Assessing lymph node status in patients with kidney cancer
Introduction
Kidney cancer represents nearly 5% of all newly diagnosed cancers, resulting in 209,000 new cases and approximately 102,000 deaths annually worldwide (1). Renal cell carcinoma (RCC), arising from epithelial tissue, is the most common type of kidney cancer, responsible for 85% of all renal cancer and its incidence has been increasing (2,3). Due to its prevalence, our examination of lymph node involvement in renal neoplasms was limited to RCC. RCC is an umbrella term which includes multiple subtypes (i.e., clear cell, papillary types I and II, and chromophobe), which all differ in their aggression and ultimately treatment course (2,4). Currently, RCC is managed surgically, through partial nephrectomy or local ablative therapies, with the patients exhibiting lymph node infiltration additionally undergoing a regional lymphadenectomy (1,5). Furthermore, RCC frequently metastasizes, with 18% of patients presenting with metastases at diagnosis and 50% of patients developing metastases following surgical nephrectomy. Of the subtypes, clear cell RCC, the most common subtype, demonstrates the highest risk of metastases, with more than 90% of metastatic RCC being of the clear cell subtype (1,6).
RCC is currently staged using the Robson Classification and the TNM Classification from the International Union against Cancer (UICC). The TNM Classification is more specific than the Robeson Classification using pre-operative imaging to determine invasion and metastasis (7). The classification systems are compared in Table 1 (7,8). Determination of lymph node (LN) involvement, a poor prognostic factor, is critical to determining TNM classification, as it greatly influences long-term survival. The presence of LN involvement increases the incidence of distant metastasis by 50%. Moreover, patients without LN involvement (N0) have a 5-year estimated survival of greater than 50%, while patients with LN involvement (N1, N2) have an estimated 5-year survival of 5–38% (7,9-11).
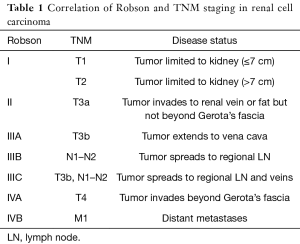
Full table
Pre-operative imaging has the greatest influence on determining treatment course, as tumor diameter and disease stage are the most important prognostic factors in RCC (12). Accurate determination of LN involvement is therefore critical to patient treatment course, pre-surgical counseling, and surgical planning (7,12-14).
With rapid advancements in multiple imaging modalities used to visualize RCC (9), the purpose of this study was to evaluate the advantages and disadvantages of each modality and determine the best modality for accurate detection of LN involvement in renal cancer rather than focusing on LN staging.
Evidence acquisition
A comprehensive computer-based literature search of English language studies of human subjects was performed to evaluate publications on the diagnostic performance of ultrasound (US), magnetic resonance imaging (MRI), lymphotropic nanoparticle-enhanced MRI (LNMRI), multidetector-row computed tomography (MDCT), F-fluoro-2-deoxyglucose positron emission tomography (FDG-PET) and PET/CT to evaluate LN status in kidney cancers. Google Scholar and MEDLINE/PubMed were used to identify relevant literature. Our search keywords consisted of: (“kidney cancer” or “renal cancer” or “renal cell carcinoma” or “renal carcinoma”) and (“lymph node” or “nodes” or “lymphatic drainage” or “lymph node metastasis” or “node metastasis” or “node infiltration” or “lymphatic involvement” or “nodal metastasis” or “lymph node staging” or “lymph node status” or “lymph node TNM” or “TNM staging” or “Robson classification”) and (“ultrasound” or “US” or “Color Doppler sonography” or “CDS” or “lymph node imaging” or “ CT scan” or “CAT” or “MRI” or “PET” or “PET/CT” or “positron-emission tomography” or “FDG” or “2-fluoro-2deoxy-D-glucose” or “FDG-PET” or “MR imaging” or “magnetic resonance imaging” or “LNMRI” or “lymphotropic MNP” or “lymphotropic nanoparticle-enhanced MRI”). This extensive review article includes articles published from 1985 to May 2018.
Only studies using US, MDCT, MRI, LNMRI, FDG-PET and PET/CT to asses LN involvement in patients with biopsy-proven kidney cancer were included in the study. The exclusion criteria were non-English language studies and studies of non-human subjects. Furthermore, all case reports, meta-analysis, review articles, and abstracts were excluded from this study.
Author names, year of publication, number of patients, number of involved LNs, technical details of imaging studies, criteria for positive LN, interpreter(s) and evidence for reference standard were noted. Sensitivity (SN) and specificity (SP) for each article were noted. If these values were not reported, they were calculated by using false positive, false negative, true positive, and true negative values.
