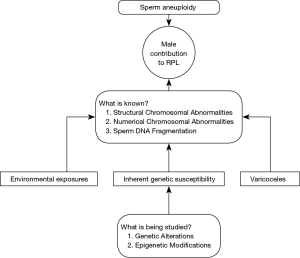
The male contribution to recurrent pregnancy loss
Introduction
Pregnancy loss is common and occurs in approximately 15–25% of clinical pregnancies. Recurrent pregnancy loss (RPL) is a distinct disorder defined as two or more failed clinical pregnancies (1). Fewer than 5% of women will experience two consecutive miscarriages and only 1% will experience three or more miscarriages (2). Our knowledge of the underlying etiologies behind RPL is still in flux. However, current evaluation of couples with RPL focuses on female factors including endocrine abnormalities such as thyroid disease, hyperprolactinemia and uncontrolled diabetes; uterine factors such as fibroids and Mullerian anomalies, acquired thrombophilia evaluation and karyotyping to evaluate for balanced translocations (3). The man, while important for conception, is investigated only with karyotype.
The semen analysis is generally not a part of the initial assessment of RPL due in part to its limitations as a functional test. However, sperm integrity is essential for sperm—egg interactions, fertilization and early embryonic development (4-6). In addition, paternally expressed genes modulate the proliferation and invasiveness of trophoblast cells and later placental proliferation (7-9). Despite some of the evidence of the effect of sperm on early embryogenesis and placental function, male factors contributory to RPL are largely unexplored.
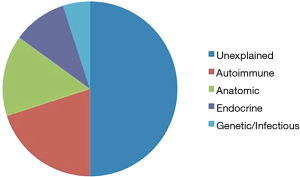
Fifty percent of couples with RPL will receive the diagnosis of unexplained RPL (Figure 1) (3). This is a frustrating diagnosis that has both physical and psychological implications. There is suspicion that some of unexplained RPL is as a result of an underlying male mechanism that is not currently understood. It is logical that since the male gamete contributes 50% of the genomic material to the embryo and placenta (10-12), the integrity of the sperm genome is essential for the initiation and maintenance of a successful pregnancy (13).
In this paper, we will discuss what is known about the male contribution to RPL. We will identify knowledge gaps and discuss the limitations of some of the findings in the literature. We will discuss some of the genes implicated in male infertility that appear to overlap with RPL, as well as some epigenetic modifications thought to be associated with RPL. Finally, we will attempt to provide a glimpse into the future and comment on what research must be done to answer some of the questions about the male contribution to RPL.
What is known?
Structural chromosomal abnormalities
Structural chromosomal abnormalities imply a different arrangement of an appropriate number of chromosomes. For example, a part of one chromosome may be found attached to a different chromosome so that all the genetic material is present but not in the right place. This is referred to as a translocation. In this situation, it is possible to create unbalanced gametes, which often result in offspring that spontaneously abort (Figure 2). Balanced translocations consist of reciprocal or Robertsonian translocations or inversions. Translocations can occur de novo in an embryo or can be inherited from either parent. If the translocation is inherited, the carrier parent is often phenotypically normal.
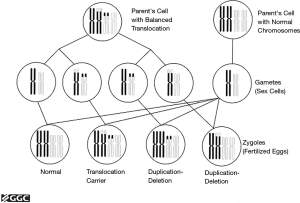
Karyotyping of couples is part of the evaluation of RPL and karyotypic abnormalities are an established cause of RPL, whether the abnormality occurs in the male partner or the female partner. It is however often the last test that is obtained because of the low likelihood of an abnormal result. A review of cytogenetic findings in multiple published surveys of couples with two or more pregnancy losses observed an overall prevalence of major chromosomal abnormalities of 2.9%, which is five to six times higher than that of the general population. Approximately 50% of the abnormalities were balanced reciprocal translocations, 24% were Robertsonian translocations and 12% were sex chromosome mosaicisms in females; the rest consisted of inversions and other sporadic abnormalities. In every group of chromosome abnormalities in the parents, there was a female to male predominance with a 2:1 ratio (14). Thus, structural chromosomal abnormalities in the male partner contribute in only a small proportion of couples with RPL.
Sperm deoxyribonucleic acid fragmentation
DNA fragmentation is the separation or breakage of DNA strands into pieces. Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) and sperm chromatin structure assays (SCSA) have been used for the identification of significant DNA fragmentation in couples with infertility and RPL (15-18). Not only does the presence of significant sperm DNA fragmentation have profound implications on embryogenesis, prenatal and postnatal growth, it has also been proposed to be associated with congenital malformations and childhood cancers (19-21).
Fertilization of an oocyte with damaged spermatozoon may result in an increase in DNA damage in the resulting embryo genome, which could result in DNA errors at different levels of embryogenesis (22). This could manifest as either being lethal to a developing embryo or as childhood diseases if the errors are non-lethal (23,24). Furthermore, this type of damage could occur in embryos with a normal chromosome complement and therefore could contribute to unexplained RPL (25). Higher sperm DNA fragmentation in couples with RPL may have its origin in poor DNA packaging at chromatin remodeling during spermiogenesis, which could leave DNA more vulnerable to oxidative stress (26-29) and DNA nucleases (30,31).
