Expanded criteria for active surveillance in prostate cancer: a review of the current data
Background
Over the last ten years, active surveillance (AS) has become increasingly utilized for patients with low-risk prostate cancer (1). Numerous studies have demonstrated the safety of AS and the vast majority of men do not progress to higher-risk disease, sparing them potentially morbid invasive treatments (2). For carefully selected patients managed with AS, 10-year prostate cancer-specific mortality (PCSM) approaches 99% (3). Given this extremely high survival, urologists have questioned whether selection criteria for AS might be safely expanded. Such a proposal would potentially spare more men the morbidity of treatment while still achieving acceptable rates of cancer progression and mortality (4-7). Herein, we will review several high-quality studies to demonstrate which patients may safely be managed with expanded criteria.
In 1994, Epstein et al. provided the criteria for “clinically insignificant” prostate cancer which can be safely observed. The criteria defining “very low-risk” (VLR) disease was clinical stage T1c, PSAd <0.15, Gleason score ≤6, ≤2 positive cores, and <50% maximum single core involvement (8). Low-risk disease has been defined as not meeting all criteria for VLR but also not meeting criteria for intermediate risk (Gleason score ≥3+4, ≥ T2b, PSA 10–20 ng/mL) (9).
There is no definitive consensus regarding when to offer definitive therapy to a patient on AS (10). Although it is beyond the scope of this review, it is worth noting that the criteria for taking a patient off AS may be as critical for oncologic outcomes as the criteria to begin AS initially. Furthermore, variation in treatment criteria may be an important confounding variable when comparing the cohorts reviewed.
Expanded criteria
For the purposes of this review, any study that included patients with baseline Gleason 3+4 disease and/or did not have strict core requirements (% and number of involved) was considered expanded criteria (Table 1). The Welty study (11) is initially classified as strict based on the defined inclusion criteria, however given that 8% of the final cohort had baseline Gleason 3+4, it was reclassified as expanded criteria in Tables 2 and 3.
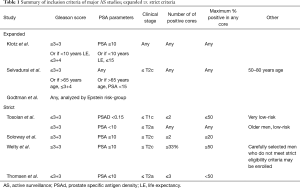
Full table

Full table

Full table
Beyond these large prospective cohorts, several studies have retrospectively analyzed baseline parameters at the initiation of AS that are predictive of adverse outcomes (12-14). These studies are included in this review because they provide insight into specifically which inclusion criteria might be safely expanded and which might expose patients to excessive oncologic risk. As AS is offered to more men with favorable-risk prostate cancer, it is critical to define our criteria with maximum inclusion but also maximum safety in mind.
Outcomes
Five AS cohorts were included that have reported sufficiently mature data with median follow up greater than five years (3,9,11,15,16). Baseline patient characteristics varied significantly between these cohorts (Table 2). Median baseline PSA and PSAd was lowest in the Tosoian cohort (3) which was also the only study with 0% baseline Gleason 7 disease. 13% of the Klotz cohort (15) had baseline Gleason 7 disease and overall 21% baseline intermediate-risk.
The major outcomes reported for these cohorts are represented in Table 3. At 10 and 15 years, Klotz reported 1.9% and 5.7% PCSM respectively compared to 0.1% and 0.1% in the Tosoian cohort. Treatment rates also differed with only 36% of the Klotz cohort having received definitive therapy at 10 years compared to 55% and 50% in the Godtman and Tosoian studies respectively. Metastasis rates were highest in the Klotz cohort at 2.8%, but similar and low in the Godtman, Welty, and Tosoian studies. Differences in biochemical recurrence (BCR) are difficult to interpret due to significant heterogeneity in reporting.
Baseline parameters predictive of reclassification, adverse final pathology, metastasis, and BCR are shown in Table 4. PSA density (PSAd) and total number of positive cores were consistent and strong predictors of reclassification into a higher risk group (Table 4). Welty reported a hazard ratio (HR) of 2.5 for PSAD >0.15 vs. <0.10 ng/mL and Tosoian reported a HR 1.47 for each additional positive core after one. Baseline PSA was not predictive of reclassification in the Welty and Bul studies. Meeting Epstein’s VLR criteria (vs. low-risk) and clinical stage T2 (vs. T1) were also not predictive.

Full table
Adverse pathology upon radical prostatectomy (RP) was studied by Reese et al. who retrospectively analyzed 8,261 men who received treatment at time of diagnosis (12). PSAd >0.15 and Gleason score ≥3+4 were found to be strong predictors of extracapsular extension, seminal vesicle invasion, and non-organ confined disease. Clinical stage T2, ≤3 positive cores, and ≤60% maximum core involvement was not predictive of adverse pathology. Kaplan-Meier analysis demonstrated no difference in BCR-free survival between men with clinical stage T2, ≤3 positive biopsy cores, 50–60% core involvement, PSAd <0.15 ng/mL and biopsy Gleason score ≤6 compared to men who met all of the Epstein VLR criteria.
