childhood have a higher risk of cardiovascular disease and premature mortality (11). This is consistent with Brenner’s hypothesis, that single-nephron hyperfiltration secondary to functional strain may initiate or perpetuate progressive deterioration of kidney function, and concomitant proteinuria hypertension, with adverse consequences only becoming apparent decades after unilateral nephrectomy (12,13). This therefore supports the argument that removal of functional kidney parenchyma is associated with a higher risk of clinically-significant kidney functional deterioration, even in healthy patients.
Radical vs. partial nephrectomy
Although a number of observational studies have reported survival benefits for patients undergoing partial compared with radical nephrectomy (3,4), a phase III randomised trial comparing radical and partial nephrectomy, which was conducted by the European Organisation for Research and Treatment of Cancer (EORTC), reported that this was not the case, and a survival benefit was actually observed for patients managed with radical nephrectomy (14). These conflicting results have generated a great deal of debate as to whether CKD due to surgical removal of nephrons has clinical significance, and because of the fact that the results of randomised controlled trials are generally thought to present higher-quality evidence compared with observational studies, a lot of emphasis has been placed on the results of the EORTC trial. Acknowledging the difficulty of conducting a methodologically-rigorous randomised trial for a surgical intervention, we argue here that because of methodological issues with the design of the trial, and potentially-flawed assumptions in the interpretation of the results, this clinical trial does not provide evidence that CKD secondary to nephrectomy is of less clinical significance than CKD due to other causes (15,16).
The EORTC trial was initially designed to rule out a 10% difference in 5-year overall survival between radical nephrectomy and partial nephrectomy for simple kidney tumours ≤5 cm at largest diameter, with a hypothesis that radical nephrectomy was associated with worse survival. There were 310 participants recruited between 1992 and 1998 in Europe and participants were randomised 1:1 to each treatment arm. The primary outcome was subsequently changed to rule out a 3% difference in 5-year overall survival. Based on power calculations (where α=0.05 and β=0.20), 300 and 1,300 patients were designated as the minimum sample size to detect a 10% and 3% difference in 5-year survival, respectively. After the change, recruitment was opened up also to patients in the USA and Canada, between 1998 and 2003. In total, 541 patients were recruited from 40 different centres, including the 310 participants recruited between 1992 and 1998 (268 randomised to partial nephrectomy, 273 randomised to radical nephrectomy; 39 patients assigned partial nephrectomy were managed with radical nephrectomy and 16 patients assigned to radical nephrectomy were managed with partial nephrectomy) (5,17). Although this trial had sufficient power to address the initial goal of ruling-out a 10% difference in 5-year survival, it was underpowered to rule-out a 3% difference. It was also underpowered to evaluate differences in the incidence of ESKD. Due to a high proportion of cross-over between treatment arms, there is a high risk that confounding by indication also impacted results.
The EORTC trial found that, with a median follow-up of 6.7 years, 85.7%, 64.7% and 1.5%; and 64.7%, 6.3% and 1.6%, of patients developed an eGFR <60, <30 or <15 mL/min per 1.73 m2, after radical and partial nephrectomy, respectively (14). It was also reported that 10-year survival was 81.1% and 75.7% in patients randomised to be managed with radical and partial nephrectomy, respectively (5.4% difference in survival) (5).
A major issue with drawing the conclusion that CKD secondary to nephrectomy is not as significant as CKD of a non-surgical aetiology from this study is simply that the trial was not designed to answer this research question: this observation would be reasonable in the context of hypothesis generation, but was far from adequate to infer causality. Although kidney function was considered as a planned secondary analysis of this trial, this was only evaluated in the context of comparing postoperative kidney function (eGFR, continuous and by CKD stage) by surgery type. It was reported that kidney function was better in patients managed with partial nephrectomy (14); however, no analysis was performed evaluating survival mediated by postoperative kidney function. It was therefore unclear how many patients who died also had impaired kidney function. Interestingly, post hoc subgroup analyses of this study showed that for patients with preoperative SCr >25% of the upper limit of normal, the risk of mortality following partial nephrectomy was lower than radical nephrectomy (aHR: 0.8, 95% CI: 0.1–3.4) compared with patients whose SCr was ≤25% of the upper limit of normal, where this effect reversed (aHR: 1.6, 95% CI: 1.0–2.3) (18).
