MRI findings guiding selection of active surveillance for prostate cancer: a review of emerging evidence
Introduction
In 2017 there will be an estimated 161,000 new cases of prostate cancer (PCa) in the United States. This represents nearly 20% of new cancer diagnoses, the most common among non-cutaneous neoplasms in men (1). Following the introduction of prostate specific antigen (PSA) screening in the 1990s, the incidence of PCa has steadily risen while cancer-specific mortality has declined considerably (2). The former has been attributed to increased detection of low-risk, localized disease which may not pose a significant threat to a patient’s longevity. Consequently, the appropriateness of aggressive intervention in the setting of increased detection has been brought under question (2,3). For those individuals with low-risk, localized disease, a less invasive management approach may be more appropriate to avoid the potential negative impact the effects of medications, radiation therapy, and surgery may have on a patient’s quality of life (4,5).
Close monitoring of low-risk patients by active surveillance (AS) includes serial serum PSA level assessments, digital rectal examinations, and transrectal ultrasound (TRUS) biopsies, and more recently, multiparametric magnetic resonance imaging (mpMRI). The decision to place a patient on AS, and ultimately determining when to depart from AS, is challenging due to limitations in both disease monitoring and reliable risk stratification criteria (6-8). It is estimated that as many as 1 in 4 patients may be improperly placed on AS based on data supporting the undergrading and underassessment of tumor volume on systematic prostate biopsy for diagnosis and risk-stratification (9,10). Indeed, nearly half of men initially placed on AS will at some point have pathologic progression and require oncologic intervention (11). Therefore, it is incumbent upon clinicians to improve risk-stratification and cancer surveillance protocols to minimize patient oncologic risk.
Over the past decade, mpMRI has emerged as a reliable diagnostic and monitoring adjunct for men on AS (12-14). This approach affords better visualization of the prostate and surrounding structures than traditional TRUS imaging used primarily for systematic tissue sampling at the time of biopsy (15). Specifically the improvements in magnetic resonance imaging technology and optimization of functional imaging sequence techniques such as diffusion weighted imaging (DWI) and volumetric estimation algorithms have permitted superior tumor characterization (16-18).
While variable, mpMRI sequences typically consist of high-resolution T2-weighted imaging, diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) mapping, and dynamic contrast-enhanced imaging (DCEI) (15,17). T2-weighted imaging reflects the generalized water content of tissues which is applicable since normal benign prostatic tissue is water-rich, cancerous tissue is water-poor, and the prostatic capsule is commonly well-defined (15). DWI quantifies the degree of water diffusing through tissues, and similarly aids in differentiating benign tissue from more densely packed malignant prostate tissue (15). The ADC, a measurement of the impedance of water molecule diffusion based on DWI sequences, further distinguishes benign from malignant prostatic tissue with a quantified value that can be mapped as a surrogate image (15). DCEI evaluates tissue vasculature and differentiates benign from malignant tissue based on the altered patterns of angiogenesis observed in cancerous lesions (19). We therefore performed a review of recently published literature to characterize the emerging evidence in support of using mpMRI to properly select patients for AS.
Methods
An English literature search was conducted on PubMed for original investigations on localized PCa, AS, and MR imaging. All articles published within the past few years (January 1st, 2014 through November 28th, 2017) were considered. Our Boolean criteria included the following terms: “PCa”, “AS”, “imaging”, “MRI”, “mpMRI”, “prospective”, “retrospective”, and “comparative”. Our search excluded publication types including commentaries, editorials, guideline statements, review articles, or interviews. We identified 71 original studies. Among these, 52 publications met our final inclusion criteria. A total of 28 studies evaluated the usefulness of mpMRI for detection of clinically significant prostate cancer (csPCa) in the setting of AS. We identified 18 studies examining the role of mpMRI specifically in guiding prostate biopsy in the context of AS management protocols. Six studies considered using serial mpMRIs in place of routine biopsy to survey men on AS meeting certain risk criteria.
