Impact of anabolic androgenic steroids on sexual function
Introduction
Anabolic-androgenic steroids (AAS) represent a class of therapies which exhibit physical effects similar to supplemental testosterone (T). Prevalence rates for AAS use in the United States are estimated at 1–3 million, with demographics of users most commonly representing Caucasian men, aged 18–44, with advanced levels of education, above average income, and employed status (1-3).
Given their impact on augmenting physical anatomy and muscle mass, AAS have been used in an off-label manner for decades for various reasons including enhanced aesthetics, improved athletic performance, increased muscle mass, or other symptomatic benefits. T, and its downstream product dihydrotestosterone (DHT), have also been shown to have several notable physiological impacts on sexual function, including growth and development of the penis, seminal vesicles, prostate, as well as impacts on libido, arousal, and orgasm as mediated by the central nervous system (4-6).
Potential benefits of T supplementation in men with low T have been widely reported, with meta-analyses of randomized controlled trials (RCTs) demonstrating modest improvements in libido, AM erections, sexual thoughts, and erectile function (7,8). In the largest RCT to date, T supplementation in hypogonadal men resulted in mild improvements in nearly all subdomains of sexual function analyzed, with 1-year treatment effects ranging from 2–10% (9). Despite the abundance of data on the physiologic role of T on sexual function and impact of supplementation in hypogonadal men, very limited data are available on the effects of supra-physiologic AAS use on libido and erectile function in the short and long-terms.
In one of the largest studies (n=45) evaluating sexual function in men taking supraphysiologic doses of T, Moss and colleagues compared current AAS users to those previously using or non-users in a survey of amateur bodybuilding athletes. Mean age for the three groups was 25.2, 23.5, and 26.3 years, respectively, and weekly doses ranged from 75–1,550 mg/week. Results demonstrated that both current and past AAS users reported increased frequency of intercourse, with no differences in morning erections, sexual thoughts, sexual enjoyment, importance, intensity, or satisfaction. Interestingly, adverse effects in this cohort included erectile dysfunction (ED), anorgasmia, and premature ejaculation (10).
Given the known physiological role for T on sexual function and the paucity of literature reporting implications of prolonged, supraphysiologic dosing, we sought to describe sexual function in a cohort of AAS users. Specifically, we sought to evaluate if supra-physiologic T supplementation is associated with improved measures of sexual function during use and subsequent sexual dysfunctions once discontinued. To evaluate our hypothesis, a sexual function survey was performed of current and previous supraphysiologic AAS users. The objective was to identify associations between AAS use, including agent, dosage, and duration of therapy, and sexual function/dysfunctions.
Methods
Participants
After institutional review board approval, participants were recruited utilizing nine online bodybuilding forums between February 1, 2015 and June 1, 2015. A link with a short description of an anonymous survey was posted on each forum, and participants were asked to answer questions related to personal patterns of T (predominantly) and other forms of AAS use. Inclusion criteria were age ≥18 years, male gender, and a current or past history of T use.
Questionnaires
All respondents were asked to complete a questionnaire via SurveyMonkey®, a secure third-party survey tool. Participant responses were collected in an anonymous fashion, with no specific identifiers obtained.
The survey included 49-items with branching logic that were designed to elicit single-answer responses. See Table S1 for a complete list of questions included. Participants were able to provide additional free-text information with select questions and had the option of not responding to questions.

Full table
Sexual function was assessed using the abbreviated, 5-item International Index of Erectile Function (IIEF-5), with erectile function classified as no ED [22–25], mild ED [17–21], mild to moderate ED [12–16], moderate ED [8–11], and severe ED [5–7] (11). Given the fluctuating and intermittent nature of AAS use, respondents were asked to respond to the questions based on their past six months rather than the standardized, one-month period. To evaluate adverse effects of therapy (including sexual symptoms), respondents were questioned on the presence of several known side effects while receiving and after stopping therapy.