Despite a clear difference in postoperative eGFR between radical and partial nephrectomy, due to the reasonably short follow-up time of this trial (6.7 years) (14), it is also unlikely that a long-enough amount of time had passed to fully appreciate the effect of nephron reduction on risk of mortality, similar to potential limitations in some studies evaluating living kidney donors. Due to these limitations in the EORTC trial’s design, it could be argued that less significance should be placed on inferences related to the clinical significance of postoperative kidney function generated from these results.
Another potential issue with drawing these conclusions about the clinical significance of CKD from this trial is that the argument relies on an inherent assumption that, apart from the amount of functional parenchyma being removed, radical and partial nephrectomy are essentially equivalent. This is not the case, and it is very likely that differences between radical nephrectomy and partial nephrectomy, which extend beyond the degree of surgical nephron reduction between the two procedures, are more than trivial. Regarding potential adverse effects, Shuch et al. discussed this eloquently, arguing that partial nephrectomy should not be viewed as “protective” but rather “less harmful” than radical nephrectomy (19).
A recent systematic review of 21 studies evaluating postoperative complications following nephrectomy reported that partial nephrectomy was associated with a higher rate of complications compared with radical nephrectomy [relative risk (RR): 1.7, 95% CI: 1.3–2.2] (4). This demonstrates that clinically-significant differences between the two procedures exist, which could affect long-term health outcomes, but which are essentially ignored in arguments relating to the association between kidney function and mortality.
It should also be noted that the safety profile of partial nephrectomy has changed significantly since its introduction into clinical practice. As the EORTC trial was conducted over more than a decade (1992 to 2003) during periods where the safety profile of partial nephrectomy was variable, it is possible that variations in surgical technique, and subsequent sequelae of this, affected results. Miller et al. demonstrated that, in an American population-based retrospective study of patients managed with both radical nephrectomy and partial nephrectomy, between 1991 and 1999, there was no statistical difference in the risk of developing ESKD following partial nephrectomy compared with radical nephrectomy (aHR: 1.3, 95% CI: 0.9–1.8); but, between 2000 and 2002 the point estimates reversed, showing ESKD was less likely in patients managed with partial nephrectomy (aHR: 0.7, 95% CI: 0.6–0.9) (20).
No data have been presented comparing mortality rates by era from the EORTC trial, and it is unlikely that these analyses would be appropriately powered, given the trial’s small sample size. It is possible that earlier cases where patients were managed with partial nephrectomy may have disproportionately influenced survival estimates compared with more recent cases, leading to an inflated estimate of the overall mortality risk associated with partial nephrectomy. It is also possible that the ESKD cases associated with partial nephrectomy were from the earlier era, where ESKD was more common after partial nephrectomy. This is particularly relevant given the low event count associated with the development of ESKD in this trial. This remains a point of concern in the interpretation of these results.
Given that the EORTC trial was not adequately powered to assess outcomes related to kidney function and ESKD, had a limited follow-up time, and did not take into account differences between the procedures outside of the amount of functional parenchyma being resected, this trial does not provide strong evidence that CKD subsequent to surgical resection of kidney tissue is of less significance than medical causes of CKD.
Downstream effects of “surgical” CKD
An argument that is often made to minimise the perceived risk of CKD following nephrectomy is that there is not strong evidence of subsequent eGFR decline (progressive CKD) in patients with “surgical” CKD. Although this statement is true, this is a common feature of a large number of patients who develop CKD (eGFR <60 mL/min per 1.73 m2) regardless of underlying aetiology. Many patients with stage 3 CKD will not experience a decline in eGFR, have prolonged periods of non-progression, will demonstrate non-linear eGFR decline, or have a widely-fluctuating eGFR or relapsing-remitting disease trajectory (21-23). Notwithstanding, all patients with stage 3 CKD have a higher mortality and cardiovascular risk, compared with patients without CKD. It is therefore important to correlate postoperative eGFR with hard clinical end-points such as mortality, as the absence of progressive CKD does not equate with the absence of risk for adverse outcomes.
