Safety and efficacy of daily Revactin® in men with erectile dysfunction: a 3-month pilot study
Introduction
Erectile dysfunction (ED) is considered an aging related condition such that the older a man gets, the probability of developing some form of ED increases (1). The presumptive functional target tissue within the penis that is primarily affected by the aging process is the corporal smooth muscle cell (CSMC) (2-5) and it is the aging related apoptosis and loss of these CSMC (6,7) that leads to what is termed aging related erectile dysfunction (ARED) (8). Studies in animals have shown that when this aging related apoptotic activity begins within the CSMC, the CSMC itself begins to counteract this apoptotic process by forming nitric oxide (NO) from the inducible nitric oxide synthase (iNOS) enzyme (7).
iNOS is one of the three isoforms of the enzyme that can make NO (9) and it is normally absent in normal CSMC. However, when the aging related apoptotic activity begins within the CSMC, iNOS production gets upregulated which translates into the ability to markedly increase intracellular NO. This newly formed intracellular NO is capable of either entering the mitochondria of the CSMC to help quell the oxidative stress inherent in the aging CSMC and/or stimulating the endogenous cytoplasmic soluble guanylate cyclase enzyme to form cGMP from GTP (10). Studies in aging animals have shown that when this iNOS related NO-cGMP pathway in the aging CSMC is upregulated as has been shown with the use of phosphodiesterase inhibitors (PDE), the apoptotic process within these CSMC can be halted or even reversed as evident by the formation of new CSMC with this translating into a decrease in cavernosal veno-occlusive dysfunction (CVOD) as measured by cavernosometry and a resultant increase in erectile function (EF) (11).
Therefore, the theoretical goal of any therapy that attempts to pre-emptively counteract or slow down the aging related apoptosis occurring within the aging CSMC is to both activate and upregulate the endogenous cellular iNOS-NO-cGMP pathway. Revactin® is a recently developed combination of four naturally occurring products that has been shown in an animal model of ARED to (I) increase production of iNOS, (II) increase CSMC content and (III) reverse CVOD (12). In addition to this in vivo data, Revactin® has been shown in a rat primary CSMC culture to stimulate the synthesis of iNOS, increase intracellular nitrite production and most importantly, to increase intracellular cGMP (13). Based on these in vitro and in vivo observations in animals, a small pilot study was conducted in adult men in order to determine primarily the safety of Revactin® and secondarily whether there was any early short term evidence of a potential efficacy of Revactin® on male sexual function.
Methods
Fifty-four middle aged men between the ages of 33 and 74 were recruited from a suburban urology clinic to participate in this safety trial which was approved by the Institutional Review Board of the Henry Mayo Hospital, Santa Clarita, CA (2010:HMNMH1012-1). After written informed consent was obtained, the men were asked to complete the International Index of Erectile Function (IIEF-15) questionnaire and were then given a bottle of Revactin® containing a 1-month supply of the capsules. Each capsule of Revactin® consisted of 125 mg each of ginger root, muira puama and Paullinia cupana as well as 400 mg of L-citrulline. The participants were requested to take two capsules of Revactin® orally twice a day for 3 months so the daily intake was 500 mg each of ginger, muira puama and Paullinia cupana and 1,600 mg of L-citrulline.
Those participants who had a diagnosis of ED were requested to refrain from taking any ED therapies during the 3-month study period. They were to return to the clinic after 1 month (M1), 2 months (M2) and three months (M3) of Revactin® use and asked to report any side effects experienced the prior month as well as filling out another IIEF-15 questionnaire. Patients who continued their participation in the study were provided an additional 1-month supply of Revactin® at each follow-up monthly clinic visit.
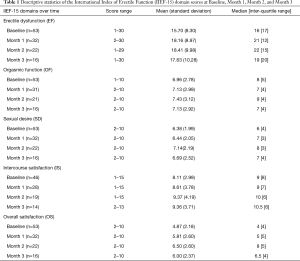
The IIEF-15 questionnaire is a highly validated multi-dimensional, self-administered instrument used in the assessment of treatment outcomes in clinical research pertaining to male sexual function (14). The IIEF-15 survey is composed of 15 questions categorized into 5 domains that include: EF, orgasmic function (OF), sexual desire (SD), intercourse satisfaction (IS) and overall satisfaction (OS). The EF domain consists of Q1, 2, 3, 4, 5 and 15 (Score range is 0–30). The OF domain consists of Q9 and 10 (score range is 0–10). The SD domain includes Q11 and 12 (score range is 2–10). The IS domain consists of Q6, 7, and 8 (score range is 0–15). The OS domain includes Q13 and 14 (score range is 2–10). Additionally, severity of ED is categorized using the EFdomain score (IIEF-EF) of ≤10 as severe, 11 to 16 as moderate, and 17 to 25 as mild ED (15). An IIEF-EF score >25 is considered as no ED.