Evidence synthesis
After an extensive computer search, 150 relevant articles were selected. After reviewing the titles and abstracts of the studies, 59 articles were potentially eligible to be included in this study. All 59 articles were reviewed in full and 10 articles were selected according to our eligibility criteria for final analysis.
Color Doppler sonography (CDS)
Doppler sonography is widely available and frequently used for the detection of urologic disease (15). CDS is an inexpensive and non-invasive method to evaluate LN infiltration. However, the use of CDS is limited by poor imaging quality in patients with obesity or in instances when the study site is covered by bowel gas (9,16). Moreover, the quality of CDS is dependent on the physician’s skill level. Our literature review only identified a single study examining LN involvement in RCC using CDS. This prospective study of 60 patients with RCC, detected 5 positive LN, later confirmed by histopathology report, providing a SN of 100% (Table 2) (16).

Full table
CT scan
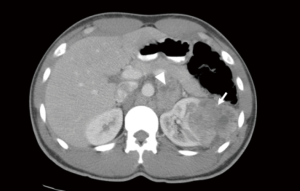
CT scan is the most commonly used method for nodal staging due to its availability, low expense, and high accuracy (72–90%) (7,17). Advancements in CT technology and the introduction of MDCT, with thin slice collimation and fast acquisition, provides an accurate representation of LN size and shape and the detailed anatomy surrounding organs (Figure 1) (12,18). On CT, a short axis diameter greater than 1 cm, shape and enhancement are the determining factors used to identify a positive LN (8,12,19-21). However, these criteria are one of the disadvantages of CT scans because micro-metastases in LN less than 1 cm may be overlooked. Moreover, in one study, 58% of patients with enlarged LN (>1 cm) had reactive hyperplasia, not positive LN (19). The reported SN and SP of conventional CT scan, ranged from 60–100% (median 83%) and 75–98.1% (median 88.5%) respectively (Table 3) (16,19,20,22,23). However, the SN and SP of MDCT ranged from 75–77% (median 76%) and 75–82% (median 78.5%), respectively (Table 3) (12,21).

Full table
MRI
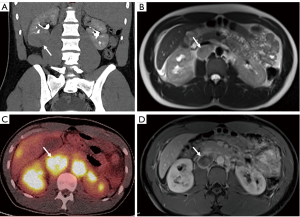
MRI provides an excellent anatomical view due to high soft-tissue contrast resolution. However, in conventional MRI the only criteria used to identify positive LN’s is the size (diameter greater than 1 cm) (Figure 2). Therefore, MRI cannot differentiate reactive enlarged LN from metastatic nodes or micro-metastases in nodes less than 1 cm (7,9,17). In recent years, use of lymphotropic nanoparticle-enhanced MRI (LNMRI), which uses lymphotropic magnetic nanoparticles (MNP) with the monocrystalline superparamagnetic iron core, has aided physicians in the detection of metastatic nodes through utilization of additional characteristics, such as heterogeneity, hyper-intensity, and single-intensity of LN. One LNMRI study reported that SN and SP for positive node detection were 100% and 95.7%, respectively (24). Comparatively, a conventional MRI study reported the SN and SP of 100% and 92%, respectively (Table 4) (23).

Full table
PET scan
FDG-PET is an established and commonly used imaging modality for cancer assessment. FDG-PET provides functional information rather than detailed anatomic features based on increased FDG tracer uptake within lesions, easily visualized due to the increased metabolic activity of most malignant lesions (25). Therefore, it can be a useful tool in evaluating metastatic lesions. However, inconsistencies in tumor uptake and lack of detailed anatomical views requires physicians to utilize conventional imaging modalities to accompany PET scans (Figure 2) (17,22,25,26). FDG-PET has a high SP in detection of distant metastases, but low SN (high false negativity) (25). In our search of the literature, we found three articles that reported the SN and SP of detecting metastatic LN using FDG-PET. In these articles, the SN ranged from 75 to 87% (median 77%) and a SP of 100% in patients with RCC (Table 5) (22,25,27).

Full table
Metastatic nodes
Presence of metastatic LN in patients with RCC is a poor prognostic factor and results in a lower 5-year survival (11). In addition, detection of positive LN is critical to the determination of treatment course and overall tumor stage. Clarification of the best imaging modality for the detection of metastatic nodes on pre-operative imaging is important to avoid missing patients with metastases or increasing the burden of unnecessary work-ups in patients lacking metastases. Detection of metastatic nodes is usually based on dimensional thresholds, with LNs less than 8–10 mm in diameter considered negative (28). In two studies in patients with bladder and prostate cancer, 25% of patients who had normal size LN were diagnosed with positive LNs during the surgery (29,30).