Several studies have suggested an association between increased sperm DNA fragmentation and unexplained RPL (32-36). The study by Bareh et al. [2016] is especially intriguing because they only included normozoospermic male partners and nonetheless detected significantly higher levels of DNA fragmentation within the RPL group compared to controls (36). The paternal genome provides the centrosome in the first mitotic division after fertilization (37). Because the paternal genome is activated between the four- and eight-cell stage in human embryos, high DNA damage may have no effect on fertilization yet manifest in later stages of embryonic development (38,39).
Although ASRM does not recommend routine sperm DNA fragmentation testing in male partners of women with unexplained RPL, current evidence since that guideline was published suggests that this could provide a potential mechanism for a male contribution to unexplained RPL. Moreover, a variety of interventions have been demonstrated to decrease sperm DNA fragmentation. Varicoceles are a known cause of sperm DNA damage (40) and many reproductive urologists will evaluate for their presence in couples with RPL. Varicocelectomy decreases sperm DNA fragmentation (41). Indeed, a randomized controlled trial demonstrated higher rates of conception and lower rates of miscarriage in couples with RPL in whom the male underwent varicocele repair (42). Furthermore, Esteves et al. demonstrated the effectiveness of using testicular sperm for ICSI over ejaculated sperm during IVF as a strategy to overcome infertility in oligozoospermic men with high sperm DNA fragmentation (43).
Additional modifiable factors that have been associated with an increase in reactive oxygen species generation and abnormal sperm DNA fragmentation include alcohol (44), smoking (45) and some environmental toxins (45-47). Furthermore, we are just beginning to explore the possibility that some men could have an unrecognized inherent genetic predisposition that causes their spermatozoa DNA to become susceptible to fragmentation. This possibility is yet to be thoroughly investigated and would require refined genetic evaluations including assessing epigenetic modifications in the sperm genome.
Despite some of the evidence for DNA fragmentation as a potential etiology of RPL, there are some limitations for its use. The threshold for what is deemed as “abnormal” DNA fragmentation varies in the literature and until there is a standardized method of measuring DNA fragmentation, it may not be widely utilized in the evaluation of couples with unexplained RPL.
Y chromosome microdeletions
The presence of severe oligospermia or azoospermia on routine semen analysis warrants further investigation including evaluation for microdeletion of the azoospermic factor (AZF) on the Y chromosome (48). Prevalence of Y-chromosome microdeletion in severely oligospermic and azoospermic men is estimated to be 8–18% (49,50). Several investigators have studied the prevalence of Y-chromosome microdeletions in their populations of couples with RPL. Three studies have demonstrated a significantly higher prevalence of microdeletion in the Y chromosome in the RPL group compared to controls and this prevalence ranged from 16% to 82% (51-53), while other studies have not demonstrated a difference in the prevalence of Y-chromosome microdeletion in the RPL group compared to the control group (54-56).
These studies on the association between Y-chromosome microdeletion and RPL are mixed in part due to the low prevalence of Y-chromosome microdeletion in the male infertile population coupled with the fact that these men are very rarely able to procreate, and almost exclusively require assisted reproductive technology (ART) for reproduction. In addition, it remains unknown how spermatozoa with a deletion influence fertilization rate and embryo quality. There are very few studies in the literature of the plausible mechanism by which AZF mutation can be implicated with miscarriages. Some of these studies implicate AZF region mutations with a meiotic defect, which may be associated with increased pregnancy loss (57-59). Another alternative explanation is that these Y-chromosome microdeletions are polymorphisms and due to the presence of palindromic areas, there are likely to be crossing over events with the X-chromosome yielding a genetic abnormality that could result in RPL (51). This is a knowledge gap that could be further investigated with animal models.
What is currently being explored?
Sperm aneuploidy
We are now beginning to understand that genetic and epigenetic contributions of sperm to early embryogenesis are extensive and have profound clinical implications. Fluorescent in situ hybridization (FISH) technology is the primary method used to study sperm chromosomes and detect aneuploidy. Sperm aneuploidy has been detected at an increased rate in male partners of women with RPL compared to controls in several studies (35,60-63). Although the data has shown increased rates of sex chromosome disomy in sperm from the male partner in couples with RPL, cytogenetic analysis of products of conception from couples with RPL does not reveal an increased rate of sex chromosome aneuploidy. This might suggest that cytogenetically abnormal sperm might be selected against during the process of fertilization (64,65). ASRM does not recommend routine sperm aneuploidy testing in couples with unexplained RPL (3). They cited the study by Stephenson et al. which demonstrated that over half of miscarriages in couples with RPL were euploid (54% vs. 46%) (64). However, one limitation of this study is the fact that they looked at a heterogeneous group with recurrent miscarriage and didn’t limit to idiopathic cases.
