Contemporary role of radiotherapy in the management of penile cancer
Introduction
Carcinoma of the penis is a rare clinic entity, which usually presents as a painless lump or ulceration on the penis. In developed nations, the incidence approximates 1/100,000 and the vast majority of cases (95%) are epithelial squamous cell carcinoma (SCC). In the United States, carcinoma of the penis accounts for less than 1% of all malignancies in men with an estimated annual incidence of 2,120 new cases per year and 360 deaths (1). In stark contrast, the incidence in developing countries in Africa, India or South America can reach 10% of all malignancies. The varying incidence may be explained by differences in risk factors including tobacco use, age distribution, cultural requirement for circumcision, sexual practices and hygiene.
As with other SCC sites, including cervix, vulva, anal, and head and neck cancers, human papilloma virus (HPV) plays an important role in carcinogenesis. In modern cohorts, HPV DNA can be found in 36% of all penile carcinomas (range, 26–47%), with HPV-16 and HPV-18 representing the most common subtypes (60% and 13%, respectively) (2,3). As with other HPV-mediated SCCs, HPV positivity in penile cancer is associated with better clinical outcome, a higher response rate to chemoradiotherapy and improved 5-year survival (4). For this reason, radiotherapy plays an important role in the management of both primary and regional disease.
While radical surgery such as total/subtotal penectomy has been employed to achieve good local control, the resultant impact on quality of life, standing micturition, psychosocial identity and sexual functioning is significant. Advances in surgical techniques have allowed for organ-sparing approaches (5), but patient selection and availability of this surgical expertise result in limited uptake (6). For patients with more advanced disease, or where surgical resection is inappropriate, treatment with radiotherapy as a non-surgical alternative for organ preservation can provide significant quality of life advantages and excellent clinical outcomes (7).
Given the low incidence of penile cancer in Western countries, large-scale trials are currently lacking. Until information from trials such as the International Penile Advanced Cancer Trial (InPACT) is available (ClinicalTrials.gov Identifier NCT02305654), clinical practice and guidelines have been informed by case-series, institutional experience and evidence from other related SCC sites.
The type of radiotherapy used in the treatment of penile cancers depends on the size and location of the lesion. For locally confined cases, options for treatment include external beam radiotherapy, interstitial brachytherapy or surface mold brachytherapy. In these instances, radiotherapy is used as the primary treatment modality with surgical resection reserved for salvage of local recurrence. Additionally, radiation may be used postoperatively after surgical resection when positive or close margins confer a high risk of local recurrence.
For more advanced (higher T-stage) and/or high-grade cancers with a significant risk of regional nodal involvement, primary radiotherapy may be combined with inguinal or sentinel node dissection for surgical nodal staging. For cases at high risk of regional relapse (multiple nodes involved, extracapsular extension or positive margins), adjuvant pelvic/inguinal radiotherapy will improve local-regional control and potentially survival outcomes. For locally or regionally advanced cases, radiotherapy combined with chemotherapy may be used neoadjuvantly to allow subsequent resection, or as definitive treatment in unresectable disease. Here we discuss the various roles of radiotherapy in localized, regional and locally advanced disease.
Carcinoma-in-situ (CIS)
SCC CIS of the penis is referred to as Bowen’s disease or erythroplasia of Queyrat. These pre-invasive lesions have identical histopathological features but vary in location at clinical presentation (8), and will progress to invasive disease. There are several penile-sparing approaches which can be employed to provide organ preservation. Since there are no randomized trials that establish superiority of any one treatment, the choice of treatment is based on patient preference, availability and cost. For small superficial lesions, topical treatment with imiquimod cream 5% has been used with complete response (9). Similarly, topical treatment with 5-fluoruracil may be used, but careful follow-up is necessary as recurrences are common, especially since there is often a background of field-change effects.
Laser therapy with carbon-dioxide or neodymium:yttrium-aluminum-garnet (Nd:YAG) laser, is another non-invasive option with a high rate of patient satisfaction, preserved sexual function (75%) and good, self-reported cosmesis (10,11). Treatment with Nd:YAG laser is preferred, due to its greater depth of penetrance (6 mm) compared to CO2 (1 mm). However, even with careful clinical mapping of lesions using acetic-acid, there is an 11–14% recurrence rate using the Nd:YAG laser (12,13). Careful follow-up and repeat treatment as required may achieve complete remission. Photodynamic therapy is an additional option, using a photosensitizing agent like methylaminolaevulinic acid which is excited by visible light, selectively destroying atypical cells. However, reported complete response rates are inferior to laser (14).