Review
Improved detection of significant PCa
We identified multiple studies that demonstrated the value of using mpMRI during the initial diagnostic workup to classify patients as AS-eligible better than traditional criteria alone without advanced imaging (14,20,21). Ouzzane et al. reviewed 281 patients who were initially deemed appropriate for AS based on clinical and biopsy results. They found 10% of the cohort was later reclassified as ineligible for AS following mpMRI and subsequent biopsy of clinically occult lesions found and sampled based on mpMRI results (20). Porpiglia et al. went one step further to suggest the use of mpMRI alone may reliably predict pathologically significant disease without confirmatory biopsy (14).
DWI sequencing, which affords calculation of the ADC values for different imaging voxels, gives providers an additional tool to evaluate suspicious prostatic lesions. In a retrospective study of 86 AS-eligible patients who eventually underwent radical prostatectomy, Henderson et al. demonstrated a low ADC, defined as lower than their single-institution median value, may independently predict time to undergoing curative intervention and/or detection of adverse histology (17). Morgan et al. compared interval suspicious lesion growth and ADC change in a cohort of 151 men on AS who underwent serial mpMRI over a median two-year interval. They found tumor growth was inversely correlated with change in the ADC, and therefore a significant decrease in ADC may be a sign of impending AS failure based on PCa progression (22).
As processing software continues to improve, there is emerging evidence to suggest even greater PCa detection sensitivity may be achieved with mpMRI. Sharif-Afshar et al. conducted a pilot trial comparing standard versus a novel high resolution DWI (HR-DWI) sequencing software in the evaluation of biopsy-confirmed PCa lesions. This technique uses smaller voxel size and also achieves a greater signal-to-noise ratio for greater spatial resolution. They found a 5-fold improvement in spatial resolution with a nearly 35% greater sensitivity in detecting biopsy-proven csPCa (23). Of note, use of 5-alpha-reductase inhibitors does not appear to alter the ability to detect cancerous tissue on prostate mpMRI (24).
It has been shown that automated calculations of lesion volume on mpMRI may correspond with PCa presence (17,25). Marin et al. found that using semi-automated sizing algorithms to measure tumor dimensions reliably correlates with actual tumor diameter on final pathology (25). Stensland et al. retrospectively evaluated 1,633 patients with available mpMRIs who underwent radical prostatectomy, and concluded tumor lesions <5 mm on mpMRI most likely represent clinically-insignificant disease on final pathology (26). However in a retrospective study of 118 patients, Dianat et al. observed 8.3% of men with mpMRI invisible tumors harbored csPCa (27). In a separate study of 298 patients by Park et al., 14% of AS-eligible patients without an identifiable lesion had their PCa upgraded on final pathology following radical prostatectomy, but just one was found to have a positive surgical margin and no patients had greater than either form of Gleason 7 disease (28). Sahibzada et al. reached a similar conclusion in their retrospective cross-sectional validation study of 100 patients, and suggest mpMRI may have greater reliability in the post-TRUS biopsy surveillance setting (29).
Incorporation of PIRADS into AS protocols
Over the past ten years, the Prostate Imaging Reporting and Data System (PIRADS) has become an increasingly useful tool for evaluating suspicious prostatic neoplasms (30-32). Suspicious lesions are rated on a five-point Likert scale with a score of 5 being the most concerning for a malignant tumor (33,34). Venderink et al. retrospectively evaluated 1,000 patients on AS, and when compared to PSA density, they found a PIRADS score of ≥3 better predicts significant PCa on repeat biopsy (31). In a study by Grey et al. that retrospectively reviewed 201 men on AS who underwent both mpMRI and prostate biopsy, they similarly concluded that those with a PIRADS score of <3 could safely forgo a subsequent repeat biopsy. However, 2.3% of the men with PIRADS <3 lesion(s) still harbored csPCa (Gleason pattern 4 or ≥6 mm cancer core length), bringing into question whether it is acceptable to potentially ‘miss’ a small number of presumably indolent cancers in the population and rely on other parameters in the surveillance protocol to pick these up at a later time point (30). A different study by Porpiglia et al. retrospectively analyzed 126 patients who underwent radical prostatectomy, and found incorporating PIRADS into the existing Epstein and/or Prostate Cancer Research International Active Surveillance (PRIAS) criteria would have increased csPCa detection by 5% and 7%, respectively (35). We identified three additional original investigations that compared a widely-accepted AS protocol with a predictive nomogram incorporating PIRADS, and they also demonstrated a significant improvement in risk-stratification (36-38).