Moreover, the 2015 ASRM practice guideline for evaluation of the infertile male suggests that patients with RPL may benefit from screening for sperm aneuploidy (48). This differing view may be due in part to uncertainty surrounding the prognostic value of FISH regarding the final progeny as well as cost considerations (66). Furthermore, most FISH studies focus on a small number of chromosomes, typically those associated with aneuploidies compatible with life, namely 13, 18, 21, X and Y (67). What is unknown is whether this limited FISH panel is sufficient for evaluation of couples with RPL or even if expanded panels would provide more information. In addition, Neusser et al. posited that in RPL, chromosomes 1, 2, 6, 15, 16 and 21 are more relevant targets for sperm aneuploidy testing with chromosome 16 being the most promising diagnostic target (68). For these reasons, some authors suggest that until more in-depth studies are performed to explore this relationship, men with RPL should be screened for sperm aneuploidy and also referred to genetic counselors (69). At present, no intervention is known to decrease sperm aneuploidy but preimplantation genetic screening (PGS) can be used to select for euploid embryos during IVF.
Methylenetetrahydrofolate reductase (MTHFR) polymorphisms
MTHFR enzyme plays an important role in catalyzing the conversion of 5,10-methylenetetraydrofolate into 5-methylenetetrahydrofolate, which provides the single-carbon for homocysteine in methionine synthesis (70,71). There have been several studies that have evaluated the association between polymorphisms in MTHFR reductase activity and unexplained RPL. The results of these epidemiologic studies have been inconsistent in the literature. Of the 40 different genetic polymorphisms of MTHFR, C677T variant is the most studied and thought to be the most clinically relevant. A recent meta-analysis of 29 articles demonstrated a significant association between the MTHFR C677T polymorphism and a susceptibility to RPL in women (72). In addition, a pooled meta-analysis of 57 articles also demonstrated that both maternal and paternal MTHFR gene C677T and A1298C variants are associated with RPL. They also observed a significant association between fetal MTHFR A1298C polymorphism and RPL, but no association with C677T (73). Due to the inconsistent literature on this association, a general consensus has not been determined on the impact of paternal MTHFR polymorphisms on RPL.
Annexin A5 M2 haplotype
In 2007, a hereditary factor for RPL was suggested (74). This factor, called the M2 haplotype, comprises four consecutive nucleotide substitutions in the core promoter of the annexin A5 (ANXA5) gene and results in reduced expression levels of ANXA5 in placenta. ANXA5 is a member of the annexin protein family. It is ubiquitously expressed in perfused ductal organs and most abundantly present at the apical surfaces of the syncytiotrophoblast covering placenta villi (75). ANXA5 has potent anticoagulant properties that have been extensively studied both in vitro and in vivo (76,77). ANXA5 is crucial for the dynamics of membrane repair within the syncytiotrophoblast and reduced expression results in various thrombophilia-related pathologies of pregnancy such as preeclampsia, fetal growth restriction and RPL (75).
Abortion risk of women carrying the M2 haplotype for ANXA5 has been demonstrated to be over 2-fold higher than the general population (74). However, the mechanism by which this occurs is not known. It is suggested that the most likely explanation is a reduced expression of ANXA5 in placenta. It has also recently been demonstrated that the genetic frequency of paternal M2 carriage is significantly higher in couples with RPL than in fertile controls in the German population and its effects occur distinctly between the 10th and 15th week of gestation (75,78). Association between Annexin A5 M2 haplotype polymorphism and RPL has been replicated in other populations including Italian, Bulgarian, Japanese and Malaysian but the mechanism by which it impacts pregnancy loss needs to be further elucidated (78-81). Genotypic evaluation of embryonic tissue obtained from pregnancy loss may be relevant in further understanding the impact of this haplotype.
Ubiquitin-specific protease (USP26) gene alterations
In recent years, increased attention has been paid to genetic causes of male infertility. In addition to Y-chromosome microdeletion and mutation of some autosomal genes, X-chromosome genes have also been found to be closely related to male fertility; however, their underlying molecular mechanisms are still largely unknown (82,83). Nishimune and Tanaka [2006] observed many genes on the X-chromosome that are related to male infertility. The ubiquitin-specific protease 26 was first identified from a screen of X-linked genes involved in spermatogenesis by Wang et al. [2001]. USP26 belongs to a family of deubiquitinating enzymes, which play an important role in several biological processes such as control of growth, differentiation, oncogenesis and genome integrity (84,85). These enzymes might be involved in the removal of histones, regulation of cell turnover during meiosis, germ cell apoptosis, and proliferation and differentiation of spermatogonial stem cells during spermatogenesis (86).
In a recent study of 166 infertile men with non-obstructive azoospermia, 72 male partners of couples with RPL and 60 fertile controls, the authors demonstrated that total frequency of mutation in three common haplotypes of the USP26 gene in the study population was significantly higher in the infertile group and RPL group compared to the fertile controls. The authors concluded that in their population of Iranian men, alterations to the USP26 could impact fertility outcomes. Mutations may lead to an increase in histone levels in sperm DNA and consequently increased sperm DNA damage (86). Further studies are required to examine this association, which could potentially be applicable to men with idiopathic RPL.