There are also a variety of penile-preserving surgical options available depending on the location of involvement. Small lesions involving the distal foreskin may be treated with circumcision alone. For lesions located on the glans or prepuptial surface, Mohs micrographic surgery can provide a good cosmetic outcome. However, since local recurrence is reported in up to 32%, careful follow-up is important and repeat procedures may be required for optimal cancer control (15). Other surgical techniques like total glans resurfacing report excellent clinical outcomes and cosmesis, with 100% complete response at a median of 30 months. The required surgical expertise may not be available at all centres (16).
Finally, radiotherapy remains an option for pre-invasive disease, with a reported local control rate of 100% (17). However, this option is usually reserved for treatment failure or invasive recurrences, given the availability and favourable toxicity profiles of other non-invasive options.
Radiotherapy for the primary tumour
Given the significant negative impact that penile cancer treatment can have on well-being, sexual function (18) and sense of sexual identity or masculinity (19), the focus of treatment of penile cancer should be on organ preservation. As expected, the extent of surgical resection is correlated to worsening sexual function, cosmesis, life interference and urinary function (20). In place of surgical resection, radiotherapy offers treatment with organ preservation using external beam radiation, interstitial brachytherapy or surface mold plesiotherapy.
External beam radiotherapy
External beam radiotherapy for localized penile cancer can provides local control and a reasonable chance of organ preservation. For T1–T2 tumours, 5-year local control is 62%, with penile preservation approaching 40% (21). For case series that include more advanced cancers (T3–T4), local control falls to 40% with a 10-year probability of penile preservation of 38% (22).
Technical challenges with external beam radiotherapy concern treatment delivery and reproducibility. For very small tumours on the glans or prepuce that are unsuitable for brachytherapy (discussed later) treatment is similar to that for skin cancers using a direct appositional electron field and lead cut out. A 2-cm margin around the gross tumour is added to allow for penumbra and coverage of microscopic disease. A layer of bolus material must be used to overcome skin-sparing and the electron energy is chosen to ensure coverage at a depth by the 90% isodose line with 100% at the skin surface.
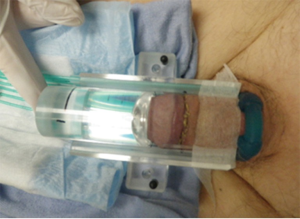
For larger tumours, treatment with external beam radiotherapy requires photons, usually of 4–6 MV energy. To isolate the penis and spare adjacent normal structures, the penis must be supported in a wax block or similar structure with tissue-equivalent electron density (Figure 1). In this technique, the patient is positioned supine, with the penis supported vertically in the central cavity of a wax block. When the bivalved block is closed, the wax provides a tissue-equivalent material that allows for adequate dose-build-up when using megavoltage photons. Typically a parallel-opposed pair of lateral fields is used. To shield the groins and testes, a lead sheet may be used, with the wax block supported on top of this. Because wax blocks are opaque and do not allow for visualization of daily treatment verification, transparent Plexiglas or Lucite chambers are now preferred. Different size blocks should be available to accommodate anatomical variation and potential penile edema, which can occur during treatment. In either case, the distal aspect of the chamber beyond the penis should be filled with tissue-equivalent material to ensure adequate dose coverage at the tip of the glans.
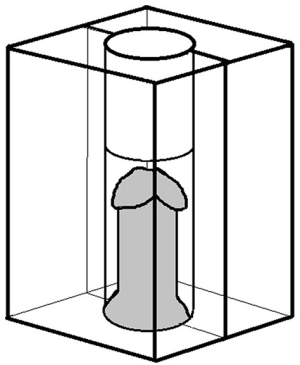
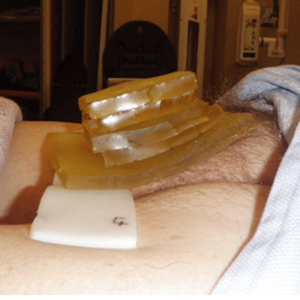
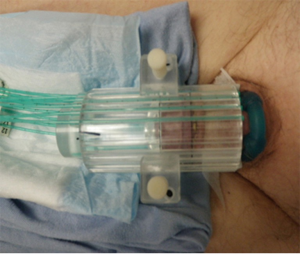
For treatment planning, the clinical target volume (CTV) includes the full thickness of the penis and is delineated with at least 1cm added proximally and distally beyond the tumour, as identified clinically and radiographically. An additional 1cm is added to allow for daily set up variations and penumbra to create a planning target volume (PTV), which thus extends a total of 2 cm beyond the gross disease. Depending on the size and location of the tumor, once treatment margins are added, the majority of the penis is often included within the treated volume. For cases where the penis (or penile remnant) is shorter, as in recurrence after partial amputation, custom wax bolus or sequential rings of bolus may be used (Figure 2).