Yamamoto et al. studied the subset of men in the Klotz cohort who developed metastatic disease (13). Gleason score ≥3+4 (HR 3.0), PSA doubling time <3 years (PSADT, HR 3.7), and ≥3 positive cores (HR 2.7) were all predictive of metastasis. Total PSA >10 ng/mL was not predictive.
Discussion
Although the Tosoian cohort was the only AS study which adhered to strict criteria, given its large sample size of 1,298 patients and 0% Gleason 7 at baseline, it provides a robust comparison for the expanded criteria cohorts. The Klotz cohort is considered to be the most robust expanded criteria study given its sample size of 993 patients and 6.4-year median follow-up. It is important to highlight that there was significant heterogeneity in the patient populations and data reporting between these studies, which limits the validity of conclusions drawn by direct comparison. Nonetheless, by integrating the prospective and retrospective data, some broad conclusions may be considered.
PCSM & metastasis
The rate of metastasis and PCSM are the most significant endpoints to consider when seeking to expand AS. The expanded criteria Klotz cohort had a higher rate of both metastasis (2.8%) and corresponding prostate cancer specific mortality (1.9% at 10 years, 5.7 at 15 years) compared to the strict Tosoian cohort (metastasis rate of 0.4%, PCSM of 0.1% at 10 years, 0.1% at 15 years). Notably, 44% of the patients that developed metastases in the Klotz cohort had baseline Gleason 7 disease and another 49% were upgraded to Gleason 7 prior to development of metastatic disease. The remaining 7% did not have final surgical pathology and thus may have harbored higher-grade disease. Given that Gleason 7 disease was identified in almost all patients who developed metastases, the variation in baseline Gleason 7 rates between the two studies (13% vs. 0%, Table 2) may account for the significantly higher rate of metastasis in the Klotz cohort.
An important possible confounder when comparing these two cohorts, however, is the significant difference in treatment rates. At 10 years, only 36% of the Klotz cohort had received definitive therapy compared to 50% in the Tosoian study—a factor that could have significantly decreased the rate of metastasis and mortality in this cohort. It is unclear whether the strict inclusion criteria or the aggressive discontinuation of AS for definitive therapy accounts for the survival differences between these two cohorts. However, the significance of higher treatment rates is supported by the data from the Godtman and Welty studies. Both of these cohorts included a significant proportion of intermediate-risk patients yet with treatment rates similar to the Tosoian cohort, achieved comparable low rates of metastasis and PCSM.
Although the rate of metastasis was high in the Klotz cohort, the median time-to-metastasis was 7.3 years. Given that 13% of this cohort had baseline Gleason 7 disease, this suggests that even intermediate-risk prostate cancer may progress relatively slowly. Considered together, these data may suggest that AS inclusion criteria could be safely expanded if combined with sufficiently conservative triggers for definitive therapy, especially in men with a shortened life expectancy.
BCR
BCR rate an important outcome in these studies since it serves as an indicator of the ability to achieve durable oncologic control after a period of AS. Unfortunately, the reporting of BCR is highly variable between studies, making it difficult to compare directly. In the Klotz cohort, 5- and 10-year BCR rates after treatment were 23% and 41% respectively which is higher than has been reported for men undergoing RP for low-risk disease at time of diagnosis (19% and 34%) (17). However, this cohort included a large proportion of intermediate-risk patients who were at a significantly higher risk of BCR after treatment (odds ratio 2.1). Increased risk of BCR in intermediate-risk patients was also found in the Godtman cohort (HR 3.7). Accounting for this confounder, these data suggest that for low-risk disease, a period of AS does not significantly compromise the ability to achieve oncologic control. Conversely, intermediate-risk patients may be at a significantly higher risk of recurrence after treatment.
Predictors of reclassification
As summarized in Table 4, PSAd was a strong and consistent predictor of reclassification into a higher risk group—this was expected given the known association of PSAd with tumor volume and aggressiveness (18). The number of positive cores was also predictive in the Bul and Tosoian cohorts. This also stands to reason since a greater number of positive cores is associated with larger tumor volume (8,19) and larger tumors are more likely to be reclassified on subsequent biopsies (20). Taken together, these two baseline parameters can identify patients on AS who may need more aggressive follow up due to the risk of an occult high-grade lesion.
Bul and Welty reported that total PSA, clinical stage T2 (vs. T1c), and meeting Epstein VLR criteria (vs. low-risk) were not predictive of reclassification. These findings are concordant with the significant base of literature demonstrating the low sensitivity and specificity of total PSA (21) and the unreliability of clinical staging (12,22). Thus, these parameters should be weighted relatively less when selecting patients for AS versus immediate treatment.