Demographic and historical data obtained included age, employment status, current income, level of education, and athletic participation in high school and college. Specific information on drug use included age of onset, duration and weekly dose, other performance enhancing drugs, and therapeutic cycling practices. Several T-related symptoms were assessed while on and off of therapy including libido, erectile function, fat gain, muscle loss, depression, decreased energy, loss of interest in working out, testicular shrinkage, gynecomastia, anger/violence, overconfidence, acne, and water retention. Participants were also asked about high-risk behaviors such as illicit drug use and criminal activities and further questioned on medical comorbidities and routine laboratory testing obtained. Given the length of the questionnaire, a separate analysis of the cohort and non-sexual dysfunction related responses was previously published as a separate manuscript (2).
Statistical analyses
Fisher’s exact and Pearson’s chi-square tests were used to compare categorical variables. Statistical significance was defined as P≤0.05, with all reported P values 2-sided. All analyses were performed using the SAS JMP (10.0; Cary, NC, USA) software package.
Results
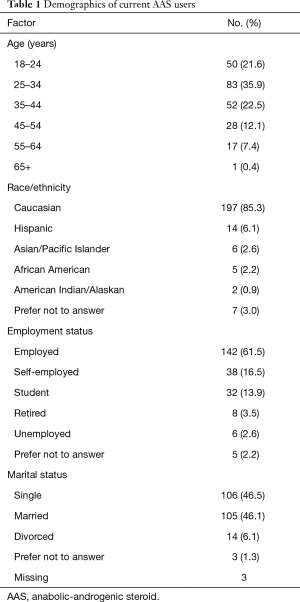
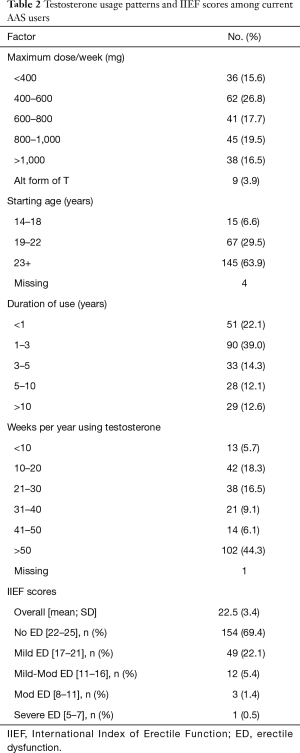
A total of 321 men responded to the survey, of which 90 failed to meet inclusion criteria, for a final cohort of 231 AAS users. Demographic variables are presented in Table 1. The majority administered weekly doses of ≥600 mg/week (54%), employed some form of post-cycle therapy (56%), and used additional substances such as anti-estrogens, 17-alpha-alkylated hormones, cutting agents, or other AAS (93%). See Table 2 for additional details on T usage patterns.
Full table
Full table
The majority of men (85%) did not initiate T due to symptoms classically associated with low T. Among those who did report low T-related symptoms, a higher percentage were older (76% ≥35 years compared to 39% of those starting T for other reasons, P<0.01), had comorbid cardiac disease (14% vs. 1%, P<0.01), had lower serum T levels (52% vs. 25%, P=0.01), and were more likely to have suffered from depression (10% vs. 1%, P=0.03). In contrast, those with low-T symptoms were less likely to compete in bodybuilding (0% vs. 10%, P=0.04), use other anabolic steroids (24% vs. 45%, P<0.05), obtain steroids from a friend (0% vs. 20%, P<0.01), or initiate T with the intent to increase muscle mass (10% vs. 38%, P<0.01).
A total of 222 men completed the IIEF-5 portion of the questionnaire, with a mean score of 22.5. Erectile function was further categorized as no ED (69.4%; 154/222), mild (22.1%; 49/222), mild-moderate (5.4%; 12/222), moderate (1.4%; 3/222), and severe ED (0.5%; 1/222). See Table 2 for IIEF scores and categorical breakdown of ED subtypes among AAS users. See Table 3 for summary of factors associated with differences in IIEF scores.

Full table
Compared to men with more severe ED, those with mild or no ED (IIEF ≥17) were more likely to use other substances including anti-estrogens (91% vs. 63% among those with mild-moderate or worse ED, P<0.001), sexual enhancement medications (63% vs. 37%, P=0.01), 17-alpha alkylated oral hormones (62% vs. 33%, P<0.01), or other anabolic substances (47% vs. 15%, P<0.01). Men with mild or no ED also had lower rates of reduced energy after stopping T (58% vs. 83%, P<0.05), and use of ≤600 mg/week of T (41% vs. 64%, P=0.03).