Telomere length
Telomeres have specialized function in maintaining chromosome integrity and in germ cells, are thought to aid in meiotic recombination and pairing of homologous chromosomes. Telomere shortening in somatic cells results in telomeres losing their capping ability at the end of chromosomes, resulting in nonreciprocal translocations, chromosomal instability, deletions, aneuploidy and DNA damage (87). Liu et al. reported that shortened telomere length in male mice resulted in apoptosis, decreased recombination and meiotic arrest, while in females shortened telomeres led to impaired embryonic viability and fetal development (88). Telomeres are hypothesized to be one of the first structures in the sperm nucleus that respond to oocyte signals for male pronucleus development at fertilization (89).
Thilagavathi et al. [2013] therefore hypothesized that if telomeres are known to play a significant role in various disorders, they might also play a role at the level of the sperm and ova genome in unexplained RPL. Their study involved analyses of telomere length by real time qPCR of leukocytes obtained from 25 couples who experienced unexplained RPL and 20 fertile controls. The authors discovered that the relative leukocyte mean telomere length in both men and women with unexplained RPL was significantly lower when compared to controls. This was an interesting finding and led the authors to conclude that shortened telomeres might play a role in unexplained RPL. However, this would need to be further substantiated by analyzing telomere lengths at the level of germ cells (90).
The era of sperm epigenetics
A growing area of research in infertility is the role of epigenetics in male fertility. Epigenetics refer to non-coding areas in the genome that do not alter the basic DNA sequence but play a regulatory role. Modifications to the epigenome can occur via several mechanisms such as methylation, micro-RNAs and histone modification (91,92). The sperm epigenetic profile might provide a historical information about the entire process of spermatogenesis. During maturation of sperm, about 90% of histones are replaced by protamines, and this allows for more efficient packaging of compacted chromatin and also protects the sperm from oxidative damage. Any modification to this process would impact the DNA integrity in sperm and render it susceptible to DNA damage (93).
For this reason, the sperm protamine 1 to protamine 2 mRNA ratio has been extensively evaluated as a parameter of sperm functionality and abnormal ratios have been suggested to be implicated in male infertility (94-96). It has also recently been demonstrated to be a prognostic indicator of IVF/ICSI outcomes (96). Furthermore, a recent study of 25 male partners of women who experienced unexplained RPL, 32 healthy sperm donors and 107 infertile cohort demonstrated significant differences between the RPL group and the healthy donors in protamine-1, protamine-2 mRNA levels as well as the protamine mRNA ratios (97). In particular, the authors discovered that spermatozoa from male partners of women with unexplained RPL contained significantly higher protamine-1 and protamine-2 and the protamine mRNA ratio was lower in the case group. The authors suggest that not only are protamines important for fertilization, they may play an additional role in early embryogenesis; albeit through an uncertain mechanism (97). Protamines warrant further investigation as its mechanism of impact on male infertility appears to dovetail with an impact on pregnancy loss.
MicroRNAs are also non-protein coding RNAs that induce post-transcriptional gene silencing and mediate translational repression (98). MicroRNAs are believed to regulate almost a third of the human genome (99). It has been suggested that single nucleotide polymorphisms (SNP) in microRNA sequences can potentially affect their regulatory function (99) and some studies have demonstrated an association with RPL (100,101). A recent comparison study of couples with unexplained RPL compared to proven controls demonstrated differences in parental microRNA polymorphisms between the cases and the controls. This was the first study to implicate male microRNAs in RPL (102). Further investigation into microRNAs is warranted.
Finally, DNA methylation is another important aspect of sperm epigenetics that plays a role in male fecundity. Recent studies have reported an association between differentially methylated areas in the sperm DNA and male fecundity (103,104). Unexplained RPL as a result of early embryogenesis defect is one degree of separation away from male fecundity and it remains to be explored, the role of differential DNA methylation profiles in male partners of women with unexplained RPL.
Conclusions and future directions
RPL is a multifactorial disease and we are just beginning to scratch the surface of understanding the male contribution to unexplained RPL. The study of epigenetic biomarkers that are contributory to unexplained RPL is greatly needed. Abnormal DNA fragmentation is likely a symptom of multiple pathways; some of which we understand and some requiring significant further investigation (Figure 3). This is an area of research that involves an overlap between genetics, epigenetics and environmental factors. In addition, there is a growing need for more reliable tests of sperm aneuploidy and research into how to overcome this obstacle for selecting sperm for use in ART. Ultimately, the mechanisms by which these genetic and epigenetic mechanisms lead to RPL must be understood in order to develop therapeutic approaches.
Furthermore, we have only begun to explore the role of genes that have been found to be associated with male infertility in unexplained RPL. There are likely other as yet unknown genetic abnormalities unrelated to male infertility that might be implicated in unexplained RPL. Some great discoveries in science have occurred serendipitously and we cannot even begin to predict how to determine these unknown male genetic contributions. Perhaps pedigree information in couples with unexplained RPL may be helpful in identifying a subset of individuals in which the disease is strongly inherited. Linkage analysis and genome wide association studies could then ensue. This is an interesting thought but would undeniably be costly and at risk of not yielding useful information.