In our review article, we have noticed that there is just one study on CDS for assessing LN in patients with RCC. The SN (100%) was high; however, the SP was not reported. CT, routinely used for tumor staging, had lower SN (median 78.2%) and SP (median 83.5%) than two different studies on MRI, which had a reported SN of 100% and median SP of 93.9%. Additionally, between MRIs the SP of LNMRI was higher than conventional MRI with gadolinium. It appears that FDG-PET had the highest SP (100%) for detection of metastatic nodes; however, it presented with a sub-optimal SN (median 77%) optimal (12,16,19-25,27).
Future directions
Overall, our review indicated FDG-PET had the highest SP compared to other modalities. Thus, PET can be used to confirm metastatic LNs. However, due to the low SN (high false negativity) of this modality, physicians should be skeptical of FDG-PET reports that are LN negative in patients with RCC (22,25,27). On the other hand, MRI has a very high SN, allowing physicians to rely on negative results and use MRI to rule out LN involvement (23,24). Overall, we recommend these two methods can be combined as a hybrid PET/MRI modality for detecting metastatic LNs. The other advantage of this hybrid modality is providing both the detailed anatomy of MRI and functional information of PET.
As we mentioned before, the criteria for metastatic LN detection is a cut-off-point of 1cm in short axis diameter in CT-scan studies which is non-specific and can be seen in non-metastatic enlarged inflammatory LNs (12,16,19-22). Thus, establishing new RCC subtype-specific size cut-offs might be helpful to differentiate benign from metastatic LNs with higher accuracy.
Additionally, MRI had a high SN but lower SP for malignant LN detection (23,24). In a DWI-MRI study on patients with prostate cancer, the apparent diffusion coefficient (ADC) value was significantly lower in malignant LNs compared to benign LNs (P<0.0001). As a result, the ADC value was reported as a marker to discriminate benign from malignant LN in the patient with prostate cancer (31). To the best of our knowledge, there is no publication using DWI-MRI for LN differentiation on RCC patients. Thus, future studies can focus on DWI-MRI to increase the capability of MRI for positive LN detection in the patient with RCC.
Conclusions
Pre-operative LN status in patients with RCC is traditionally evaluated using CT and MRI studies (28). However, our comprehensive review indicates that while MRI has the highest SN and is useful for evaluating the absence or presence of LN involvement, FDG-PET has the highest SP and is useful for confirming involvement and extent of disease. Utilization of a combination of modalities, the establishment of new cut-off points for LN involvement in CT and MRI, and use of new techniques, such as the incorporation of diffusion-weighted imaging (DWI), should improve our capability to accurately detect LN involvement in RCC patients. In conclusion, there is a capability to differentiate malignant from benign LNs in RCC patients with higher accuracy through the use of new techniques, a combination of modalities, or new cut off point interoperations.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009;373:1119-32. [Crossref] [PubMed]
- Sánchez-Gastaldo A, Kempf E, González Del Alba A, et al. Systemic treatment of renal cell cancer: A comprehensive review. Cancer Treat Rev 2017;60:77-89. [Crossref] [PubMed]
- Ljungberg B, Campbell SC, Cho HY, et al. The epidemiology of renal cell carcinoma. Eur Urol 2011;60:615-21. Erratum in: Eur Urol 2011;60:1317. Cho, Han Yong [corrected to Choi, Han Yong]. [Crossref] [PubMed]
- Ng CS, Wood CG, Silverman PM, et al. Renal cell carcinoma: diagnosis, staging, and surveillance. AJR Am J Roentgenol 2008;191:1220-32. [Crossref] [PubMed]
- Capitanio U, Becker F, Blute ML, et al. Lymph Node Dissection in Renal Cell Carcinoma. Eur Urol 2011;60:1212-20. [Crossref] [PubMed]
- Brufau BP, Cerqueda CS, Villalba LB, et al. Metastatic Renal Cell Carcinoma: Radiologic Findings and Assessment of Response to Targeted Antiangiogenic Therapy by Using Multidetector CT. Radiographics 2013;33:1691-716. [Crossref] [PubMed]
- Reznek RH. CT/MRI in staging renal cell carcinoma. Cancer Imaging 2004;4 Spec No A:S25-32.
