Urinary markers aiding in the detection and risk stratification of prostate cancer
Introduction
Prostate cancer (PCa) is the most common cancer in men after skin cancers and is the second leading cause of cancer related deaths in males in the United States, causing an estimated 26,730 deaths in 2017 alone (1). Definitive diagnosis of PCa is made based upon the results of a prostate biopsy. For almost 30 years, the decision to biopsy a patient has relied upon elevated prostate-specific antigen (PSA) levels (generally greater than 4 ng/mL, though age-specific PSA thresholds are commonly utilized) or abnormal findings on a digital rectal exam (DRE). It has become clear that neither PSA nor DRE alone, or even together, are accurate enough to reliably detect clinically significant prostate cancer (csPCa), defined as any PCa with Gleason Score (GS) ≥7. Thus, there has been a movement to develop new biomarkers for more effective detection of PCa. Several are now commercially available, others await FDA approval, and a multitude more are still being investigated. Particularly, great efforts have been expended to identify urinary biomarkers, often to be used as an adjunct to PSA screening. Prostatic secretions merge with the urine where the ejaculatory duct joins the urethra. In patients with PCa, tumor cells are similarly excreted through the ejaculatory ducts, and thus can be collected in the urine for analysis. Unlike other sources of biomarkers, such as tissue or blood, urine collection is inexpensive and easy to obtain in large volumes without invasive procedures. Additionally, isolation of biomarkers is easier in urine, whose composition is less complex than that of blood (2).
Current screening standards: PSA and the DRE
PSA [also called gamma-seminoprotein or kallikrein-3 (KLK3)] is an enzyme secreted exclusively by prostatic epithelial cells and aids in liquefaction of the seminal coagulum (3). PSA is present in small amounts in the blood of healthy men, but can be elevated in individuals with PCa. Evaluation of serum PSA levels has been the standard of practice for PCa screening since 1989. However, it is has become clear that PSA alone is not an adequate metric for assessing cancer risk. Elevations in PSA are not specific for the presence of PCa and can be elevated in a variety of other conditions, including benign prostatic hypertrophy (BPH), prostatitis, or urinary tract infections (UTIs). Additionally, PSA carries a low positive predictive value (PPV) for PCa of approximately 30%, meaning less than one in three men with elevated PSA will have positive pathology on subsequent biopsy (4-7). Additionally, men with low PSA levels can develop PCa. The Prostate Cancer Prevention Trial (PCPT) identified high-grade PCa in 12.5% of men with PSA <0.5 ng/mL (8). Even in PCa-positive men, PSA values can fluctuate throughout the clinical course, making it an ineffective tool for tumor staging and grading and subsequent risk-stratification (9). Although attempts have been made to overcome these inadequacies by developing other PSA-based tests, metrics such as percent free PSA, PSA velocity, PSA density, and age-specific PSA ranges similarly suffer from lack of general specificity as conventional PSA measurement (10).
The other common indication for prostate biopsy, an abnormal DRE, has also been shown to be ineffective for detection of PCa. During a DRE, the physician examines for changes in prostate size, shape, and texture. Suspicion for cancer is increased when the examination reveals a hardened prostate gland or nodularity. In the Prostate, Lung, Colorectal, and Ovarian (PLCO) Screening Trial, only 99 of 5,064 men (2%) who had an abnormal DRE in the setting of normal PSA were diagnosed with clinically significant PCa (11). Furthermore, though difficult to quantify, patient anxiety surrounding DRE can be very high, and has been reported as a significant barrier to PCa screening in the past (12,13).
A preliminary report from the United States Preventative Services Task Force (USPSTF) in April 2017 gave routine PSA screening in men ages 55–69 a grade C recommendation, an advisement to only screen on a case-by-case basis. In men age ≥70, PSA screening was given a grade D recommendation advising against routine screening. The American Urological Association’s (AUA) recommendations differ slightly, and do recommend routine PSA screening in men ages 55–69 after informed discussion and shared-decision making. The downstream effects of these screening recommendations are as yet unknown, but indicate a need for better biomarkers for PCa detection. Additionally, current screening methods often lead to the diagnosis of early, localized, clinically insignificant cancers which may lead to intense anxiety on the part of the patient or overtreatment, with current treatment options carrying the risk of significant morbidity such as incontinence, erectile dysfunction, and hematuria (13-17).