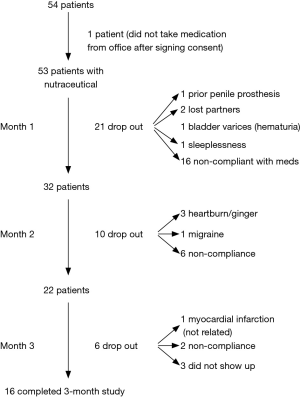
Figure 1 represents the flow of the patients in the study over the three-month period. Of the 54 patients initially enrolled in the study, 53 completed their baseline IIEF-15 question survey and were given a one month’s supply of Revactin®. Due to either non-compliance with the instruction (n=16) or other causes (n=5), there were 32 patients at month 1. At month 2, 10 patients dropped out (n=6 due to non-compliance, and 4 due to other reasons) and 22 patients remained in the study. At month 3, 6 additional patients had dropped out of the study leaving 16 patients who completed the entire 3 months of the study (Figure 1).
Data analysis
Data were analyzed using descriptive statistics to summarize the data and examine the distribution of the score of the domains. Patient’s age was presented as mean, standard deviation (SD) and range. IIEF-15 domain scores were expressed as means, SD, medians, and inter-quartile ranges (IQR) at baseline, M1, M2, and M3.
Based on the skewed distribution of the domain scores, median domain scores for EF, OF, SD, IS, and OS were used to analyze the change in domain scores over time. The change in domain scores at M1, M2, M3 compared to baseline was analyzed using the non-parametric Wilcoxon test for paired data. In order to test the trend in the domains’ scores, the Friedman rank chi square test was used.
Fisher exact test was used to analyze the statistically significant difference in the proportion of patients with IIEF-EF score >25 at M1, M2, and M3 relative to baseline (B). The decline in number patients over time was accounted for in determining significance in proportional percentage of patients with and without ED at baseline (B) and subsequent monthly intervals.
In addition, the same statistical analysis tests were utilized for the subset of the patients who had complete data at the end of month 3 (n=16).
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS©, version 22; SPSS Inc., IBM Corp., Armonk, NY, USA). A P value<0.05 was considered statistically significant.
Results
Fifty-four adult men were initially enrolled in the study with a mean age of 57.8±10.7 (range, 33–77) years. Table 1 shows the descriptive statistics of the IIEF-domain scores at B, M1, M2, and M3. It is evident that there is an increase in median domain score values for EF, OF, SD, IS, and OS over 3 months compared to baseline median scores.
Full table
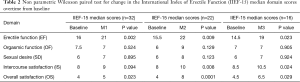
However, non-parametric Wilcoxon paired test showed statistical significance in only the EF, IS, and OS median domain scores at M1, M2, and M3 relative to baseline score (Table 2). For the EF domain, the median scores were: M1 =21, M2 =22, M3 =19 relative to B of 16, 15.5, and 14.5 respectively (P<0.05). The median scores for the IS domain were: M1 =9, M2 =10, M3 =10.5 relative to a B score of 8, 8 and 8.5, respectively (P<0.05 only for M2 and M3 relative to baseline. The median OS domain scores were: M1 =5, M2 =8, M3 =6.5 relative to B of 4, 4, and 4.5 respectively (P<0.05 only for M2, M3 relative to baseline). Of note, despite overall increase in domain score over time compared to baseline, there is a decrease in the EF, IS, and OS median score from M2 to M3 (Table 2).
Full table
Trend analysis using Friedman test indicated a significant improved trend in EF domain over time from B to M3 (mean rank =1.69, 2.75, 2.75 and 2.81, respectively; chi square =9.4, P=0.03), OS domains (mean rank = 1.56, 2.97, 2.88, 2.59, respectively; chi square =17.3, P=0.001) and IS domains (mean rank = 1.73, 2.58, 2.77, 2.92, respectively; chi square = 8.3, P=0.04).
Subset analysis of patients with complete data (n=16) using non-parametric Wilcoxon paired test indicated statistically significant difference in the EF and OS domain scores in M1, M2 and M3 relative to B (P<0.05). For the IS domain score, statistical significance was found in only M2 and M3 compared to B (P<0.05). Trend analysis using Friedman test indicated a significant improved trend in EF, OS, and IS domains over time from B to M3 (P<0.05) (data not shown).