With the information we currently have, testing for sperm chromosomal abnormalities and DNA fragmentation appears to be a reasonable option for male partners of women with unexplained RPL, with referral to genetic counselor if results are positive (69). Although, it remains difficult to predict the exact risk of unfavorable outcomes in the presence of positive findings from the available tests we have especially with the limitations in the methods of testing, this information provides for more detailed discussion about the risks and potential impacts on subsequent pregnancy outcomes. Psychologically, there may be benefit to couples in understanding the reasons for their losses, and diagnosis of a male factor may help couple in considering alternative reproductive options, including the use of donor sperm. Clinicians currently counsel couples with unexplained RPL that the chance of a livebirth in a subsequent pregnancy is approximately 75% (3). However, given the heterogeneity of unexplained RPL, this may not be accurate in a subset of patients. Further understanding of this complicated ailment will allow couples to make more educated, albeit difficult reproductive decisions.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- van den Boogaard E, Kaandorp SP, Franssen MT, et al. Consecutive or non-consecutive recurrent miscarriage: is there any difference in carrier status? Hum Reprod 2010;25:1411-4. [Crossref] [PubMed]
- Stirrat GM. Recurrent miscarriage. Lancet 1990;336:673-5. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril 2012;98:1103-11. [Crossref] [PubMed]
- Simerly C, Wu GJ, Zoran S, et al. The paternal inheritance of the centrosome, the cell's microtubule-organizing center, in humans, and the implications for infertility. Nat Med 1995;1:47-52. Erratum in: Nat Med 1995;1:599. [Crossref] [PubMed]
- Van Blerkom J. Sperm centrosome dysfunction: a possible new class of male factor infertility in the human. Mol Hum Reprod 1996;2:349-54. [Crossref] [PubMed]
- Moomjy M, Colombero LT, Veeck LL, et al. Sperm integrity is critical for normal mitotic division and early embryonic development. Mol Hum Reprod 1999;5:836-44. [Crossref] [PubMed]
- Janny L, Menezo YJ. Evidence for a strong paternal effect on human preimplantation embryo development and blastocyst formation. Mol Reprod Dev 1994;38:36-42. [Crossref] [PubMed]
- Check JH, Katsoff D, Check ML. Some semen abnormalities may cause infertility by impairing implantation rather than fertilization. Med Hypotheses 2001;56:653-7. [Crossref] [PubMed]
- Goshen R, Ben-Rafael Z, Gonik B, et al. The role of genomic imprinting in implantation. Fertil Steril 1994;62:903-10. [PubMed]
- Sutovsky P, Schatten G. Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int Rev Cytol 2000;195:1-65. [PubMed]
- Miozzo M, Simoni G. The role of imprinted genes in fetal growth. Biol Neonate 2002;81:217-28. [Crossref] [PubMed]
- Puscheck EE, Jeyendran RS. The impact of male factor on recurrent pregnancy loss. Curr Opin Obstet Gynecol 2007;19:222-8. [Crossref] [PubMed]
- Bedford JM, Bent MJ, Calvin H. Variations in the structural character and stability of the nuclear chromatin in morphologically normal human spermatozoa. J Reprod Fertil 1973;33:19-29. [Crossref] [PubMed]
- Tharapel AT, Tharapel SA, Bannerman RM. Recurrent pregnancy losses and parental chromosome abnormalities: a review. Br J Obstet Gynaecol 1985;92:899-914. [Crossref] [PubMed]
- Evenson DP, Jost LK, Marshall D, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod 1999;14:1039-49. [Crossref] [PubMed]
- Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril 2010;93:1027-36. [Crossref] [PubMed]
- Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod 2005;20:226-30. [Crossref] [PubMed]
- Bungum M, Bungum L, Giwercman A. Sperm chromatin structure assay (SCSA): a tool in diagnosis and treatment of infertility. Asian J Androl 2011;13:69-75. [Crossref] [PubMed]
- Nanassy L, Carrell DT. Paternal effects on early embryogenesis. J Exp Clin Assist Reprod 2008;5:2. [Crossref] [PubMed]
- Cho C, Jung-Ha H, Willis WD, et al. Protamine 2 deficiency leads to sperm DNA damage and embryo death in mice. Biol Reprod 2003;69:211-7. [Crossref] [PubMed]
- Lewis SE, Aitken RJ. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res 2005;322:33-41. [Crossref] [PubMed]
- Lewis SE, Simon L. Clinical implications of sperm DNA damage. Hum Fertil (Camb) 2010;13:201-7. [Crossref] [PubMed]
- Cooke MS, Evans MD, Dizdaroglu M, et al. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 2003;17:1195-214. [Crossref] [PubMed]