Advances in genomics and proteomics have allowed us to understand the molecular biology of cancer to a level previously unattainable. Herein we discuss novel urinary biomarkers (Table 1) which promise to change future screening standards and improve the detection of clinically csPCa.
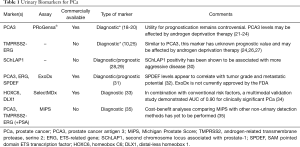
Full table
Methods
PubMed and MEDLINE database searches were conducted. Terms searched included “prostate cancer”, “biomarkers”, “urinary markers”, “diagnosis”, “prognostication”, “risk-stratification”, “urine assay”, “gene panel”, and “screening”. All relevant studies were considered, regardless of year of publication. Biomarkers were selected for inclusion based on the amount of promise they show for use in clinical practice, based generally on commercial availability of the assay, the wealth of literature available regarding the marker, and the apparent effectiveness of the marker in regards to specificity, sensitivity, and area under the curve for diagnosis or prognostication of PCa.
Urinary biomarkers for PCa
Prostate cancer antigen 3 (PCA3)
One of the most widely studied urinary PCa biomarkers is PCA3, also called “differential display code 3” (DD3). PCA3 is a long non-coding RNA (lncRNA) that is specific to the prostate and was first reported in 1999 by Bussemakers et al. (36). PCA3 is overexpressed in PCa relative to normal prostate tissue, and is present in up to 95% of PCa (36-39). Quantitative real-time polymerase chain reaction (qRT-PCR) for PCA3 detection was approved by the FDA in 2012 for clinical use in the USA, marketed as the PRoGensa® and later the uPM3TM assay. It is indicated for men age ≥50 with prior negative biopsies for whom standard of care includes repeat biopsy.
The “PCA3 score”, calculated as an adjusted ratio of PCA3 to PSA mRNA (PCA3/PSA ×1,000), was found to be associated with the probability of diagnosing PCa in subsequent prostate Bx (40,41). There remains controversy as to the ideal cutoff score to use in order to rule out the need for repeat biopsy. PCA3 score values of 20, 35, and 50 have been examined in numerous studies, with sensitivities and specificities ranging from 52–82% and 79–89% depending on the selected cutoff point (10,25,42-47). A 2011 meta-analysis of 11 clinical studies suggested an optimal cutoff PCA3 score of 20, which imparted a sensitivity of 72% and a specificity of 53% for men undergoing repeat biopsy (40).
More recently, efforts have been made to integrate PCA3 score as a component of diagnostic nomograms. One study by Hansen et al. in 2013 found that a nomogram incorporating PCA3 was far more accurate than one which did not include PCA3 score; concluding that 55% of men would be able to avoid a biopsy with ≤2% missed cases of csPCa (18). Additional studies have reported similar results, suggesting an important role for PCA3 in detection of PCa (19,20). Although PCA3 appears to be a reliable tool for the detection of PCa, studies disagree as to its prognostic value. Leyten et al. found that PCA3 score did not correlate with GS on biopsy or clinical tumor stage, while another group reported increasing tumor aggressiveness with higher PCA3 scores (21,22). Additionally, PCA3 levels in the urine may be significantly affected by androgen deprivation therapy, thus limiting the ability to monitor the clinical course of disease (23,24).
TMPRSS2-ERG
TMPRSS2-ERG is a chimeric protein composed of the androgen-related transmembrane protease serine 2 (TMPRSS2) and ETS-related gene (ERG). At the time of its discovery in 2005, TMPRSS2-ERG was the first known fusion gene implicated in PCa. This mutated protein results in aberrant expression of ERG, which contributes to oncogenesis by forcing progression through the cell cycle (48). Though TMPRSS2-ERG is the most common gene recombination seen in PCa, it’s prevalence appears to vary widely between ethnic groups—as high as 50% in western countries and as low as 11% in China (49-51).