More recently, a proposed PIRADS version 2 (PIRADSv2) was developed to capture the increasingly complex MRI characterization of a single lesion with the application of multiple sequences such as DWI and DCEI for interpretation (32,34,39). Studies comparing it to the original PIRADS algorithm are underway in the setting of AS. Yim et al. recently found that using the PIRADSv2 scoring system may reliably classify suspicious lesions as clinically-insignificant PCa, therefore permitting safe selection for AS (40). Lim et al. found patients with PIRADSv2 scores ≥3 on mpMRI with a prior TRUS biopsy of 3+4=7 PCa have a higher chance of pathological upgrading at the time of radical prostatectomy. Therefore, the PIRADSv2 system could predict AS failure in this select patient population (41).
Alternatively, Nougaret et al. suggest PCa may be overlooked as often as 5% of the time when using PIRADSv2 scores of ≥3 as a threshold cutoff (32). In addition, there is concern that central zone (CZ) lesions may not be accurately characterized when using the PIRADS algorithm. In a review of 73 patients who underwent MRI-fusion biopsy, only two (7.7%) of 26 CZ lesions that were designated PIRADS ≥3 actually contained clinically-significant disease (42). Since there is evidence to suggest PCa originating from the CZ can be more aggressive, relying on the PIRADS score alone may overcall lesions and lead to unnecessary confirmatory biopsy sessions (43,44).
Prostate biopsy in the era of MRI-US fusion
MRI-US fusion technology utilizes the improved visualization of anatomy afforded by MRI to perform targeted biopsy of suspicious prostatic lesions (45,46). Multiple investigations suggest performing MRI-US fusion targeted biopsy may be superior to standard systematic template TRUS-guided tissue sampling to detect new csPCa for men on AS (47-52). Siddiqui et al. suggest performing MRI-US fusion targeted biopsy may reduce the number of insignificant PCa diagnoses, thus sparing patients from unnecessary biopsies (53). We identified two studies showing improved csPCa detection when utilizing mpMRI-US fusion technology for transperineal prostate biopsy (54,55). Penzkofer et al. found this approach to be of particular benefit in the setting of anteriorly located tumors when utilizing an in-bore MRI-guided approach (55). Felker et al. also achieved satisfactory cancer detection rates when performing in-bore MRI-guided biopsy via transrectal approach (56).
However, there is conflicting data over whether performing MRI targeted biopsy in isolation, abandoning the standard twelve core template sampling approach, is a safe monitoring strategy for men on AS (48-50,57). Nassiri et al. retrospectively evaluated 250 patients undergoing MRI-fusion biopsy and observed that 32 of 33 cases with pathological upgrading were a result of positive MRI-targeted cores (50). Da Rosa et al. reported a 100% negative predictive value for detecting a Gleason score 6 to 7 upgrade when using MRI-fusion technology in their cohort of 72 men on AS (49). Conversely, Marliere et al. observed that standard template biopsy at the time of MRI-targeted sampling still has utility for the detection of new and significant cancer foci. In their cohort of 41 men on AS undergoing combined standard template and MRI-targeted confirmatory biopsy, pathological upstaging was attributable to standard template core tissue more than half of the time (57).
Of all of the prostate biopsy modalities, there is strong evidence in support of saturation biopsy (24 or 30 cores templated sampling) as the technique with greatest sensitivity for detecting significant PCa in the initial AS period (58-60). However, it subjects patients to the burden of acquiring a significant amount of tissue, and may not reliably predict the location or extent of disease on pathology following radical prostatectomy (61). Alternatively, Galosi et al. proposed a hybrid approach to saturation biopsy called ‘cognitive zonal fusion biopsy’ (60). For this, patients undergo mpMRI prior to fusion biopsy. If during the biopsy there is a discrepancy between what was found on MRI and what is seen on US, several cores are obtained from the MRI region of interest. This approach reliably detected csPCa in their prospective study of 58 men who were either biopsy naïve, had a prior negative biopsy, or were on AS (60). In a study of 48 men on AS with prior negative TRUS biopsy, Lai et al. also achieved satisfactory PCa detection using this technique (62).
Could mpMRI allow safe surveillance without repeat tissue biopsy?