Predictors of adverse oncologic outcomes
The Reese, Yamamato, and Godtman studies analyzed baseline predictors of adverse final pathology, metastasis, and BCR respectively. Reese et al. found maximum positive core involvement, total positive cores, Gleason score, and PSAd to be predictive of adverse pathology. While the Epstein cutoffs of Gleason score ≤6 and PSAd <0.15 were supported, maximum positive core involvement and total positive cores were not associated with increased risk of adverse pathology up to 60% and 4 cores respectively. There was no association with clinical stage. Although the pathologic outcomes analyzed in this study have not been definitively correlated with poor clinical outcomes, this does suggest that several of the commonly used criteria for AS may be expanded without obvious oncologic risk.
The Yamamoto study largely supported these findings but found that ≥3 total positive cores was associated with an increased risk of metastatic disease. Similarly, the Godtman study found that intermediate-risk disease was predictive BCR compared to low and VLR. Overall, these analyses support the expansion beyond Epstein’s VLR criteria for core involvement and clinical stage but not for Gleason score or PSAd.
Future research
So long as the traditional baseline parameters (clinical stage, number of cores, percent involvement, Gleason score, PSA/PSAd) are used to stratify patients at time of diagnosis, more data is needed to determine the optimal cutoff values. One method of determining these cutoffs would be to generate receiver operating characteristic (ROC) curves for each parameter to define values that maximize sensitivity and specificity for clinically significant versus insignificant prostate cancer. Furthermore, by combining these ROC curves with number needed to treat and number needed to harm analyses, physicians and patients can have maximally informed discussions about the risks and benefits of treatment versus AS (23,24).
Moving beyond the limitations of these traditional parameters, multiparametric magnetic resonance imaging (mpMRI) is increasingly being used to better delineate the extent of a patient’s disease and guide targeted biopsies. This way, patients are more accurately stratified at baseline and the risk of putting a patient with an occult intermediate or high-grade tumor on AS is diminished. The role of mpMRI may continue to expand due to these benefits and radiographic features may be incorporated into baseline risk stratification.
Another possible advancement in risk stratification may come from cancer genomics. An increasing amount of genetic tests are becoming available which can be used to predict the aggressiveness of a neoplastic clone (25-28). These tests can go beyond the subjective histopathologic appearance of a tumor (Gleason score) and detect specific mutations which have been associated with either a more aggressive or more indolent clinical course. As the prognostic significance of individual mutations are better understood, prostate cancer risk stratification will become even more accurate.
Conclusions
Defining the optimal criteria for AS is an ongoing debate in the field of urology. Although the existing literature cannot define the precise extent to which AS criteria may be safely expanded, some tentative conclusion can be drawn from the data presented in this review. As is well known, it is clear that patients with intermediate-risk disease have poorer oncologic outcomes compared to low and VLR. However, the data also suggest that select intermediate-risk patients may be reasonably managed with AS if the treatment triggers are sufficiently conservative. Nonetheless, the risk of losing the opportunity for definitive oncologic control is certainly higher in these patients and the costs and benefits of intervention must be carefully considered.
Comparing Epstein’s VLR to low-risk patients, in contrast to standard practice at many institutions, the data strongly suggest that not all VLR criteria must be met in order to safely initiate AS. Specifically, the clinical stage and core criteria (number and percent involvement) have not been shown to be predictive of adverse pathologic or clinical outcomes. That being so, many patients who do not meet these parameters might safely be managed with AS and spared the morbidity associated with definitive therapy. On the contrary, expansion of the PSAd and Gleason score criteria (<0.15 ng/mL and ≤6) should be considered more carefully given the strong association with poor oncologic outcomes. Ultimately, the decision between AS and immediate treatment will continue to be a discussion between physicians and patients with a strong emphasis on choosing a course of action that is appropriate for each individual patient.