Some studies have suggested that the expression of TMPRSS2-ERG in PCa carries a worse clinical prognosis. Hägglöf et al. showed that TMPRSS2-ERG was associated with increased cancer-specific mortality compared to patients whose tumors lacked ERG staining (52). However, other studies have failed to show clinical significance (53). Assays screening for TMPRSS2-ERG have been shown in multiple studies to improve detection of PCa. In one study, the combination of TMPRSS2-ERG with PSA allowed for a 50% reduction in repeat biopsies, with no reduction in either 10-year overall survival or 15-year PCa-specific survival (10). In particular, TMPRSS2-ERG is often combined with PCA3 due to the fact that PCA3 has high sensitivity for PCa and TMPRSS2-ERG has high specificity (25). The prognostic value of TMPRSS2-ERG is still under investigation, and results from multiple studies have been inconsistent. TMPRSS2-ERG was shown to improve predictive power in the European Randomised Study of Screening for Prostate Cancer risk calculator (ERSPCrc) in terms of predicting GS and stage (10). Another large multi-institutional trial by Tomlins et al. demonstrated that the addition of TMPRSS2-ERG and PCA3 to the PCPT nomogram significantly improved PCa risk prediction (AUC increased by 9–16%) (35). In contrast, studies by Cornu et al. and Leyten et al. showed that while TMPRSS2-ERG + PCA3 greatly increased PCa detection sensitivity, TMPRSS2-ERG was not correlated with GS in pathology specimens (26,27). Data also indicate that TMPRSS2-ERG may suffer from the same limitations as PCA3 when combined with androgen deprivation therapy (24).
SChLAP1
SChLAP1 (second chromosome locus associated with prostate-1) (also called LINC00913) was first characterized by Prensner et al. in 2013, and was shown to promote development of PCa by inhibiting SWI/SNF, a tumor-suppressing protein complex (30). SChLAP1 is expressed in a subset of PCa and is associated with more aggressive disease (30). Mehra et al. demonstrated that high levels of SChLAP1 expression were correlated with increasing GS and tumor stage (P<0.005) and increased time to development of lethal PCa (28). Additionally, quantifying SChLAP1 expression levels has been shown to be useful for predicting PCa metastasis within 10 years (OR 2.45; 95% CI, 1.70–3.53, P<0.0001) (29). Assays utilizing SChLAP1 for PCa screening are not yet commercially available.
ExoDx prostate Intelliscore urine exosome assay
ExoDx is a urine exosome gene expression assay which measures a combination of ERG, PCA3, and SPDEF (SAM Pointed Domain ETS Transcription Factor). Higher level of SPDEF is thought to correlate with increased PCa aggressiveness and metastatic potential (32). One study by McKiernan et al. demonstrated that the ExoDx panel used in combination with PSA, age, race, and family history was better able to detect GS ≥7 PCa than these other variables alone (AUC 0.71), suggesting that 27% of biopsies could be avoided. In this study, the assay demonstrated a negative predictive value (NPV) of 91% and a sensitivity of 92%, missing only 12 of 148 GS ≥7 cancers (8%) (31). This gene panel became clinically available in September 2016; however, this test is only available from the manufacturer through a CLIA-approved (Clinical Laboratory Improvement Amendments) lab. It has not been approved by the FDA, and thus may not be covered by health insurance plans.
SelectMDx
SelectMDx is another commercially available combination assay utilizing HOXC6 and DLX1. HOXC6 (homeobox C6) on chromosome 12 and DLX1 (Distal-less homeobox 1) on chromosome 2 are two homeobox genes that have been found to be upregulated in PCa (54). HOXC6 plays a role in epithelial proliferation41, but little is known about the oncogenic function of DLX1 (33). HOXC6 + DLX1 has been shown to be highly effective at predicting the presence of csPCa, with an overall AUC of 0.76 (33). When combined with other predictors including PSA density, DRE results, previous Bx, PSA, family history, and age, it reached an overall AUC of 0.90 (95% CI, 0.85–0.95) (34).
Though not currently included in the SelectMDx panel, there have been studies of HOXC6 + DLX1 combined with the protein TDRD1 (Tudor domain containing 1). TDRD1 was the first identified target of ERG, and upregulation of TDRD1 is highly correlated with ERG upregulation. One study found that this combination was more accurate in detecting clinically significant PCa than either PCA3 or PSA alone (AUC 0.77 vs. 0.62 PCA3 vs. 0.72 PSA) (24). Furthermore, the panel became even more effective when combined with PSA, with an AUC of 0.82 (24). NCCN 2017 practice guidelines state that SelectMDx is officially still under investigation, and information will be reviewed as it becomes available.