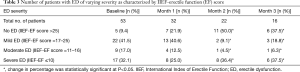
Patients’ severity of ED was classified into no dysfunction, mild, moderate, and severe dysfunction at baseline, M1, M2, and M3 according to their IIEF-EF scores. The change in the percentage of patients without ED (IIEF-EF score >25) and with ED (IIEF-EF score ≤25 which includes mild, moderate, and severe ED) at M1, M2, and M3 relative to baseline is presented in Table 3 and shows that there is an overall increase in the percent of patients with non-ED over the 3-month period (P<0.05). Conversely, there is a decrease in percentage of patients with ED over M1 to M3 relative to baseline (Table 3). The increase in percentage of patients with no ED was noted over M1 and M2 with 22.6% and 50.0% respectively (P<0.05). There is a decrease in the percentage of patients without ED between M2 and M3 at 50.0% and 37.5% (P<0.05) (Table 3).
Full table
Subset analysis of patients with complete data (n=16) indicated there was a change in the percent of patients with ED over time. Of those who had ED at B (IIEF-EF score <25), improvement (an IIEF-EF score >25) was seen in 14.3% at M1, 35.7% at M2 and 28.6% at M3 but it was not statistically significant (P>0.05).
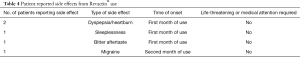
A total of 5 patients reported side effects between one to three months of Revactin® use (Table 4). Side effects included dyspepsia/heartburn, bitter after taste, sleeplessness, and migraine. Patients did not seek medical attention for the side effects nor did they or the investigators feel the side effects were life-threatening.
Full table
Discussion
This short-term pilot study, conducted primarily to answer the question whether the combination of the four naturally occurring constituents comprising Revactin® was safe when ingested orally, showed that it was. The nutraceutical combination had minimal side effects and was acceptable to patients both with and without pre-existing ED.
Since the original premise for the development of Revactin®, which consists of a combination of ginger, muira puama, Paullinia cupana and L-citrulline, was to enhance the NO-cGMP pathway via the iNOS enzyme within the cytoplasm of the CSMC, an attempt was also made in this trial to determine in this initial cohort of middle aged patients whether up-regulation of this iNOS directed NO-cGMP pathway, until now recognized within the penis solely as an anti-fibrotic anti-apoptotic pathway (7), has any salutatory effect on sexual function. Indeed, it was determined that within the first month of taking Revactin®, approximately 50% of men who were compliant with the treatment, reported a significant improvement in EF as measured by the EF domain of the IIEF-15. This approximate 50% significantly better response rate over baseline also persisted throughout the final 2 months of the trial. The early improvement in EF in these patients taking Revactin® came as a surprise because it was assumed that the up-regulation of the iNOS enzyme and its potential downstream stimulation of the NO-cGMP pathway (via iNOS) within the cytoplasm of the CSMC would simply result in an anti-fibrotic and anti-apoptotic response by the CSMC. Although it is unknown how long such an anti-fibrotic and anti-apoptotic response would take to become clinically evident, i.e., by improving one’s EF, it seems almost physiologically unimaginable for such histological responses to transpire within the short time period of 1 to 3 months. The mechanism(s) responsible for this clinical observation of an early pro-erectile response to Revactin® within this short time period remains to be determined.
It is now generally accepted that the first and earliest change in a man’s EF is the increase in his refractory period that begins to occur and becomes recognizable early in his life. This physiological phenomenon was made evident by the response of men with normal EF who were given sildenafil and reported that although their EF remained normal, they noticed a significant decrease in their refractory period (16,17). Indeed, when men under 40 years of age with a complaint of ED are evaluated to determine the cause of their ED, the most common vascular etiology found is not arterial but CVOD (18,19) which occurs as the result of either a dysfunction of or a decrease in the total CSMC mass. The relatively common prevalence of CVOD as a cause of ED in this under 40 year of age group could explain the findings from the MMAS that approximately 40% of the men during their 40’s will have some form of ED (1). Obviously, the condition causing ED in many of these men in their 40’s must have started prior to them entering this 5th decade of their life.