There is emerging evidence to suggest that serial mpMRIs alone may be sufficient to monitor men on AS, and the time interval for repeat confirmatory biopsy could be prolonged (7,63-67). Both Walton Diaz et al. and Felker et al. reported that stable mpMRI findings reliably correlated with Gleason score stability in their cohorts of men on AS meeting standard inclusion criteria (63,65). Frye et al. also appreciated reliable detection of cancer progression using mpMRI alone in a retrospective review of 162 men (7).
Several recent investigations evaluated the usefulness of predicting pathologic progression of an index lesion based on size criteria. In a review of more than 150 men on AS, those with index lesions 7mm or less were found to have no change in either size or pathologic characteristics during a two-year follow-up period (66). Thus, men otherwise meeting AS criteria could potentially defer PCa surveillance for up to a two-year interval of time without compromising care. Based on serial mpMRIs on a cohort of men who underwent regularly scheduled biopsies, Siddiqui et al. developed a predictive nomogram for pathological progression. Their nomogram would have theoretically avoided repeat biopsy in 68% of men in their study (64). Lai et al. developed a similar predictive nomogram for low-risk men on AS with certain mpMRI criteria but also incorporating clinical parameters for each case (38).
Discussion
AS for low-risk, localized PCa has become a widely adopted strategy, and offers adequate disease control while optimizing quality of life. Nonetheless, this strategy remains a challenge due to disease variability, inconsistencies on optimal surveillance regimens and a wide variety of available diagnostic tests. A majority of AS protocols use serial digital rectal examinations, serum PSA levels, and TRUS-guided systematic biopsies to monitor patients (3-5). However, since nearly half of all patients on AS have been shown to ultimately require some form of intervention, improved surveillance strategies are needed to monitor for disease progression in an effective and efficient manner (6,7,9-11,68).
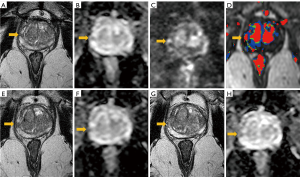
Novel MRI sequencing techniques and improved technology has allowed for meticulous characterization of suspicious intraprostatic lesions (Figure 1). Recent evidence suggests that using mpMRI to characterize suspicious foci within the prostate allows for better detection than prior imaging techniques and systematic sampling alone, and may allow for safe AS while potentially decreasing the frequency of invasive biopsy sessions (14,16-18,20,21). Furthermore, mpMRI may even detect more aggressive disease not found on the initial standard template biopsy, and, therefore, may keep the patient from being inappropriately placed on AS (20). For these reasons, there has been a push towards formally incorporating mpMRI findings into existing AS criteria such as the Epstein criteria and PRIAS (35-38).
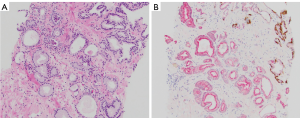
When performing a prostate biopsy is warranted, novel MRI fusion technology permits targeted tissue sampling with unparalleled accuracy (Figure 2) (48-51). Compared to saturation biopsies, recent evidence suggests that MRI-targeted biopsies may be just as reliable for csPCa detection with improved efficiency (58-60,69). This would spare patients, urologists and pathologists the task of acquiring and interpreting numerous cores obtained from a saturation biopsy approach and may decrease the frequency of finding insignificant disease (59). Furthermore, we identified two studies suggesting that in-bore MRI-guided biopsy may offer an even greater detection rate (55,56). Conversely, other evidence suggests MRI-US fusion alone may not be adequate in the initial diagnostic setting, and standard template biopsy may be equally diagnostic in biopsy naïve men (57). Several other studies suggest that men with small enough index lesions could avoid repeat biopsy altogether, and instead be followed by serial mpMRIs with equivocal detection and omission rates (7,64,66).