Michigan Prostate Score (MiPS)
The MiPS is a combination of PSA + PCA3 + TMPRSS2-ERG developed in 2016 by Tomlins et al. MiPS uses a transcription-mediated amplification assay that generates scores for TMPRSS2-ERG and PCA3 by normalizing urinary mRNA with urinary PSA mRNA. A final multivariable regression generates the combined MiPS score. Tomlins et al. demonstrated significantly higher prediction rates for the presence of both PCa and csPCa (GS ≥7) when compared to PSA alone, PCA3 + PSA, and TMPRSS2-ERG + PSA (AUC 0.751, 0.585, 0.726, and 0.693 respectively) (35). Utilization of MiPS with the PCPT nomogram would have avoided 35–47% of biopsies, while only delaying the diagnosis of 1–2.3% of clinically significant PCa (35). MiPS is currently not commercially available, and the authors note that a cost-benefit analysis between MiPS and other non-urinary detection methods has not yet been performed (35).
Conclusions
In light of a mounting body of evidence highlighting the inadequacies of PSA and the DRE as screening tools for PCa, the development and utilization of novel biomarkers for better cancer detection is critical. Urine specimens in particular represent a low-cost, non-invasive, convenient source from which to measure the presence of biomarkers. Although utilization of the assays discussed in this review has not yet become standard of practice, they offer promising alternatives for both effective diagnosis and prognostication of PCa.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Wu D, Ni J, Beretov J, et al. Urinary biomarkers in prostate cancer detection and monitoring progression. Crit Rev Oncol Hematol 2017;118:15-26. [Crossref] [PubMed]
- Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol 2003;21:383-91. [Crossref] [PubMed]
- Ferro MA, Barnes I, Roberts JB, et al. Tumour markers in prostatic carcinoma. A comparison of prostate-specific antigen with acid phosphatase. Br J Urol 1987;60:69-73. [Crossref] [PubMed]
- Gann PH, Hennekens CH, Stampfer MJ. A prospective evaluation of plasma prostate-specific antigen for detection of prostatic cancer. JAMA 1995;273:289-94. [Crossref] [PubMed]
- Stamey TA, Yang N, Hay AR, et al. Prostate-specific antigen as a serum marker for adenocarcinoma of the prostate. N Engl J Med 1987;317:909-16. [Crossref] [PubMed]
- Vickers AJ, Cronin AM, Aus G, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Göteborg, Sweden. BMC Med 2008;6:19. [Crossref] [PubMed]
- Thompson IM, Pauler DK, Goodman PJ, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or =4.0 ng per milliliter. N Engl J Med 2004;350:2239-46. Erratum in: N Engl J Med 2004;351:1470. [Crossref] [PubMed]
- McGrath S, Christidis D, Perera M, et al. Prostate cancer biomarkers: Are we hitting the mark? Prostate Int 2016;4:130-5. [Crossref] [PubMed]
- Wei JT, Feng Z, Partin AW, et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J Clin Oncol 2014;32:4066-72. [Crossref] [PubMed]
- Nyitray AG, Chiao EY. Re: Cui T, Kovell RC, Terlecki RP. Is it time to abandon the digital rectal examination? Lessons from the PLCO Cancer Screening Trial and peer-reviewed literature. Curr Med Res Opin 2016;32:1-7. Curr Med Res Opin 2017;33:315-6. [Crossref] [PubMed]
- Consedine NS, Morgenstern AH, Kudadjie-Gyamfi E, et al. Prostate cancer screening behavior in men from seven ethnic groups: the fear factor. Cancer Epidemiol Biomarkers Prev 2006;15:228-37. [Crossref] [PubMed]
- Consedine NS, Horton D, Ungar T, et al. Fear, knowledge, and efficacy beliefs differentially predict the frequency of digital rectal examination versus prostate specific antigen screening in ethnically diverse samples of older men. Am J Mens Health 2007;1:29-43. [Crossref] [PubMed]
- Rosser CJ, Parker A. Re: The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol 2005;174:1154-5; author reply 1155-6. [Crossref] [PubMed]