We recommended using conventional fractionation to a total dose of 65–70 Gy. Fraction sizes <2 Gy prolong treatment courses beyond 45 days, and/or deliver a biologically equivalent dose (BED: value α/β of 10) <60 Gy and are reported to be less effective (22,23). Furthermore, protracted treatment courses become problematic for set-up as patients invariably develop moist dermatitis during treatment.
As lymph node status is such an important prognostic factor, surgical staging is the preferred approach for high risk patients. “Prophylactic coverage” of the inguinal lymph nodes is not recommended but could be considered for those who have declined, or are unsuitable for, surgical management. Treatment of the primary tumour may be combined with concomitant treatment of the nodal regions in regionally advanced cases but radio-sensitization with concurrent chemotherapy should be considered. These topics will be discussed separately under the section on locally advanced disease.
Brachytherapy
Brachytherapy is an alternative to penectomy in T1 and T2 tumours, especially those confined to the glans and less than 4 cm. In a recent meta-analysis, Hasan et al. compared outcomes for 2,178 males, 1,505 treated surgically and 673 with brachytherapy (24). The penile preservation rate for brachytherapy was 74%. For stage I/II tumours, the 5-year local control rate was 84% for brachytherapy and 86% for surgery (ns), while 5 year OS was 79% and 80% (ns), respectively. Even when including stage III and IV patients, the penile preservation rate for brachytherapy was 74%, with no difference in OS compared to surgery. This supports the teaching that recurrences after brachytherapy are salvageable with surgical resection, and that initial management with a goal of penile preservation does not adversely affect overall survival. Brachytherapy is typically performed with an interstitial technique with low-dose rate (LDR), pulsed-dose rate (PDR) or high-dose rate (HDR) implants but a surface mold technique has been described. All patients should have a circumcision prior to the procedure to allow for complete tumour visualization and to avoid problems with painful necrosis and ulceration of the prepuce. In many cases, this will also “debulk” the tumour, facilitating implantation. Surface mold plesiotherapy will be discussed later.
For interstitial implants, tumours should ideally be confined to the glans and maximum 4 cm in diameter (25,26). While larger tumours can be technically suitable, there is an increased risk of ulceration or necrosis. Results of local control for larger tumours are good. In a multi-institutional French experience (n=184 patients) there was slightly decreased local control for tumours >3 cm (20% local failure rate vs. 14% for tumours <3 cm, P=0.05). In contrast, Crook et al. experience on 49 patients (19 with tumours >3 cm) did not show a correlation between tumour size and local control (P=0.43). Treatment options providing an opportunity for potential penile preservation should be discussed with patients since the risk of possible adverse side effects is often deemed preferable to penectomy.
Patients with high-grade disease are also candidates for brachytherapy. In a series published by Crook et al. on 74 patients treated with brachytherapy, there was no difference in local control for well differentiated or moderately/poorly differentiated (27) tumours. However, higher grade is a strong predictor for lymph node involvement: moderately or poorly differentiated cancers demonstrate rates of lymph node metastases of 60–70% (28). For these patients regardless of preoperative imaging, surgical regional staging is recommended, either sentinel lymph node sampling or inguinal nodal dissection.
Low dose rate interstitial brachytherapy
LDR interstitial brachytherapy is an effective treatment for organ confined T1–T2 penile cancers as well as select T3 lesions (less than 4 cm). In contrast to fractionated external beam radiotherapy, treatment with LDR brachytherapy is done over a relatively short period of time (4–5 days) and allows delivery of a high dose of radiation (60 Gy) with superior conformality and sparing of normal tissue (26). The expected skin reaction develops after treatment is complete and thus does not interfere with treatment completion. In the hands of a skilled practitioner, the outcomes are excellent with 5- and 10-year penile preservation rates of 88% and 67%, respectively (8 local failures requiring penectomy and 2 necrosis in 67 patients at a median follow-up of 4 years) (27). Cause specific survival was 83.6%.