Conclusions
In the era of ever increasing use of AS for men with low-risk PCa, improved strategies for proper stratification are needed to balance overtreatment with underassessment of true risk. mpMRI has dramatically enhanced the detection of clinically-significant PCa, and may permit less-invasive surveillance strategies compared to currently accepted protocols. Further investigation is warranted to determine the most appropriate utilization of mpMRI in the setting of serial imaging and to also identify to what extent targeted versus templated systematic prostate biopsy should be performed.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Etzioni R, Gulati R, Cooperberg MR, et al. Limitations of basing screening policies on screening trials: The US Preventive Services Task Force and Prostate Cancer Screening. Med Care 2013;51:295-300. [Crossref] [PubMed]
- Eggener SE, Badani K, Barocas DA, et al. Gleason 6 Prostate Cancer: Translating Biology into Population Health. J Urol 2015;194:626-34. [Crossref] [PubMed]
- Ritch CR, Graves AJ, Keegan KA, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol 2015;193:801-6. [Crossref] [PubMed]
- Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005;293:2095-101. [Crossref] [PubMed]
- Leyh-Bannurah SR, Abou-Haidar H, Dell'Oglio P, et al. Primary Gleason pattern upgrading in contemporary patients with D'Amico low-risk prostate cancer: implications for future biomarkers and imaging modalities. BJU Int 2017;119:692-9. [Crossref] [PubMed]
- Frye TP, George AK, Kilchevsky A, et al. Magnetic Resonance Imaging-Transrectal Ultrasound Guided Fusion Biopsy to Detect Progression in Patients with Existing Lesions on Active Surveillance for Low and Intermediate Risk Prostate Cancer. J Urol 2017;197:640-6. [Crossref] [PubMed]
- Glaser ZA, Gordetsky JB, Porter KK, et al. Prostate Cancer Imaging and Biomarkers Guiding Safe Selection of Active Surveillance. Front Oncol 2017;7:256. [Crossref] [PubMed]
- Berglund RK, Masterson TA, Vora KC, et al. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol 2008;180:1964-7; discussion 1967-8.
- Freedland SJ, Kane CJ, Amling CL, et al. Upgrading and downgrading of prostate needle biopsy specimens: risk factors and clinical implications. Urology 2007;69:495-9. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Oberlin DT, Casalino DD, Miller FH, et al. Dramatic increase in the utilization of multiparametric magnetic resonance imaging for detection and management of prostate cancer. Abdom Radiol (NY) 2017;42:1255-8. [Crossref] [PubMed]
- Almeida GL, Petralia G, Ferro M, et al. Role of Multi-Parametric Magnetic Resonance Image and PIRADS Score in Patients with Prostate Cancer Eligible for Active Surveillance According PRIAS Criteria. Urol Int 2016;96:459-69. [Crossref] [PubMed]
- Porpiglia F, Cantiello F, De Luca S, et al. In-parallel comparative evaluation between multiparametric magnetic resonance imaging, prostate cancer antigen 3 and the prostate health index in predicting pathologically confirmed significant prostate cancer in men eligible for active surveillance. BJU Int 2016;118:527-34. [Crossref] [PubMed]
- Yoo S, Kim JK, Jeong IG. Multiparametric magnetic resonance imaging for prostate cancer: A review and update for urologists. Korean J Urol 2015;56:487-97. [Crossref] [PubMed]
- Salami SS, Ben-Levi E, Yaskiv O, et al. Risk stratification of prostate cancer utilizing apparent diffusion coefficient value and lesion volume on multiparametric MRI. J Magn Reson Imaging 2017;45:610-6. [Crossref] [PubMed]