- Wagenlehner FM, van Oostrum E, Tenke P, et al. Infective complications after prostate biopsy: outcome of the Global Prevalence Study of Infections in Urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol 2013;63:521-7. [Crossref] [PubMed]
- Carignan A, Roussy JF, Lapointe V, et al. Increasing risk of infectious complications after transrectal ultrasound-guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol 2012;62:453-9. [Crossref] [PubMed]
- Wallis CJD, Glaser A, Hu JC, et al. Survival and Complications Following Surgery and Radiation for Localized Prostate Cancer: An International Collaborative Review. Eur Urol 2018;73:11-20. [Crossref] [PubMed]
- Hansen J, Auprich M, Ahyai SA, et al. Initial prostate biopsy: development and internal validation of a biopsy-specific nomogram based on the prostate cancer antigen 3 assay. Eur Urol 2013;63:201-9. [Crossref] [PubMed]
- Ruffion A, Devonec M, Champetier D, et al. PCA3 and PCA3-based nomograms improve diagnostic accuracy in patients undergoing first prostate biopsy. Int J Mol Sci 2013;14:17767-80. [Crossref] [PubMed]
- Vedder MM, de Bekker-Grob EW, Lilja HG, et al. The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men. Eur Urol 2014;66:1109-15. [Crossref] [PubMed]
- Leyten GH, Hessels D, Smit FP, et al. Identification of a Candidate Gene Panel for the Early Diagnosis of Prostate Cancer. Clin Cancer Res 2015;21:3061-70. [Crossref] [PubMed]
- Merola R, Tomao L, Antenucci A, et al. PCA3 in prostate cancer and tumor aggressiveness detection on 407 high-risk patients: a National Cancer Institute experience. J Exp Clin Cancer Res 2015;34:15. [Crossref] [PubMed]
- Martínez-Piñeiro L, Schalken JA, Cabri P, et al. Evaluation of urinary prostate cancer antigen-3 (PCA3) and TMPRSS2-ERG score changes when starting androgen-deprivation therapy with triptorelin 6-month formulation in patients with locally advanced and metastatic prostate cancer. BJU Int 2014;114:608-16. [Crossref] [PubMed]
- van Gils MP, Hessels D, Peelen WP, et al. Preliminary evaluation of the effect of dutasteride on PCA3 in post-DRE urine sediments: a randomized, open-label, parallel-group pilot study. Prostate 2009;69:1624-34. [Crossref] [PubMed]
- Fradet Y, Saad F, Aprikian A, et al. uPM3, a new molecular urine test for the detection of prostate cancer. Urology 2004;64:311-5; discussion 315-6. [Crossref] [PubMed]
- Cornu JN, Cancel-Tassin G, Egrot C, et al. Urine TMPRSS2:ERG fusion transcript integrated with PCA3 score, genotyping, and biological features are correlated to the results of prostatic biopsies in men at risk of prostate cancer. Prostate 2013;73:242-9. [Crossref] [PubMed]
- Leyten GH, Hessels D, Jannink SA, et al. Prospective multicentre evaluation of PCA3 and TMPRSS2-ERG gene fusions as diagnostic and prognostic urinary biomarkers for prostate cancer. Eur Urol 2014;65:534-42. [Crossref] [PubMed]
- Mehra R, Udager AM, Ahearn TU, et al. Overexpression of the Long Non-coding RNA SChLAP1 Independently Predicts Lethal Prostate Cancer. Eur Urol 2016;70:549-52. [Crossref] [PubMed]
- Lin D. Commentary on "RNA biomarkers associated with metastatic progression in prostate cancer: A multi-institutional high-throughput analysis of SChLAP1." Prensner JR, Zhao S, Erho N, Schipper M, Iyer MK, Dhanasekaran SM, Magi-Galluzzi C, Mehra R, Sahu A, Siddiqui J, Davicioni E, Den RB, Dicker AP, Karnes RJ, Wei JT, Klein EA, Jenkins RB, Chinnaiyan AM, Feng FY, University of Washington-Urology, Seattle, WA. Lancet Oncol 2014; 15(13):1469-80. Urol Oncol 2016;34:521-2. [PubMed]
- Prensner JR, Iyer MK, Sahu A, et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat Genet 2013;45:1392-8. [Crossref] [PubMed]
- McKiernan J, Donovan MJ, O'Neill V, et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol 2016;2:882-9. [Crossref] [PubMed]
- Osisami M, Keller ET. SPDEF: a molecular switch for E-cadherin expression that promotes prostate cancer metastasis. Asian J Androl 2013;15:584-5. [Crossref] [PubMed]