One of the largest databases of nephrectomy patients is maintained by the Cleveland Clinic in the USA, and in this section, we will primarily discuss studies which utilise these data. General findings of these studies demonstrate that, compared with patients who do not develop CKD after nephrectomy, patients who do develop CKD, and had CKD prior to surgery, have a higher risk of all-cause mortality (for example, aHR: 1.2, 95% CI: 1.0–1.4; and aHR: 2.0, 95% CI: 1.7–2.3, respectively, with a median follow-up of 9.4 years, n=4,299) (24). A common conclusion of these studies is that “medical”, “surgical” and “medical/surgical” CKD (referring to the broad underlying aetiology, or combination thereof, driving reductions in kidney function) are associated with different risks of clinically-significant end-points. We will argue that these distinctions are arbitrary, and that the key factor that determines risk is the postoperative eGFR, regardless of the underlying cause.
Nephrectomy in patients with preoperative CKD, and patients without preoperative CKD who go on to develop postoperative CKD, can lead to adverse outcomes and progressive eGFR decline, caused by either maladaptation to nephron mass reduction, or because of underlying damage to the kidneys. In patients with clinically-evident CKD prior to nephrectomy, this damage has already been identified. In patients without clinically-evident CKD prior to nephrectomy, this damage may not be present, or may only be mild, and may never have become symptomatic in the absence of surgical mass reduction. Undergoing nephrectomy modifies this, and patients are subsequently classed as having CKD. Although the distinction of CKD before surgery is important in terms of individual patient prognosis: that patients with CKD prior to undergoing nephrectomy are at higher risk of adverse events compared with patients who do not; extrapolating this to infer that new-onset CKD after surgery is of less significance than new-onset CKD of other causes is not appropriate, as these patient groups are not comparable in this respect.
If patients who develop CKD after nephrectomy are considered to have had subclinical CKD before surgery, then the reason for the difference in terms of overall survival between the two groups (patients with CKD before surgery and patients with incident CKD after nephrectomy) could be attributed to lead-time bias. If the underlying pathological processes are considered to be essentially equivalent between subclinical and clinically-evident CKD, then the only difference is the initial time of onset. As patients with evidence of CKD before surgery are more likely to have had an earlier onset of the underlying pathology contributing to CKD, they are therefore more likely to die as a consequence of CKD than patients who develop clinically-evident CKD only after undergoing nephrectomy. Therefore, if causal inference is intended, it cannot be concluded that new-onset CKD after nephrectomy is a less significant disease process compared with prevalent CKD, based on the findings that patients with “medical/surgical” CKD have a higher mortality rate than patients with just “surgical” CKD. Although it is more likely that patients with prevalent CKD will experience adverse events, this finding is not unexpected. Mortality risk is inversely proportional to eGFR, regardless of the population of interest (25-28). In terms of evaluating the clinical significance of post-nephrectomy CKD, the important comparison in these studies was between patients with incident CKD after nephrectomy and patients who did not develop CKD after nephrectomy—this comparison showed that patients with incident CKD had significantly higher mortality rates.
Therefore, the next relevant question is: how does the risk of mortality for patients with new-onset CKD after surgery compare to that of patients with medical causes of CKD who do not undergo nephrectomy? This was partially addressed using the Cleveland Clinic Surgical Registry dataset by Demirjian et al., who compared all-cause mortality and non-renal cancer mortality in patients with “surgical” and “medical/surgical” CKD (n=1,097 and 1,053, respectively) and a cohort of 42,658 patients with CKD which was not secondary to nephrectomy, who were managed by nephrologists at the Cleveland Clinic (29). The authors showed that patients with “medical” and “medical/surgical” CKD had similar risk of all-cause and non-renal cancer mortality, and that the risk was higher than for patients who developed CKD only after nephrectomy. This study was limited by the fact that patients who did not develop CKD after nephrectomy were not included. The risk of bias introduced by performing a head-to-head comparison of these patients was also quite large, given that patients referred to a nephrologist will typically have at least moderately-severe CKD, which again introduces the risk of lead-time bias (30). It is unclear what the exclusion criteria were for the comparison group of patients with CKD of a medical aetiology and, unlike patients undergoing nephrectomy, they have no clear precipitating event to designate T0, which makes the interpretation of time-to-event analyses difficult.