- Henderson DR, de Souza NM, Thomas K, et al. Nine-year Follow-up for a Study of Diffusion-weighted Magnetic Resonance Imaging in a Prospective Prostate Cancer Active Surveillance Cohort. Eur Urol 2016;69:1028-33. [Crossref] [PubMed]
- Kim TH, Jeong JY, Lee SW, et al. Diffusion-weighted magnetic resonance imaging for prediction of insignificant prostate cancer in potential candidates for active surveillance. Eur Radiol 2015;25:1786-92. [Crossref] [PubMed]
- Buckley DL, Roberts C, Parker GJ, et al. Prostate cancer: evaluation of vascular characteristics with dynamic contrast-enhanced T1-weighted MR imaging--initial experience. Radiology 2004;233:709-15. [Crossref] [PubMed]
- Ouzzane A, Renard-Penna R, Marliere F, et al. Magnetic Resonance Imaging Targeted Biopsy Improves Selection of Patients Considered for Active Surveillance for Clinically Low Risk Prostate Cancer Based on Systematic Biopsies. J Urol 2015;194:350-6. [Crossref] [PubMed]
- Radtke JP, Kuru TH, Bonekamp D, et al. Further reduction of disqualification rates by additional MRI-targeted biopsy with transperineal saturation biopsy compared with standard 12-core systematic biopsies for the selection of prostate cancer patients for active surveillance. Prostate Cancer Prostatic Dis 2016;19:283-91. [Crossref] [PubMed]
- Morgan VA, Parker C, MacDonald A, et al. Monitoring Tumor Volume in Patients With Prostate Cancer Undergoing Active Surveillance: Is MRI Apparent Diffusion Coefficient Indicative of Tumor Growth? AJR Am J Roentgenol 2017;209:620-8. [Crossref] [PubMed]
- Sharif-Afshar AR, Nguyen C, Feng TS, et al. Prospective Pilot Trial to Evaluate a High Resolution Diffusion-Weighted MRI in Prostate Cancer Patients. EBioMedicine 2016;7:80-4. [Crossref] [PubMed]
- Giganti F, Gambarota G, Moore CM, et al. Prostate cancer detection using quantitative T2 and T2 -weighted imaging: The effects of 5-alpha-reductase inhibitors in men on active surveillance. J Magn Reson Imaging 2018;47:1646-53. [Crossref] [PubMed]
- Marin L, Ezziane M, Comperat E, et al. Comparison of semi-automated and manual methods to measure the volume of prostate cancer on magnetic resonance imaging. Diagn Interv Imaging 2017;98:423-8. [Crossref] [PubMed]
- Stensland KD, Coutinho K, Hobbs AR, et al. Are magnetic resonance imaging undetectable prostate tumours clinically significant? Results of histopathological analyses. Arab J Urol. 2016;14:256-61. [Crossref] [PubMed]
- Dianat SS, Carter HB, Pienta KJ, et al. Magnetic resonance-invisible versus magnetic resonance-visible prostate cancer in active surveillance: a preliminary report on disease outcomes. Urology 2015;85:147-53. [Crossref] [PubMed]
- Park BH, Jeon HG, Choo SH, et al. Role of multiparametric 3.0-Tesla magnetic resonance imaging in patients with prostate cancer eligible for active surveillance. BJU Int 2014;113:864-70. [Crossref] [PubMed]
- Sahibzada I, Batura D, Hellawell G. Validating multiparametric MRI for diagnosis and monitoring of prostate cancer in patients for active surveillance. Int Urol Nephrol 2016;48:529-33. [Crossref] [PubMed]
- Grey AD, Chana MS, Popert R, et al. Diagnostic accuracy of magnetic resonance imaging (MRI) prostate imaging reporting and data system (PI-RADS) scoring in a transperineal prostate biopsy setting. BJU Int 2015;115:728-35. [Crossref] [PubMed]
- Venderink W, van Luijtelaar A, Bomers JG, et al. Results of Targeted Biopsy in Men with Magnetic Resonance Imaging Lesions Classified Equivocal, Likely or Highly Likely to Be Clinically Significant Prostate Cancer. Eur Urol 2017. [Epub ahead of print].