When not taking T, 27% of men reported de novo ED, and 57% de novo decreased libido. Among the 127 men who reported de novo decreased libido when not taking AAS, several significant factors were notable including a greater frequency (>40 weeks a year of use) and duration (>3 years) of T supplementation and increased utilization of adjunctive therapies such as 17-alpha alkylated oral hormones, research pharmaceuticals, and human growth hormone. Interestingly, the use of post-cycle therapy was associated with higher rates of preserved libido when not taking T, suggesting a possible protective effect. De novo ED was also associated with various factors, including other traditional low-T symptoms, duration of T use >10 years, and use >40 weeks per year. See Table 4 for summary of variables associated with de novo ED and decreased libido when not taking T.

Full table
Discussion
The current study represents the largest evaluation of sexual dysfunction in a cohort of AAS users and demonstrates several notable findings. Not surprisingly, increasing use of T was associated with higher rates of preserved erectile function in men currently using the therapy. However, surprisingly, a high percentage of men reported de novo sexual dysfunctions, including ED (27%) and decreased libido (57%) when not taking AAS. Additionally, longer durations of use and higher frequency of use per year were associated with experiencing these symptoms. De novo ED was also associated with multiple other classic low-T symptoms such as reduced libido, decreased energy, depression, subjective reduction in muscle mass, and increased subjective adiposity.
These findings may suggest that to some degree, the body becomes dependent upon hyper-supplementation of T (suppression of hypothalamic-pituitary-gonadal axis, possible change in androgen receptor density, possible down regulation at nuclear level), an effect that is only recognized after discontinuing. The study however is not able to address if these symptoms remain persistent for an extended period of time or whether symptoms return to baseline after a further period of recovery. These findings do support our clinical impression from our practice, in which men often do present with symptoms of sexual dysfunctions after an extended history of AAS use. Further study is required to assess this important clinical question.
As would be expected, results also demonstrated that those with increased comorbid conditions and higher rates of low T related symptoms were found to have lower IIEF scores. Similarly, those experiencing low-T symptoms when not receiving T were more likely to have moderate to severe ED, suggesting a shared mechanism for ED and other low T symptoms.
The use of estrogen-modulating therapies were found to be a protective factor in maintaining erectile function after discontinuing AAS. Although this requires further evaluation to determine its significance, the mechanism behind commonly used selective estrogen receptor modulators, such as clomiphene citrate, includes partial estrogen receptor agonist activity. This is noteworthy, as T and estrogen have recently been shown by Finkelstein and colleagues to independently exhibit physiological effects on sexual function (12). In their study of 400 men (aged 20–50 years), each was administered Goserelin acetate to deplete gonadal steroids. Next, participants were randomly assigned to be given placebo, varying doses of topical T alone, or topical T with anastrozole (to prevent conversion of T to estradiol). Results demonstrated preservation of sexual function in men receiving T, with greater improvements noted among those not receiving anastrozole. Findings suggested that both T and estrogen have important effects on sexual function and desire, which provides a potential mechanism for outcomes of the current study.
Findings from the current study are consistent with other reported literature. In a small series of 33 prior AAS users, Rasmussen et al. reported similar rates of ED among former AAS abusers (27% of former AAS users compared to 29% in our cohort overall) (13). The study found that participants suffered persistent low T levels after discontinuing AAS abuse, and there were also higher rates of decreased libido and ED among former AAS abusers than participants who were currently taking the substance as well as those in the control group, all of which were also found in our larger series.
Another small study of 36 weightlifters examined the long-term effects of AAS abuse on sexual function and prolonged hypogonadism (14). Consistent with our findings, results demonstrated that former AAS abusers experienced lower sexual libido along with displaying an overall decreased testicular volume and serum T levels when compared with the weightlifters that had never used the substance. Two of the participants failed to regain erectile function or normal libido despite receiving T treatment.