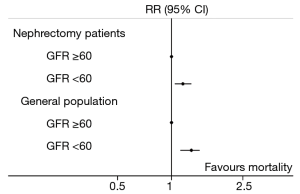
A different approach is to compare the RR for mortality for patients with and without new-onset CKD after nephrectomy to a well-established value in the literature, comparing patients with and without medical CKD in the general population, with a comparable follow-up period. Tonelli et al. conducted a systematic review of 39 studies which included populations of patients who were at risk of CKD, and had kidney function and mortality data recorded (31). Overall there were 1,371,990 patients included, with a median follow-up of 4.5 (0.8–16.0) years. They reported that patients with CKD (eGFR <60 mL/min per 1.73 m2) had an increased risk of all-cause mortality (RR: 1.8, 95% CI: 1.3–2.3) compared with patients with a postoperative eGFR ≥60 mL/min per 1.73 m2.
Data from another study which used the Cleveland Clinic Surgical Registry conducted by Wu et al., who evaluated mortality after nephrectomy in patients who did and did not develop CKD after surgery (n=931 and 2,202, respectively), was compared with the mortality estimates from the general population discussed above (26). We calculated the RR comparing non-kidney cancer-related mortality in patients who did and did not develop new-onset CKD after nephrectomy, using methods described previously (32). This analysis demonstrated that patients who developed new-onset CKD had an increased risk of mortality than those who did not (RR: 1.4, 95% CI: 1.1–1.8). The 10-year mortality risk was reasonably similar (RR: 1.5, 95% CI: 1.2–1.8). Although the point-estimates are slightly lower than those reported by Tonelli et al., there was substantial overlap between the 95% CIs, which tends to indicate that patients with new-onset CKD after surgery were at increased risk of death compared with patients who did not develop CKD after nephrectomy, and at comparable risk to patients with CKD of a medical aetiology compared with patients without CKD in the general population (Figure 1). As this is a relative measure, there is an underlying assumption that patients who do not develop CKD following surgery (i.e., postoperative eGFR >60 mL/min per 1.73 m2) were at similarly comparable risk of death compared with patients without CKD who do not undergo nephrectomy. Based on the results of studies using the Cleveland Clinic Surgical Registry, it could be extrapolated that new-onset CKD after nephrectomy was associated with an increased risk of mortality compared with having an eGFR >60 mL/min per 1.73 m2 after surgery. Although this single-centre database is limited by lack of generalisability, the findings from these studies are consistent with reports from other population groups managed surgically for kidney cancer (33,34).
Summary
There is a flaw in the argument that CKD secondary to surgical nephron reduction is of less clinical significance than CKD due to other causes.
We have demonstrated that, based on data from living kidney donors, loss of functional kidney parenchyma, even in healthy individuals, is associated with an increased long-term risk of progressive CKD, ESKD and all-cause and cardiovascular mortality. We also showed that, due to methodological constraints and untested assumptions, using the EORTC trial to argue that CKD following surgical reduction of nephron mass is of less clinical significance than CKD of other causes does not have a strong evidence base. Finally, we showed that, even in the absence of progressive CKD, developing CKD (eGFR <60 mL/min per 1.73 m2) after nephrectomy is associated with a higher risk of mortality compared with patients who do not develop CKD after nephrectomy, and that this risk is essentially equivalent with the risk of mortality for patients with CKD of any cause.