- Aitken RJ, De Iuliis GN, McLachlan RI. Biological and clinical significance of DNA damage in the male germ line. Int J Androl 2009;32:46-56. [Crossref] [PubMed]
- Alvarez JG. DNA fragmentation in human spermatozoa: significance in the diagnosis and treatment of infertility. Minerva Ginecol 2003;55:233-9. [PubMed]
- Agarwal A, Makker K, Sharma R. Clinical relevance of oxidative stress in male factor infertility: an update. Am J Reprod Immunol 2008;59:2-11. [Crossref] [PubMed]
- Makker K, Agarwal A, Sharma R. Oxidative stress & male infertility. Indian J Med Res 2009;129:357-67. [PubMed]
- Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod 2010;16:3-13. [Crossref] [PubMed]
- Aitken RJ, Koppers AJ. Apoptosis and DNA damage in human spermatozoa. Asian J Androl 2011;13:36-42. [Crossref] [PubMed]
- Maione B, Pittoggi C, Achene L, et al. Activation of endogenous nucleases in mature sperm cells upon interaction with exogenous DNA. DNA Cell Biol 1997;16:1087-97. [Crossref] [PubMed]
- Sailer BL, Sarkar LJ, Bjordahl JA, et al. Effects of heat stress on mouse testicular cells and sperm chromatin structure. J Androl 1997;18:294-301. [PubMed]
- Carrell DT, Liu L, Peterson CM, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl 2003;49:49-55. [Crossref] [PubMed]
- Kumar K, Deka D, Singh A, et al. Predictive value of DNA integrity analysis in idiopathic recurrent pregnancy loss following spontaneous conception. J Assist Reprod Genet 2012;29:861-7. [Crossref] [PubMed]
- Ribas-Maynou J, García-Peiró A, Fernandez-Encinas A, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One 2012;7. [Crossref] [PubMed]
- Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril 2016;105:58-64. [Crossref] [PubMed]
- Bareh GM, Jacoby E, Binkley P, et al. Sperm deoxyribonucleic acid fragmentation assessment in normozoospermic male partners of couples with unexplained recurrent pregnancy loss: a prospective study. Fertil Steril 2016;105:329-36.e1. [Crossref] [PubMed]
- Sathananthan AH. Mitosis in the human embryo: the vital role of the sperm centrosome (centriole). Histol Histopathol 1997;12:827-56. [PubMed]
- Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril 2004;81:1289-95. [Crossref] [PubMed]
- Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 1988;332:459-61. [Crossref] [PubMed]
- Wang YJ, Zhang RQ, Lin YJ, et al. Relationship between varicocele and sperm DNA damage and the effect of varicocele repair: a meta-analysis. Reprod Biomed Online 2012;25:307-14. [Crossref] [PubMed]
- Kadioglu TC, Aliyev E, Celtik M. Microscopic varicocelectomy significantly decreases the sperm DNA fragmentation index in patients with infertility. Biomed Res Int 2014;2014. [Crossref] [PubMed]
- Mansour Ghanaie M, Asgari SA, Dadrass N, et al. Effects of varicocele repair on spontaneous first trimester miscarriage: a randomized clinical trial. Urol J 2012;9:505-13. [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- La Vignera S, Condorelli RA, Balercia G, et al. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013;15:221-5. [Crossref] [PubMed]
- Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online 2014;28:684-703. [Crossref] [PubMed]
- Sengupta P, Banerjee R. Environmental toxins: alarming impacts of pesticides on male fertility. Hum Exp Toxicol 2014;33:1017-39. [Crossref] [PubMed]
- Wirth JJ, Mijal RS. Adverse effects of low level heavy metal exposure on male reproductive function. Syst Biol Reprod Med 2010;56:147-67. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Chandley AC. Chromosome anomalies and Y chromosome microdeletions as causal factors in male infertility. Hum Reprod 1998;13 Suppl 1:45-50. [Crossref] [PubMed]
- Van Assche E, Bonduelle M, Tournaye H, et al. Cytogenetics of infertile men. Hum Reprod 1996;11 Suppl 4:1-24; discussion 25-6. [Crossref] [PubMed]
- Dewan S, Puscheck EE, Coulam CB, et al. Y-chromosome microdeletions and recurrent pregnancy loss. Fertil Steril 2006;85:441-5. [Crossref] [PubMed]
- Karaer A, Karaer K, Ozaksit G, et al. Y chromosome azoospermia factor region microdeletions and recurrent pregnancy loss. Am J Obstet Gynecol 2008;199:662.e1-5. [Crossref] [PubMed]
- Agarwal S, Agarwal A, Khanna A, et al. Microdeletion of Y chromosome as a cause of recurrent pregnancy loss. J Hum Reprod Sci 2015;8:159-64. Erratum in: J Hum Reprod Sci 2016;9:131. [Crossref] [PubMed]
- Wettasinghe TK, Jayasekara RW, Dissanayake VH. Y chromosome microdeletions are not associated with spontaneous recurrent pregnancy loss in a Sinhalese population in Sri Lanka. Hum Reprod 2010;25:3152-6. [Crossref] [PubMed]