If it is simply the aging process that is the primary culprit in the development of ED in young men resulting in a dysfunctional CSMC mass, what could be the mechanisms responsible for this change? It is known that aging is a multifactorial process that is genetically determined and influenced epigenetically by the environment (20). For those who adhere to the oxidative stress theory of aging, the process most likely begins around 28 years of age in men (21). Specifically, reactive oxygen species (ROS) causes damage to the mitochondria which affects cellular function leading ultimately to dysfunction and apoptosis of the cell (22). The initiation of such oxidative stress in men in their late 20’s could explain the finding of CVOD in men under 40 years of age (18,19). Our theory is that when such aging related changes begin to occur within the CSMC, the cell itself attempts to combat this by initiating the endogenous production of NO via iNOS (7) in order to accomplish two goals that together combat the increased stress within the aging cell. First of all, NO from iNOS is known to act directly on the mitochondria to quench ROS (23). Secondly, NO is well known to induce the production of cGMP within the cytoplasm. This production of cGMP by NO from iNOS is believed to be the catalyst responsible for initiating the reparative mechanisms within the CSMC which have been impacted by the aging process.
It is also known that in the penis an increase in intracellular cGMP, whether it is due to the release of NO emanating extracellularly from the cavernosal nerves to stimulate guanylate cyclase to form cGMP or due to a decrease in the degradation of cGMP once formed within the cytoplasm of the CSMC as is seen with the oral PDE5 inhibitors, can enhance one’s EF. The observation of an improved EF in those men who took Revactin® as early as the first month that seems to persist for all three months in those patients who continued taking the Revactin® suggests that the putative increase in production of intracellular cGMP that is known to occur with Revactin®, initially designed to work as a long term strategy to combat or halt the histological changes ongoing in the CSMC, may end up having an early beneficial effect on the EF of some patients. The explanation for this early pro-erectile response may reside in the in vitro experimental finding of a rapid rise in intracellular cGMP within the CSMC as early as 4 hours after exposure to Revactin® (personal data).
Of the participants in this study who had a history of impotence and started taking Revactin® in this 3-month clinical trial, the monthly drop out rate was high. This high drop-out was primarily due to the fact that the impaneled patients were told to withhold their ongoing pro-erectogenic therapy during this time period of the trial; however, many of them (n=24) were not compliant with this request because they wanted to continue being sexually active and began using their PDE 5 inhibitors. In fact, 17 of these 24 non-compliant patients began taking the PDE5 inhibitors within the first month of starting Revactin® underlying the importance of ongoing sexual activity in this age group. With respect to adverse events, there was one patient who had a myocardial infarction (MI) during the third month of the study and assessment by his cardiologist determined that the MI was not due to the ingestion of Revactin®. The most common complaint related to Revactin® was that of a ginger aftertaste following ingestion of Revactin® in three patients.
The results of this short-term 3-month pilot study demonstrate that Revactin® appears to be an extremely safe product for human use. This is not surprising since all four constituents of Revactin® have an excellent safety profile when taken individually (24,25).
The product was originally conceived to stimulate the endogenous production of iNOS within the CSMC in the hope that this would increase intracellular NO which would not only quench ROS within the mitochondria but also increase intracellular cGMP to act as an anti-fibrotic and anti-apoptotic stimulus. This dual targeted approach with Revactin® had as its design the ability to slow down, retard or even reverse the ongoing pro-apoptotic and pro-fibrotic changes occurring within the aging CSMC. The one surprise of this trial was that some of the men reported an early improvement in EF which could possibly be due to the increase in intracellular cGMP seen in vitro when the CSMC are exposed to Revactin®. Further studies with larger cohorts of patients are needed to determine whether longer term treatment with Revactin® will also result in these observed effects.
Conclusions
Revactin® is a product comprised of four natural ingredients that has been shown to stimulate endogenous iNOS production and upregulate of the NO-cGMP pathway in the CSMC. Although scientifically unproven as of this date, the NO thus formed from iNOS may also be working as an anti-oxidative molecule combating the increase in ROS associated with aging in the CSMC. Further studies are needed to determine whether longer term treatment with Revactin® particularly in men less than 40 years of age will result in halting or even reversing the histological and functional changes within the cavernosa that is known to occur with aging.
Acknowledgments
Funding: This work supported in part by grants UL1TR001881, U54MD007598 and S21 MD000103 from the National Institutes of Health.
Footnote
Conflicts of Interest: J Rajfer is a stockholder in KLRM, LLC which supplied Revactin for the study and has a US patent pending on Revactin®. The other authors have no conflicts of interest to declare.
Ethical Statement: The research was approved by the Institutional Review Board of the Henry Mayo Hospital, Santa Clarita, CA (2010:HMNMH1012-1) and written informed consent was obtained.