Thresholds for CKD stages in clinical guidelines are based on the fact that 60 mL/min per 1.73 m2 is approximately half of the maximum physiological eGFR of the average person, is able to be distinguished accurately by estimating equations, and is associated with increased risk of adverse events (23). In clinical practice, the distinction of CKD stages is arbitrary, and patients can experience eGFR changes in a fluctuating or relapsing-remitting fashion. It is clear that not all patients with an eGFR <60 mL/min per 1.73 m2 from a medical cause will experience further decline in kidney function, and many even experience improvements (21), not unlike patients undergoing nephrectomy; but the fact that not all patients who experience new-onset CKD after nephrectomy will experience a progressive decline in kidney function is cited as a reason for not considering it to have the same clinical significance as CKD of a medical cause (35). In the community, a patient with an eGFR fluctuating around 60 mL/min per 1.73 m2 will not be referred to a nephrologist, unless a clear pattern of rapid decline is present, or there is another indication such as uncontrolled hypertension; but, they should undergo regular monitoring of eGFR and urinary albumin-creatinine ratio, and subsequently referred if function begins to deteriorate (30).
Conclusions
Patients who develop new-onset CKD after surgical management of kidney tumours should be considered differently to patients who do not, as, at a population level, they have increased risk of adverse events, including all-cause and cardiovascular mortality, and ESKD. Although a patient who ends up with an eGFR around 50–60 mL/min per 1.73 m2 after nephrectomy should not be rushed off to a nephrologist, risk factors for CKD should be modified where possible, and patients should be monitored regularly by evaluating serum creatinine, eGFR, and albumin-creatinine ratio. If there are signs that kidney function is deteriorating, referral should then be considered.
Acknowledgments
RJ Ellis was supported by an Australian Government Research Training Scholarship.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Znaor A, Lortet-Tieulent J, Laversanne M, et al. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 2015;67:519-30. [Crossref] [PubMed]
- Ellis RJ, Cho Y, Del Vecchio SJ, et al. Outcome measures used to report kidney function in studies investigating surgical management of kidney tumours: a systematic review. Eur Urol Focus 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Kim SP, Thompson RH, Boorjian SA, et al. Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: a systematic review and meta-analysis. J Urol 2012;188:51-7. [Crossref] [PubMed]
- Mir MC, Derweesh I, Porpiglia F, et al. Partial nephrectomy versus radical nephrectomy for clinical T1b and T2 renal tumors: a systematic review and meta-analysis of comparative studies. Eur Urol 2017;71:606-17. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2011;59:543-52. [Crossref] [PubMed]
- Fehrman-Ekholm I, Elinder CG, Stenbeck M, et al. Kidney donors live longer. Transplantation 1997;64:976-8. [Crossref] [PubMed]
- Garg AX, Meirambayeva A, Huang A, et al. Cardiovascular disease in kidney donors: matched cohort study. BMJ 2012;344:e1203. [Crossref] [PubMed]
- Mjøen G, Hallan S, Hartmann A, et al. Long-term risks for kidney donors. Kidney Int 2014;86:162-7. [Crossref] [PubMed]
- Muzaale AD, Massie AB, Wang MC, et al. Risk of end-stage renal disease following live kidney donation. JAMA 2014;311:579-86. [Crossref] [PubMed]
- Wainright JL, Robinson AM, Wilk AR, et al. Risk of ESRD in prior living kidney donors. Am J Transplant 2018;18:1129-39. [Crossref] [PubMed]
- Cozzi DA, Ceccanti S, Cozzi F. Renal function up to the 5th decade of life after nephrectomy in childhood: a literature review. Nephrology 2018;23:397-404. [Crossref] [PubMed]
- Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1988;1:335-47. [Crossref] [PubMed]