- Dunnick NR. Renal cell carcinoma: staging and surveillance. Abdom Radiol (NY) 2016;41:1079-85. [Crossref] [PubMed]
- Kwee RM, Kwee TC. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009;12:6-22. [Crossref] [PubMed]
- Golimbu M, Al-Askari S, Tessler A, et al. Aggressive Treatment of Metastatic Renal Cancer. J Urol 1986;136:805-7. [Crossref] [PubMed]
- Thrasher JB, Paulson DF. Prognostic factors in renal cancer. Urol Clin North Am 1993;20:247-62. [PubMed]
- Nazim SM, Ather MH, Hafeez K, et al. Accuracy of multidetector CT scans in staging of renal carcinoma. Int J Surg 2011;9:86-90. [Crossref] [PubMed]
- Mahesan T, Coscione A, Ayres B, et al. Sentinel lymph node biopsy in renal malignancy: The past, present and future. World J Nephrol 2016;5:182-8. [Crossref] [PubMed]
- Hutterer GC, Patard JJ, Perrotte P, et al. Patients with renal cell carcinoma nodal metastases can be accurately identified: External validation of a new nomogram. Int J Cancer 2007;121:2556-61. [Crossref] [PubMed]
- Bos SD, Mensink HJ. Can duplex Doppler ultrasound replace computerized tomography in staging patients with renal cell carcinoma? Scand J Urol Nephrol 1998;32:87-91. [Crossref] [PubMed]
- Spahn M, Portillo FJM, Michel MS, et al. Color Duplex Sonography vs. Computed Tomography: Accuracy in the Preoperative Evaluation of Renal Cell Carcinoma. Eur Urol 2001;40:337-42. [Crossref] [PubMed]
- Malayeri AA, Pattanayak P, Apolo AB. Imaging muscle-invasive and metastatic urothelial carcinoma. Curr Opin Urol 2015;25:441-8. [Crossref] [PubMed]
- Catalano C, Fraioli F, Laghi A, et al. High-Resolution Multidetector CT in the Preoperative Evaluation of Patients with Renal Cell Carcinoma. AJR Am J Roentgenol 2003;180:1271-7. [Crossref] [PubMed]
- Studer UE, Scherz S, Scheidegger J, et al. Enlargement of Regional Lymph Nodes in Renal Cell Carcinoma is Often not Due to Metastases. J Urol 1990;144:243-5. [Crossref] [PubMed]
- Johnson CD, Dunnick NR, Cohan RH, et al. Renal adenocarcinoma: CT staging of 100 tumors. AJR Am J Roentgenol 1987;148:59-63. [Crossref] [PubMed]
- Türkvatan A, Akdur PO, Altinel M, et al. Preoperative staging of renal cell carcinoma with multidetector CT. Diagn Interv Radiol 2009;15:22-30. [PubMed]
- Kang DE, White RL, Zuger JH, et al. Clinical use of fluorodeoxyglucose F 18 positron emission tomography for detection of renal cell carcinoma. J Urol 2004;171:1806-9. [Crossref] [PubMed]
- Constantinides C, Recker F, Bruehlmann W, et al. Accuracy of magnetic resonance imaging compared to computerized tomography and renal selective angiography in preoperatively staging renal cell carcinoma. Urol Int 1991;47:181-5. [Crossref] [PubMed]
- Guimaraes AR, Tabatabei S, Dahl D, et al. Pilot study evaluating use of lymphotrophic nanoparticle-enhanced magnetic resonance imaging for assessing lymph nodes in renal cell cancer. Urology 2008;71:708-12. [Crossref] [PubMed]
- Majhail NS, Urbain JL, Albani JM, et al. F-18 fluorodeoxyglucose positron emission tomography in the evaluation of distant metastases from renal cell carcinoma. J Clin Oncol 2003;21:3995-4000. [Crossref] [PubMed]
- Boellaard R, O'Doherty MJ, Weber WA, et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: version 1.0. Eur J Nucl Med Mol Imaging 2010;37:181-200. [Crossref] [PubMed]
- Safaei A, Figlin R, Hoh CK, et al. The usefulness of F-18 deoxyglucose whole-body positron emission tomography (PET) for re-staging of renal cell cancer. Clin Nephrol 2002;57:56-62. [Crossref] [PubMed]
- McMahon CJ, Rofsky NM, Pedrosa I. Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 2010;254:31-46. [Crossref] [PubMed]
- Fleischmann A, Thalmann GN, Markwalder R, et al. Extracapsular extension of pelvic lymph node metastases from urothelial carcinoma of the bladder is an independent prognostic factor. J Clin Oncol 2005;23:2358-65. [Crossref] [PubMed]
- Schumacher MC, Burkhard FC, Thalmann GN, et al. Good outcome for patients with few lymph node metastases after radical retropubic prostatectomy. Eur Urol 2008;54:344-52. [Crossref] [PubMed]
- Giannarini G, Petralia G, Thoeny HC. Potential and limitations of diffusion-weighted magnetic resonance imaging in kidney, prostate, and bladder cancer including pelvic lymph node staging: a critical analysis of the literature. Eur Urol 2012;61:326-40. [Crossref] [PubMed]