- Piña-Aguilar RE, Martínez-Garza SG, Kohls G, et al. Y chromosome microdeletions in Mexican males of couples with idiopathic recurrent pregnancy loss. J Obstet Gynaecol Res 2012;38:912-7. [Crossref] [PubMed]
- Ghorbian S, Saliminejad K, Sadeghi MR, et al. The Association between Y Chromosome Microdeletion and Recurrent Pregnancy Loss. Iran Red Crescent Med J 2012;14:358-62. [PubMed]
- Perrin J, Metzler-Guillemain C, Karsenty G, et al. Meiotic arrest at the midpachytene stage in a patient with complete azoospermia factor b deletion of the Y chromosome. Fertil Steril 2006;85:494.e5-8. [Crossref] [PubMed]
- Geoffroy-Siraudin C, Aknin-Seiffer I, Metzler-Guillemain C, et al. Meiotic abnormalities in patients bearing complete AZFc deletion of Y chromosome. Hum Reprod 2007;22:1567-72. [Crossref] [PubMed]
- Guichaoua MR, Perrin J, Metzler-Guillemain C, et al. Meiotic anomalies in infertile men with severe spermatogenic defects. Hum Reprod 2005;20:1897-902. [Crossref] [PubMed]
- Bernardini LM, Costa M, Bottazzi C, et al. Sperm aneuploidy and recurrent pregnancy loss. Reprod Biomed Online 2004;9:312-20. [Crossref] [PubMed]
- Ramasamy R, Scovell JM, Kovac JR, et al. Fluorescence in situ hybridization detects increased sperm aneuploidy in men with recurrent pregnancy loss. Fertil Steril 2015;103:906-9.e1. [Crossref] [PubMed]
- Rubio C, Simón C, Blanco J, et al. Implications of sperm chromosome abnormalities in recurrent miscarriage. J Assist Reprod Genet 1999;16:253-8. [Crossref] [PubMed]
- Carrell DT, Wilcox AL, Lowy L, et al. Elevated sperm chromosome aneuploidy and apoptosis in patients with unexplained recurrent pregnancy loss. Obstet Gynecol 2003;101:1229-35. [PubMed]
- Stephenson MD, Awartani KA, Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage: a case-control study. Hum Reprod 2002;17:446-51. [Crossref] [PubMed]
- Rubio C, Gil-Salom M, Simón C, et al. Incidence of sperm chromosomal abnormalities in a risk population: relationship with sperm quality and ICSI outcome. Hum Reprod 2001;16:2084-92. [Crossref] [PubMed]
- Tempest HG, Martin RH. Cytogenetic risks in chromosomally normal infertile men. Curr Opin Obstet Gynecol 2009;21:223-7. [Crossref] [PubMed]
- Ramasamy R, Besada S, Lamb DJ. Fluorescent in situ hybridization of human sperm: diagnostics, indications, and therapeutic implications. Fertil Steril 2014;102:1534-9. [Crossref] [PubMed]
- Neusser M, Rogenhofer N, Dürl S, et al. Increased chromosome 16 disomy rates in human spermatozoa and recurrent spontaneous abortions. Fertil Steril 2015;104:1130-7.e1-10.
- Kohn TP, Kohn JR, Darilek S, et al. Genetic counseling for men with recurrent pregnancy loss or recurrent implantation failure due to abnormal sperm chromosomal aneuploidy. J Assist Reprod Genet 2016;33:571-6. [Crossref] [PubMed]
- Kobashi G, Kato EH, Morikawa M, et al. MTHFR C677T Polymorphism and factor V Leiden mutation are not associated with recurrent spontaneous abortion of unexplained etiology in Japanese women. Semin Thromb Hemost 2005;31:266-71. [Crossref] [PubMed]
- Wu W, Shen O, Qin Y, et al. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of male infertility: a meta-analysis. Int J Androl 2012;35:18-24. [Crossref] [PubMed]
- Rai V. Methylenetetrahydrofolate Reductase C677T Polymorphism and Recurrent Pregnancy Loss Risk in Asian Population: A Meta-analysis. Indian J Clin Biochem 2016;31:402-13. [Crossref] [PubMed]
- Yang Y, Luo Y, Yuan J, et al. Association between maternal, fetal and paternal MTHFR gene C677T and A1298C polymorphisms and risk of recurrent pregnancy loss: a comprehensive evaluation. Arch Gynecol Obstet 2016;293:1197-211. [Crossref] [PubMed]
- Bogdanova N, Horst J, Chlystun M, et al. A common haplotype of the annexin A5 (ANXA5) gene promoter is associated with recurrent pregnancy loss. Hum Mol Genet 2007;16:573-8. [Crossref] [PubMed]
- Rogenhofer N, Engels L, Bogdanova N, et al. Paternal and maternal carriage of the annexin A5 M2 haplotype are equal risk factors for recurrent pregnancy loss: a pilot study. Fertil Steril 2012;98:383-8. [Crossref] [PubMed]
- Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem 1990;265:17420-3. [PubMed]
- Römisch J, Seiffge D, Reiner G, et al. In-vivo antithrombotic potency of placenta protein 4 (annexin V). Thromb Res 1991;61:93-104. [Crossref] [PubMed]
- Tüttelmann F, Ivanov P, Dietzel C, et al. Further insights into the role of the annexin A5 M2 haplotype as recurrent pregnancy loss factor, assessing timing of miscarriage and partner risk. Fertil Steril 2013;100:1321-5. [Crossref] [PubMed]