- Brenner BM, Lawler EV, Mackenzie HS. The hyperfiltration theory: a paradigm shift in nephrology. Kidney Int 1996;49:1774-7. [Crossref] [PubMed]
- Scosyrev E, Messing EM, Sylvester R, et al. Renal function after nephron-sparing surgery versus radical nephrectomy: results from EORTC randomized trial 30904. Eur Urol 2014;65:372-7. [Crossref] [PubMed]
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [Crossref] [PubMed]
- Sylvester RJ, Canfield SE, Lam TB, et al. Conflict of evidence: resolving discrepancies when findings from randomized controlled trials and meta-analyses disagree. Eur Urol 2017;71:811-9. [Crossref] [PubMed]
- Van Poppel H, Da Pozzo L, Albrecht W, et al. A prospective randomized EORTC intergroup phase 3 study comparing the complications of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 2007;51:1606-15. [Crossref] [PubMed]
- Scosyrev E, Messing EM, Sylvester R, et al. Exploratory subgroup analyses of renal function and overall survival in European Organization for Research and Treatment of Cancer randomized trial of nephron-sparing surgery versus radical nephrectomy. Eur Urol Focus 2017;3:599-605. [Crossref] [PubMed]
- Shuch B, Hanley JM, Lai JC, et al. Adverse health outcomes associated with surgical management of the small renal mass. J Urol 2014;191:301-8. [Crossref] [PubMed]
- Miller DC, Schonlau M, Litwin MS, et al. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer 2008;112:511-20. [Crossref] [PubMed]
- Mahmood U, Healy HG, Kark A, et al. Spectrum (characteristics) of patients with chronic kidney disease (CKD) with increasing age in a major metropolitan renal service. BMC Nephrol 2017;18:372. [Crossref] [PubMed]
- Li L, Astor BC, Lewis J, et al. Longitudinal progression trajectory of GFR among patients with CKD. Am J Kidney Dis 2012;59:504-12. [Crossref] [PubMed]
- Kidney Disease: Improving Global Outcomes CKD Workgroup. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150.
- Lane BR, Demirjian S, Derweesh IH, et al. Survival and functional stability in chronic kidney disease due to surgical removal of nephrons: importance of the new baseline glomerular filtration rate. Eur Urol 2015;68:996-1003. [Crossref] [PubMed]
- Holzmann MJ, Carlsson AC, Hammar N, et al. Chronic kidney disease and 10-year risk of cardiovascular death. Eur J Prev Cardiol 2016;23:1187-94. [Crossref] [PubMed]
- Wu J, Suk-Ouichai C, Dong W, et al. Analysis of survival for patients with chronic kidney disease primarily related to renal cancer surgery. BJU Int 2018;121:93-100. [Crossref] [PubMed]
- Mafham M, Emberson J, Landray MJ, et al. Estimated glomerular filtration rate and the risk of major vascular events and all-cause mortality: a meta-analysis. PLoS One 2011;6:e25920. [Crossref] [PubMed]
- Donfrancesco C, Palleschi S, Palmieri L, et al. Estimated glomerular filtration rate, all-cause mortality and cardiovascular diseases incidence in a low risk population: the MATISS study. PLoS One 2013;8:e78475. [Crossref] [PubMed]
- Demirjian S, Lane BR, Derweesh IH, et al. Chronic kidney disease due to surgical removal of nephrons: relative rates of progression and survival. J Urol 2014;192:1057-62. [Crossref] [PubMed]
- Johnson DW, Atai E, Chan M, et al. KHA-CARI guideline: Early chronic kidney disease: detection, prevention and management. Nephrology 2013;18:340-50. [Crossref] [PubMed]
- Tonelli M, Wiebe N, Culleton B, et al. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 2006;17:2034-47. [Crossref] [PubMed]
- Deeks JJ, Higgins JPT. Statistical Algorithms in Review Manager 5. Statistical Methods Group of the Cochrane Collaboration, 2010.
- Weight CJ, Larson BT, Fergany AF, et al. Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 2010;183:1317-23. [Crossref] [PubMed]
- Streja E, Kalantar-Zadeh K, Molnar MZ, et al. Radical versus partial nephrectomy, chronic kidney disease progression and mortality in US veterans. Nephrol Dial Transplant 2018;33:95-101. [PubMed]
- Lane BR, Demirjian S, Derweesh IH, et al. Is all chronic kidney disease created equal? Curr Opin Urol 2014;24:127-34. [Crossref] [PubMed]