References
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Lue TF, Takamura T, Schmidt RA, et al. Hemodynamics of erection in the monkey. J Urol 1983;130:1237-41. [Crossref] [PubMed]
- Lue TF, Tanagho EA. Physiology of erection and pharmacological management of impotence. J Urol 1987;137:829-36. [Crossref] [PubMed]
- Fournier GR Jr, Juenemann KP, Lue TF, et al. Mechanisms of venous occlusion during canine penile erection: an anatomic demonstration. J Urol 1987;137:163-7. [Crossref] [PubMed]
- Wespes E. Smooth muscle pathology and erectile dysfunction. Int J Impot Res 2002;14 Suppl 1:S17-21. [Crossref] [PubMed]
- Jevtich MJ, Khawand NY, Vidic B. Clinical significance of ultrastructural findings in the corpora cavernosa of normal and impotent men. J Urol 1990;143:289-93. [Crossref] [PubMed]
- Ferrini M, Magee TR, Vernet D, et al. Aging-related expression of inducible nitric oxide synthase and markers of tissue damage in the rat penis. Biol Reprod 2001;64:974-82. [Crossref] [PubMed]
- Sexual inadequacy in the Aging Male. In: Masters WH, Johnson VE. editors. Human Sexual Inadequacy, Chapter 12. Boston: Little, Brown & Co.; 1970:316-22.
- Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 1992;256:225-8. [Crossref] [PubMed]
- Nesbitt JA 3rd, Anderson WB, Miller Z, et al. Guanylate cyclase and cyclic guanosine 3':5'-monophosphate phosphodiesterase activities and cyclic guanosine 3':5'-monophosphate levels in normal and transformed fibroblasts in culture. J Biol Chem 1976;251:2344-52. [PubMed]
- Ferrini MG, Kovanecz I, Sanchez S, et al. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod 2007;76:915-23. [Crossref] [PubMed]
- Ferrini MG, Hlaing SM, Chan A, et al. Treatment with a combination of ginger, L-citrulline, muira puama and Paullinia cupana can reverse the progression of corporal smooth muscle loss, fibrosis and veno-occlusive dysfunction in the aging rat. Andrology (Los Angel) 2015;4:132. [Crossref] [PubMed]
- Ferrini MG, Garcia E, Abraham A, et al. Effect of ginger, Paullinia cupana, muira puama and L-citrulline, singly or in combination, on modulation of the inducible nitric oxide NO-cGMP pathway in rat penile smooth muscle cells. Nitric Oxide 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Rosen RC, Riley A, Wagner G, et al. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology 1997;49:822-30. [Crossref] [PubMed]
- Cappelleri JC, Rosen RC, Smith MD, et al. Diagnostic evaluation of the erectile function domain of the International Index of Erectile Function. Urology 1999;54:346-51. [Crossref] [PubMed]
- Aversa A, Mazzilli F, Rossi T, et al. Effects of sildenafil (Viagra) administration on seminal parameters and post-ejaculatory refractory time in normal males. Hum Reprod 2000;15:131-4. [Crossref] [PubMed]
- Mondaini N, Ponchietti R, Muir GH, et al. Sildenafil does not improve sexual function in men without erectile dysfunction but does reduce the postorgasmic refractory time. Int J Impot Res 2003;15:225-8. [Crossref] [PubMed]
- Donatucci CF, Lue TF. Erectile dysfunction in men under 40: etiology and treatment choice. Int J Impot Res 1993;5:97-103. [PubMed]
- Rajfer J, Valeriano J, Sinow R. Early onset erectile dysfunction is usually not associated with abnormal cavernosal arterial Inflow. Int J Impot Res 2013;25:217-20. [Crossref] [PubMed]
- Kirkwood TB. Understanding the odd science of aging. Cell 2005;120:437-47. [Crossref] [PubMed]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol 1956;11:298-300. [Crossref] [PubMed]
- Yamaguchi R, Perkins G. Dynamics of mitochondrial structure during apoptosis and the enigma of Opa1. Biochim Biophys Acta 2009;1787:963-72. [Crossref] [PubMed]
- Cui H, Kong Y, Zhang H. Oxidative stress, mitochondrial dysfunction, and aging. J Signal Transduct 2012;2012:646354. [PubMed]
- Gruenwald J, Brendler T, Jaenicke C. editors. PDR for Herbal Medicines. 2nd ed. Montvale, New Jersey: Medical Economics Co.; 2000:531-2.
- Oliveira CH, Moraes ME, Moraes MO, et al. Clinical toxicology study of an herbal medicinal extract of Paullinia cupana, Trichilia catigua, Ptychopetalum olacoides and Zingiber officinale (Catuama) in healthy volunteers. Phytother Res 2005;19:54-7. [Crossref] [PubMed]