- Nougaret S, Robertson N, Golia Pernicka J, et al. The performance of PI-RADSv2 and quantitative apparent diffusion coefficient for predicting confirmatory prostate biopsy findings in patients considered for active surveillance of prostate cancer. Abdom Radiol (NY) 2017;42:1968-74. [Crossref] [PubMed]
- Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol 2012;22:746-57. [Crossref] [PubMed]
- Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40. [Crossref] [PubMed]
- Porpiglia F, Cantiello F, De Luca S, et al. Multiparametric magnetic resonance imaging and active surveillance: How to better select insignificant prostate cancer? Int J Urol 2016;23:752-7. [Crossref] [PubMed]
- Hoeks CM, Somford DM, van Oort IM, et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance-guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: a prospective multicenter cohort study. Invest Radiol 2014;49:165-72. [Crossref] [PubMed]
- Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 2013;119:3359-66. [Crossref] [PubMed]
- Lai WS, Gordetsky JB, Thomas JV, et al. Factors predicting prostate cancer upgrading on magnetic resonance imaging-targeted biopsy in an active surveillance population. Cancer 2017;123:1941-8. [Crossref] [PubMed]
- Vargas HA, Hotker AM, Goldman DA, et al. Updated prostate imaging reporting and data system (PIRADS v2) recommendations for the detection of clinically significant prostate cancer using multiparametric MRI: critical evaluation using whole-mount pathology as standard of reference. Eur Radiol 2016;26:1606-12. [Crossref] [PubMed]
- Yim JH, Kim CK, Kim JH. Clinically insignificant prostate cancer suitable for active surveillance according to Prostate Cancer Research International: Active surveillance criteria: Utility of PI-RADS v2. J Magn Reson Imaging 2018;47:1072-9. [Crossref] [PubMed]
- Lim CS, McInnes MDF, Flood TA, et al. Prostate Imaging Reporting and Data System, Version 2, Assessment Categories and Pathologic Outcomes in Patients With Gleason Score 3 + 4 = 7 Prostate Cancer Diagnosed at Biopsy. AJR Am J Roentgenol 2017;208:1037-44. [Crossref] [PubMed]
- Tan WP, Mazzone A, Shors S, et al. Central zone lesions on magnetic resonance imaging: Should we be concerned? Urol Oncol 2017;35:31.e7-31.e12. [Crossref] [PubMed]
- Cohen RJ, Shannon BA, Phillips M, et al. Central zone carcinoma of the prostate gland: a distinct tumor type with poor prognostic features. J Urol 2008;179:1762-7; discussion 1767.
- Vargas HA, Akin O, Franiel T, et al. Normal central zone of the prostate and central zone involvement by prostate cancer: clinical and MR imaging implications. Radiology 2012;262:894-902. [Crossref] [PubMed]
- Rais-Bahrami S, Siddiqui MM, Turkbey B, et al. Utility of multiparametric magnetic resonance imaging suspicion levels for detecting prostate cancer. J Urol 2013;190:1721-7. [Crossref] [PubMed]
- Logan JK, Rais-Bahrami S, Turkbey B, et al. Current status of magnetic resonance imaging (MRI) and ultrasonography fusion software platforms for guidance of prostate biopsies. BJU Int 2014;114:641-52. [Crossref] [PubMed]
- Okoro C, George AK, Siddiqui MM, et al. Magnetic Resonance Imaging/Transrectal Ultrasonography Fusion Prostate Biopsy Significantly Outperforms Systematic 12-Core Biopsy for Prediction of Total Magnetic Resonance Imaging Tumor Volume in Active Surveillance Patients. J Endourol 2015;29:1115-21. [Crossref] [PubMed]
- Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: a randomized study. Urol Oncol 2015;33:17.e1-17.e7. [Crossref] [PubMed]
- Da Rosa MR, Milot L, Sugar L, et al. A prospective comparison of MRI-US fused targeted biopsy versus systematic ultrasound-guided biopsy for detecting clinically significant prostate cancer in patients on active surveillance. J Magn Reson Imaging 2015;41:220-5. [Crossref] [PubMed]
- Nassiri N, Margolis DJ, Natarajan S, et al. Targeted Biopsy to Detect Gleason Score Upgrading during Active Surveillance for Men with Low versus Intermediate Risk Prostate Cancer. J Urol 2017;197:632-9. [Crossref] [PubMed]
- Abdi H, Pourmalek F, Zargar H, et al. Multiparametric magnetic resonance imaging enhances detection of significant tumor in patients on active surveillance for prostate cancer. Urology 2015;85:423-8. [Crossref] [PubMed]