- Chan DW, Hui WW, Wang JJ, et al. DLX1 acts as a crucial target of FOXM1 to promote ovarian cancer aggressiveness by enhancing TGF-β/SMAD4 signaling. Oncogene 2017;36:1404-16. [Crossref] [PubMed]
- Van Neste L, Hendriks RJ, Dijkstra S, et al. Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur Urol 2016;70:740-8. [Crossref] [PubMed]
- Tomlins SA, Day JR, Lonigro RJ, et al. Urine TMPRSS2:ERG Plus PCA3 for Individualized Prostate Cancer Risk Assessment. Eur Urol 2016;70:45-53. [Crossref] [PubMed]
- Bussemakers MJ, van Bokhoven A, Verhaegh GW, et al. DD3: a new prostate-specific gene, highly overexpressed in prostate cancer. Cancer Res 1999;59:5975-9. [PubMed]
- de Kok JB, Verhaegh GW, Roelofs RW, et al. DD3(PCA3), a very sensitive and specific marker to detect prostate tumors. Cancer Res 2002;62:2695-8. [PubMed]
- Hessels D, Klein Gunnewiek JM, van Oort I, et al. DD3(PCA3)-based molecular urine analysis for the diagnosis of prostate cancer. Eur Urol 2003;44:8-15; discussion 15-6. [Crossref] [PubMed]
- Yang Z, Yu L, Wang Z. PCA3 and TMPRSS2-ERG gene fusions as diagnostic biomarkers for prostate cancer. Chin J Cancer Res 2016;28:65-71. [PubMed]
- Luo Y, Gou X, Huang P, et al. The PCA3 test for guiding repeat biopsy of prostate cancer and its cut-off score: a systematic review and meta-analysis. Asian J Androl 2014;16:487-92. [Crossref] [PubMed]
- Crawford ED, Rove KO, Trabulsi EJ, et al. Diagnostic performance of PCA3 to detect prostate cancer in men with increased prostate specific antigen: a prospective study of 1,962 cases. J Urol 2012;188:1726-31. [Crossref] [PubMed]
- Groskopf J, Aubin SM, Deras IL, et al. APTIMA PCA3 molecular urine test: development of a method to aid in the diagnosis of prostate cancer. Clin Chem 2006;52:1089-95. [Crossref] [PubMed]
- Tinzl M, Marberger M, Horvath S, et al. DD3PCA3 RNA analysis in urine--a new perspective for detecting prostate cancer. Eur Urol 2004;46:182-6; discussion 187. [Crossref] [PubMed]
- Marks LS, Fradet Y, Deras IL, et al. PCA3 molecular urine assay for prostate cancer in men undergoing repeat biopsy. Urology 2007;69:532-5. [Crossref] [PubMed]
- Tombal B, Ameye F, de la Taille A, et al. Biopsy and treatment decisions in the initial management of prostate cancer and the role of PCA3; a systematic analysis of expert opinion. World J Urol 2012;30:251-6. [Crossref] [PubMed]
- Haese A, de la Taille A, van Poppel H, et al. Clinical utility of the PCA3 urine assay in European men scheduled for repeat biopsy. Eur Urol 2008;54:1081-8. [Crossref] [PubMed]
- Ramos CG, Valdevenito R, Vergara I, et al. PCA3 sensitivity and specificity for prostate cancer detection in patients with abnormal PSA and/or suspicious digital rectal examination. First Latin American experience. Urol Oncol 2013;31:1522-6. [Crossref] [PubMed]
- Wang Z, Wang Y, Zhang J, et al. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol Med Rep 2017;16:5450-8. [Crossref] [PubMed]
- Esgueva R, Perner S, J, LaFargue C, et al. Prevalence of TMPRSS2-ERG and SLC45A3-ERG gene fusions in a large prostatectomy cohort. Mod Pathol 2010;23:539-46. [Crossref] [PubMed]
- Cary KC, Cooperberg MR. Biomarkers in prostate cancer surveillance and screening: past, present, and future. Ther Adv Urol 2013;5:318-29. [Crossref] [PubMed]
- Jiang H, Mao X, Huang X, et al. TMPRSS2:ERG fusion gene occurs less frequently in Chinese patients with prostate cancer. Tumour Biol 2016;37:12397-402. Erratum in: Tumour Biol 2016;37:14331. [Crossref] [PubMed]
- Hägglöf C, Hammarsten P, Strömvall K, et al. TMPRSS2-ERG expression predicts prostate cancer survival and associates with stromal biomarkers. PLoS One 2014;9. [Crossref] [PubMed]
- Ullman D, Dorn D, Rais-Bahrami S, et al. Clinical Utility and Biologic Implications of Phosphatase and Tensin Homolog (PTEN) and ETS-related Gene (ERG) in Prostate Cancer. Urology 2018;113:59-70. [Crossref] [PubMed]
- Hamid AR, Hoogland AM, Smit F, et al. The role of HOXC6 in prostate cancer development. Prostate 2015;75:1868-76. [Crossref] [PubMed]