Acknowledgements
We would like to thank all the men who participated in the trials on which this review is based.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Murphy DG, Loeb S. Prostate cancer: Growth of AS in the USA signals reduction in overtreatment. Nat Rev Urol 2015;12:604-5. [Crossref] [PubMed]
- Popiolek M, Rider JR, Andren O, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol 2013;63:428-35. [Crossref] [PubMed]
- Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol 2015;33:3379-85. [Crossref] [PubMed]
- Bill-Axelson A, Holmberg L, Ruutu M, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med 2011;364:1708-17. [Crossref] [PubMed]
- Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med 2012;367:203-13. [Crossref] [PubMed]
- Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol 2014;65:1046-55. [Crossref] [PubMed]
- Cooperberg MR, Carroll PR, Klotz L. Active surveillance for prostate cancer: progress and promise. J Clin Oncol 2011;29:3669-76. [Crossref] [PubMed]
- Epstein JI, Walsh PC, Carmichael M, et al. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA 1994;271:368-74. [Crossref] [PubMed]
- Godtman RA, Holmberg E, Khatami A, et al. Outcome following active surveillance of men with screen-detected prostate cancer. Results from the Goteborg randomised population-based prostate cancer screening trial. Eur Urol 2013;63:101-7. [Crossref] [PubMed]
- Tosoian JJ, Carter HB, Lepor A, et al. Active surveillance for prostate cancer: current evidence and contemporary state of practice. Nat Rev Urol 2016;13:205-15. [Crossref] [PubMed]
- Welty CJ, Cowan JE, Nguyen H, et al. Extended followup and risk factors for disease reclassification in a large active surveillance cohort for localized prostate cancer. J Urol 2015;193:807-11. [Crossref] [PubMed]
- Reese AC, Landis P, Han M, et al. Expanded criteria to identify men eligible for active surveillance of low risk prostate cancer at Johns Hopkins: a preliminary analysis. J Urol 2013;190:2033-8. [Crossref] [PubMed]
- Yamamoto T, Musunuru B, Vesprini D, et al. Metastatic Prostate Cancer in Men Initially Treated with Active Surveillance. J Urol 2016;195:1409-14. [Crossref] [PubMed]
- Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol 2013;63:597-603. [Crossref] [PubMed]
- Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol 2015;33:272-7. [Crossref] [PubMed]
- Selvadurai ED, Singhera M, Thomas K, et al. Medium-term outcomes of active surveillance for localised prostate cancer. Eur Urol 2013;64:981-7. [Crossref] [PubMed]
- Kane CJ, Im R, Amling CL, et al. Outcomes after radical prostatectomy among men who are candidates for active surveillance: results from the SEARCH database. Urology 2010;76:695-700. [Crossref] [PubMed]
- Kundu SD, Roehl KA, Yu X, et al. Prostate specific antigen density correlates with features of prostate cancer aggressiveness. J Urol 2007;177:505-9. [Crossref] [PubMed]
- Stamey TA, Freiha FS, McNeal JE, et al. Localized prostate cancer. Relationship of tumor volume to clinical significance for treatment of prostate cancer. Cancer 1993;71:933-8. [Crossref] [PubMed]
- Newcomb LF, Thompson IM Jr, Boyer HD, et al. Outcomes of Active Surveillance for Clinically Localized Prostate Cancer in the Prospective, Multi-Institutional Canary PASS Cohort. J Urol 2016;195:313-20. [Crossref] [PubMed]
- Thompson IM, Ankerst DP, Chi C, et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006;98:529-34. [Crossref] [PubMed]
- Leibovici D, Shikanov S, Gofrit ON, et al. How accurate is our clinical prediction of "minimal prostate cancer"? Isr Med Assoc J 2013;15:359-63. [PubMed]
- Russo GI, Cimino S, Castelli T, et al. Percentage of cancer involvement in positive cores can predict unfavorable disease in men with low-risk prostate cancer but eligible for the prostate cancer international: active surveillance criteria. Urol Oncol 2014;32:291-6. [Crossref] [PubMed]
- Jin BS, Kang SH, Kim DY, et al. Pathological upgrading in prostate cancer patients eligible for active surveillance: Does prostate-specific antigen density matter? Korean J Urol 2015;56:624-9. [Crossref] [PubMed]
- Cuzick J, Berney DM, Fisher G, et al. Prognostic value of a cell cycle progression signature for prostate cancer death in a conservatively managed needle biopsy cohort. Br J Cancer 2012;106:1095-9. [Crossref] [PubMed]
- Klein EA, Cooperberg MR, Magi-Galluzzi C, et al. A 17-gene assay to predict prostate cancer aggressiveness in the context of Gleason grade heterogeneity, tumor multifocality, and biopsy undersampling. Eur Urol 2014;66:550-60. [Crossref] [PubMed]
- Cullen J, Rosner IL, Brand TC, et al. A Biopsy-based 17-gene Genomic Prostate Score Predicts Recurrence After Radical Prostatectomy and Adverse Surgical Pathology in a Racially Diverse Population of Men with Clinically Low- and Intermediate-risk Prostate Cancer. Eur Urol 2015;68:123-31. [Crossref] [PubMed]
- Blume-Jensen P, Berman DM, Rimm DL, et al. Development and clinical validation of an in situ biopsy-based multimarker assay for risk stratification in prostate cancer. Clin Cancer Res 2015;21:2591-600. [Crossref] [PubMed]