- Weaver JK, Kim EH, Vetter JM, et al. Presence of Magnetic Resonance Imaging Suspicious Lesion Predicts Gleason 7 or Greater Prostate Cancer in Biopsy-Naive Patients. Urology 2016;88:119-24. [Crossref] [PubMed]
- Siddiqui MM, Rais-Bahrami S, Turkbey B, et al. Comparison of MR/ultrasound fusion-guided biopsy with ultrasound-guided biopsy for the diagnosis of prostate cancer. JAMA 2015;313:390-7. [Crossref] [PubMed]
- Hansen N, Patruno G, Wadhwa K, et al. Magnetic Resonance and Ultrasound Image Fusion Supported Transperineal Prostate Biopsy Using the Ginsburg Protocol: Technique, Learning Points, and Biopsy Results. Eur Urol 2016;70:332-40. [Crossref] [PubMed]
- Penzkofer T, Tuncali K, Fedorov A, et al. Transperineal in-bore 3-T MR imaging-guided prostate biopsy: a prospective clinical observational study. Radiology 2015;274:170-80. [Crossref] [PubMed]
- Felker ER, Lee-Felker SA, Feller J, et al. In-bore magnetic resonance-guided transrectal biopsy for the detection of clinically significant prostate cancer. Abdom Radiol (NY) 2016;41:954-62. [Crossref] [PubMed]
- Marliere F, Puech P, Benkirane A, et al. The role of MRI-targeted and confirmatory biopsies for cancer upstaging at selection in patients considered for active surveillance for clinically low-risk prostate cancer. World J Urol 2014;32:951-8. [Crossref] [PubMed]
- Li YH, Elshafei A, Li J, et al. Potential benefit of transrectal saturation prostate biopsy as an initial biopsy strategy: decreased likelihood of finding significant cancer on future biopsy. Urology 2014;83:714-8. [Crossref] [PubMed]
- Pepe P, Cimino S, Garufi A, et al. Detection rate for significant cancer at confirmatory biopsy in men enrolled in Active Surveillance protocol: 20 cores vs 30 cores vs MRI/TRUS fusion prostate biopsy. Arch Ital Urol Androl 2016;88:300-3. [Crossref] [PubMed]
- Galosi AB, Maselli G, Sbrollini G, et al. Cognitive zonal fusion biopsy of the prostate: Original technique between target and saturation. Arch Ital Urol Androl 2016;88:292-5. [Crossref] [PubMed]
- Gordetsky J, Rais-Bahrami S, Epstein JI. Pathological Findings in Multiparametric Magnetic Resonance Imaging/Ultrasound Fusion-guided Biopsy: Relation to Prostate Cancer Focal Therapy. Urology 2017;105:18-23. [Crossref] [PubMed]
- Lai WJ, Wang HK, Liu HT, et al. Cognitive MRI-TRUS fusion-targeted prostate biopsy according to PI-RADS classification in patients with prior negative systematic biopsy results. J Chin Med Assoc 2016;79:618-24. [Crossref] [PubMed]
- Walton Diaz A, Shakir NA, George AK, et al. Use of serial multiparametric magnetic resonance imaging in the management of patients with prostate cancer on active surveillance. Urol Oncol 2015;33:202.e1-202.e7. [Crossref] [PubMed]
- Siddiqui MM, Truong H, Rais-Bahrami S, et al. Clinical implications of a multiparametric magnetic resonance imaging based nomogram applied to prostate cancer active surveillance. J Urol 2015;193:1943-9. [Crossref] [PubMed]
- Felker ER, Wu J, Natarajan S, et al. Serial Magnetic Resonance Imaging in Active Surveillance of Prostate Cancer: Incremental Value. J Urol 2016;195:1421-7. [Crossref] [PubMed]
- Rais-Bahrami S, Turkbey B, Rastinehad AR, et al. Natural history of small index lesions suspicious for prostate cancer on multiparametric MRI: recommendations for interval imaging follow-up. Diagn Interv Radiol 2014;20:293-8. [Crossref] [PubMed]
- Moore CM, Giganti F, Albertsen P, et al. Reporting Magnetic Resonance Imaging in Men on Active Surveillance for Prostate Cancer: The PRECISE Recommendations-A Report of a European School of Oncology Task Force. Eur Urol 2017;71:648-55. [Crossref] [PubMed]
- Thaxton CS, Loeb S, Roehl KA, et al. Treatment outcomes of radical prostatectomy in potential candidates for 3 published active surveillance protocols. Urology 2010;75:414-8. [Crossref] [PubMed]
- Siddiqui MM, George AK, Rubin R, et al. Efficiency of Prostate Cancer Diagnosis by MR/Ultrasound Fusion-Guided Biopsy vs Standard Extended-Sextant Biopsy for MR-Visible Lesions. J Natl Cancer Inst 2016.108. [PubMed]