The procedure can be performed under general or regional anesthesia and begins with a clinical exam to help define the target volume. The radiation oncologist will define the CTV around the gross tumour using a 1-cm margin. An in-dwelling Foley catheter is inserted at the time of brachytherapy and remains for the duration of the treatment. The positioning and spacing of the needles is determined by the radiation oncologist to allow for appropriate tumor coverage while avoiding transfixing the urethra. A minimum of a 2-plane template is required in order to ensure coverage at a depth. It should be noted that the depth of invasion is difficult to assess clinically and is often under-estimated on biopsies.
It is recommended that the Paris system of dosimetry (29) be followed to design the geometry of the implant such that the location of the prescription isodose can be accurately predicted. The lateral safety margin, or distance of the prescription isodose from the most superficial needles, is generally about 1/3 of the spacing between the needles, provided the liner activity is uniform and the needles and planes of needles are equally spaced. With a spacing of 12–15 mm the superficial needle plane (within the penis) can be placed at a depth of 4–5 mm from the skin surface; the prescription isodose will cover the tumor but the needles are deep enough in tissue to avoid ulceration and linear scarring. Larger implants with three planes, or an additional plesiotherapy plane, may be needed to ensure appropriate dose coverage. If a plesiotherapy plane is added, tissue-equivalent bolus must be used to fill the air gap between the plesiotherapy needles and surface of the penis.
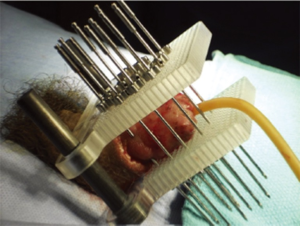
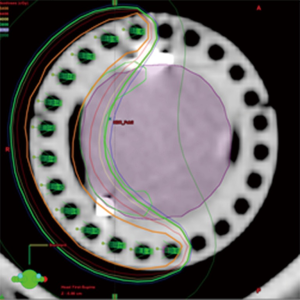
For implant guidance, the use of a “universal template” (Figure 3) with holes predrilled at 3 mm grid spacing is recommended. Ideal intersource spacing is 12, 15 or 18 mm for LDR implants. The spacing should be uniform between sources and between planes. Two predrilled plates are positioned parallel on either side of the penis. Usually an anterior-posterior arrangement is used but in some circumstances depending on tumor location, a right-left orientation may be suitable. The steel needles are individually locked to each template. For treatment planning, CT simulation with needle reconstruction is currently the standard-of-care. However, given the rigid positioning provided by the template, dosimetry can be calculated using the needle/plane spacing and the measured distance from each needle tip to the outer surface of the template.
Needle placement is guided by classic Paris system rules: needles must be parallel, equidistant from one another, and the linear source strength must be uniform and identical for all sources (Figure 4). The lateral safety margin depends on the intersource spacing, being larger for wider spacing. The length of the treated volume along the axis of the needles is 0.75 of the active length of the wire sources due to in-drawing of the isodoses between the wires. When using a remote afterloading system with a stepping source, such as in pulse dose rate brachytherapy, dose optimization is possible; the dwell times within the templates can be increased to reduce the difference between the active and treated lengths. The basal dose rate is the average of the minimum dose rates located in the central plane of the implant between the sources. The dose is then prescribed at 85% of the basal dose rate. This keeps the “hyperdose volume” (170% of the basal dose rate, or 2× the prescription isodose. This is equivalent to V200 or the volume enclosed within the 200% isodose) to an acceptable level. The dose prescribed is 60–65 Gy over 5 days with 50–60 cGy delivered per hour for classic LDR implants and similarly 0.5–0.6 Gy/h with PDR brachytherapy. Patients are generally admitted and bed rest is recommended; however, analgesia requirements are often minimal. Antithrombotic therapy is recommended for the 5-day duration of the implant. Needle removal can occur at the bedside with appropriate premedication with narcotic analgesia.