The current study has several notable limitations. The data were obtained from a survey posted on body-building forums and is therefore not necessarily representative of the population as a whole. However, this was done intentionally, as data on men using high doses of AAS for extended periods of time cannot reasonably or ethically be obtained in other ways. The data are also captured at a single time point, with inability to track findings long-term and limited ability to compare findings between current and former AAS users. Despite these limitations, the current study represents the largest series of current and former AAS users with data on sexual function, utilizes a standardized IIEF questionnaire, and includes a detailed analysis of AAS frequency, duration, and dosage. This permits a more in depth and higher power analysis on factors associated with de novo sexual dysfunctions compared to any prior study.
Conclusions
The current study represents the largest series to date evaluating the impact of high dose, extended duration AAS supplementation on sexual function. Results demonstrate that increasing duration and frequency of AAS are associated with higher rates of de novo ED and decreased libido following discontinuation. Men with de novo ED were also more likely to report other low T symptoms, such as reduced libido, decreased energy, depression, subjective reduction in muscle mass, and increased subjective adiposity. Inversely, current use of higher T dosage and anti-estrogens (i.e., selective estrogen receptor modulators) are associated with higher current IIEF scores. Overall, findings suggest that increased frequency and duration of high-dose AAS may result in sexual dysfunctions following discontinuation and warrants further study.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Committee reviewed the above referenced application and determined it to be exempt from IRB review under 45 CFR 46.101(b), item 2. Continued IRB review of this study is not required as it is currently written. However, any modifications to the study design or procedures must be submitted to the IRB to determine whether the study continues to be exempt. Review: The Committee noted receipt of the study protocol (undated). As protected health information is not being requested from subjects, HIPAA authorization is not required in accordance with 45 CFR 160.103.
References
- Sjoqvist F, Garle M, Rane A. Use of doping agents, particularly anabolic steroids, in sports and society. Lancet 2008;371:1872-82. [Crossref] [PubMed]
- Westerman ME, Charchenko CM, Ziegelmann MJ, et al. Heavy Testosterone Use Among Bodybuilders: An Uncommon Cohort of Illicit Substance Users. Mayo Clinic proceedings 2016;91:175-82. [Crossref] [PubMed]
- Cohen J, Collins R, Darkes J, et al. A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States. J Int Soc Sports Nutr 2007;4:12. [Crossref] [PubMed]
- Melcangi RC, Magnaghi V, Martini L. Steroid metabolism and effects in central and peripheral glial cells. J Neurobiol 1999;40:471-83. [Crossref] [PubMed]
- Thigpen AE, Silver RI, Guileyardo JM, et al. Tissue distribution and ontogeny of steroid 5 alpha-reductase isozyme expression. J Clin Invest 1993;92:903-10. [Crossref] [PubMed]
- Traish AM, Goldstein I, Kim NN. Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007;52:54-70. [Crossref] [PubMed]
- Bolona ER, Uraga MV, Haddad RM, et al. Testosterone use in men with sexual dysfunction: a systematic review and meta-analysis of randomized placebo-controlled trials. Mayo Clin Proc 2007;82:20-8. [Crossref] [PubMed]
- Isidori AM, Giannetta E, Gianfrilli D, et al. Effects of testosterone on sexual function in men: results of a meta-analysis. Clin Endocrinol (Oxf) 2005;63:381-94. [Crossref] [PubMed]
- Snyder PJ, Bhasin S, Cunningham GR, et al. Effects of Testosterone Treatment in Older Men. N Engl J Med 2016;374:611-24. [Crossref] [PubMed]
- Moss HB, Panzak GL, Tarter RE. Sexual functioning of male anabolic steroid abusers. Arch Sex Behav 1993;22:1-12. [Crossref] [PubMed]
- Rosen RC, Cappelleri JC, Smith MD, et al. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res 1999;11:319-26. [Crossref] [PubMed]
- Finkelstein JS, Yu EW, Burnett-Bowie SA. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med 2013;369:2457. [Crossref] [PubMed]
- Rasmussen JJ, Selmer C, Ostergren PB, et al. Former Abusers of Anabolic Androgenic Steroids Exhibit Decreased Testosterone Levels and Hypogonadal Symptoms Years after Cessation: A Case-Control Study. PLoS One 2016;11. [Crossref] [PubMed]
- Kanayama G, Hudson JI, DeLuca J, et al. Prolonged hypogonadism in males following withdrawal from anabolic-androgenic steroids: an under-recognized problem. Addiction 2015;110:823-31. [Crossref] [PubMed]