- Tiscia G, Colaizzo D, Chinni E, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost 2009;102:309-13. [Crossref] [PubMed]
- Miyamura H, Nishizawa H, Ota S, et al. Polymorphisms in the annexin A5 gene promoter in Japanese women with recurrent pregnancy loss. Mol Hum Reprod 2011;17:447-52. [Crossref] [PubMed]
- Ang KC, Kathirgamanathan S, Ch'ng ES, et al. Genetic analysis of the M2/ANXA5 haplotype as recurrent pregnancy loss predisposition in the Malay population. J Assist Reprod Genet 2017;34:517-24. [Crossref] [PubMed]
- Yang Y, Xiao CY. DAZ1/DAZ2 cluster deletion mediated by gr/gr recombination per se may not be sufficient for spermatogenesis impairment: a study of Chinese normozoospermic men. Asian J Androl 2006;8:183-7. [Crossref] [PubMed]
- Fernando L, Gromoll J, Weerasooriya TR, et al. Y-chromosomal microdeletions and partial deletions of the Azoospermia Factor c (AZFc) region in normozoospermic, severe oligozoospermic and azoospermic men in Sri Lanka. Asian J Androl 2006;8:39-44. [Crossref] [PubMed]
- Glickman MH, Ciechanover A. The ubiquitin-proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev 2002;82:373-428. [Crossref] [PubMed]
- Amerik AY, Hochstrasser M. Mechanism and function of deubiquitinating enzymes. Biochim Biophys Acta 2004;1695:189-207. [Crossref] [PubMed]
- Asadpor U, Totonchi M, Sabbaghian M, et al. Ubiquitin-specific protease (USP26) gene alterations associated with male infertility and recurrent pregnancy loss (RPL) in Iranian infertile patients. J Assist Reprod Genet 2013;30:923-31. [Crossref] [PubMed]
- Murnane JP. Telomeres and chromosome instability. DNA Repair (Amst) 2006;5:1082-92. [Crossref] [PubMed]
- Liu L, Franco S, Spyropoulos B, et al. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A 2004;101:6496-501. [Crossref] [PubMed]
- Zalenskaya IA, Bradbury EM, Zalensky AO. Chromatin structure of telomere domain in human sperm. Biochem Biophys Res Commun 2000;279:213-8. [Crossref] [PubMed]
- Thilagavathi J, Kumar M, Mishra SS, et al. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet 2013;287:803-7. [Crossref] [PubMed]
- Hotaling J, Carrell DT. Clinical genetic testing for male factor infertility: current applications and future directions. Andrology 2014;2:339-50. [Crossref] [PubMed]
- Carrell DT. Epigenetics of the male gamete. Fertil Steril 2012;97:267-74. [Crossref] [PubMed]
- Oliva R. Protamines and male infertility. Hum Reprod Update 2006;12:417-35. [Crossref] [PubMed]
- Steger K, Wilhelm J, Konrad L, et al. Both protamine-1 to protamine-2 mRNA ratio and Bcl2 mRNA content in testicular spermatids and ejaculated spermatozoa discriminate between fertile and infertile men. Hum Reprod 2008;23:11-6. [Crossref] [PubMed]
- Nanassy L, Liu L, Griffin J, et al. The clinical utility of the protamine 1/protamine 2 ratio in sperm. Protein Pept Lett 2011;18:772-7. [Crossref] [PubMed]
- Rogenhofer N, Dansranjavin T, Schorsch M, et al. The sperm protamine mRNA ratio as a clinical parameter to estimate the fertilizing potential of men taking part in an ART programme. Hum Reprod 2013;28:969-78. [Crossref] [PubMed]
- Rogenhofer N, Ott J, Pilatz A, et al. Unexplained recurrent miscarriages are associated with an aberrant sperm protamine mRNA content. Hum Reprod 2017;32:1574-82. [Crossref] [PubMed]
- Lim LP, Lau NC, Garrett-Engele P, et al. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005;433:769-73. [Crossref] [PubMed]
- Mishra PJ, Bertino JR. MicroRNA polymorphisms: the future of pharmacogenomics, molecular epidemiology and individualized medicine. Pharmacogenomics 2009;10:399-416. [Crossref] [PubMed]
- Jeon YJ, Choi YS, Rah H, et al. Association study of microRNA polymorphisms with risk of idiopathic recurrent spontaneous abortion in Korean women. Gene 2012;494:168-73. Erratum in: Gene 2012;498:336. [Crossref] [PubMed]
- Jeon YJ, Kim SY, Rah H, et al. Association of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and miR-499A>G polymorphisms with risk of spontaneously aborted fetuses. Am J Reprod Immunol 2012;68:408-17. [Crossref] [PubMed]
- Amin-Beidokhti M, Mirfakhraie R, Zare-Karizi S, et al. The role of parental microRNA alleles in recurrent pregnancy loss: an association study. Reprod Biomed Online 2017;34:325-30. [Crossref] [PubMed]
- Hammoud SS, Purwar J, Pflueger C, et al. Alterations in sperm DNA methylation patterns at imprinted loci in two classes of infertility. Fertil Steril 2010;94:1728-33. [Crossref] [PubMed]
- Jenkins TG, Aston KI, Meyer TD, et al. Decreased fecundity and sperm DNA methylation patterns. Fertil Steril 2016;105:51-7.e1-3.