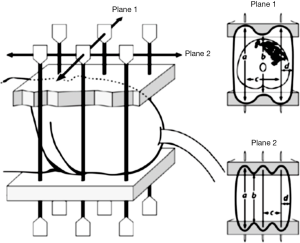
Table 1 shows the results of the larger series. Five year local control ranges from 70–96% and 10-year from 70–80%, with penile preservation at 10 years being 70%. Continued surveillance and patient-awareness regarding self-examination are essential as some local failures may be quite delayed, up to 10 years post treatment.
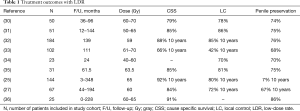
Full table
High dose rate interstitial brachytherapy
The use of manually loaded temporary low dose rate brachytherapy has declined in favour of automated after-loading. Although this can be achieved with pulse dose rate brachytherapy, HDR brachytherapy treatment units are much more common in today’s radiotherapy departments. There are several advantages for HDR treatment over LDR including radioprotection, cost effectiveness and versatility for treating many tumour sites. The use of a stepping source allows treatment optimization with precisely adjusted dose distributions. Although experience is short and the numbers small, there are a few published series reporting encouraging results with a variety of fractionation schemes for HDR penile brachytherapy including: 54 Gy in 18 fractions BID (37), and 42–51 Gy in 14–17 fractions (38) The ABS-GEC-ESTRO consensus guideline recommends 38.4 Gy in 12 fractions (39). Twice-daily fractionation with a minimum of 6 h between fractions is consistently recommended. This should allow treatment to be completed within a reasonable length of time (6 days).
In one of the largest published case series, Kellas-Sleczka et al. treated 55 men with HDR interstitial brachytherapy with the majority having T1 or T2 tumours (49% and 29%, respectively) (40). A median of 4 needles were used with a treatment time of 11 days. The total dose ranged from 30–45.5 Gy (median 36 Gy) for patients with gross total resection and 30–54 Gy (median 49 Gy) for gross disease. After a median follow-up of 59 months, 4 patients did not achieve complete response and there were 7 subsequent local recurrences (12.7%). Organ preservation was maintained in 44 patients (80%) and the 5- and 10-year local control were 84% and 63%, respectively. Further experience with HDR interstitial penile brachytherapy is required to establish treatment parameters for total dose and fractionation in order to replicate results seen with LDR. While the aforementioned studies are promising, further experience is required with longer follow-up before validated guidelines can be established.
Selected results for HDR penile brachytherapy are displayed in Table 2. Follow-up and series size are smaller than with LDR as this an evolving modality.

Full table
Surface mold plesiotherapy
Surface mold plesiotherapy is a technique developed in the era of LDR, where a patient would wear an appliance around the penis containing iridium wire sources for several hours daily for up to 1 week. The rapid dose fall-off made this appropriate for only the most superficial tumors. In one of the largest reported series on 24 patients treated with iridium mould, 79% of patients experience a complete response, compared to 53% in the external beam arm (42). However, there were more favourable tumours in the brachytherapy group and a subset analysis looking at stage 1 patients did not demonstrate a difference. Despite the satisfactory initial response rate, almost 45% of patients required salvage surgery. This high rate of failure can be explained by a lower total dose when compared to interstitial implants, as well as the dose-gradient within the tumour. One of the benefits of interstitial implants is the reliable distribution of dose throughout the tumour, making interstitial implants highly efficacious while being able to spare normal tissues.
As with interstitial implants, there has been renewed interest in surface mould plesiotherapy using HDR afterloading (Figures 5,6). With CT-based planning and custom moulds using 3D printing, custom plans can be created with very high conformality and dose-homogeneity (Figure 7) (43,44). Plesiobrachytherapy should be limited to superficial tumours (Tis, T1a or b) and should not be used for those invading the corpus spongiosum or thicker than 5 mm as adequate coverage at depth is not possible without exceeding skin tolerance.
With this technique, it is possible to limit dose delivered to the skin to less than 120%; with the source catheters embedded within the applicator at a depth of 5 mm from the penile surface, one is able to avoid the most rapid region of dose fall-off at the surface yet still achieve 100% coverage of the tumor at depth (usually to a maximum of 5 mm with 90% to 10 mm). The typical dose delivered is 4 Gy, twice daily at least 6 h apart over 5 days to a total dose of 40 Gy. We use a clear Lucite applicator in which the penis is positioned for planning and can be readily verified for each treatment. A constriction ring can be added to displace cranially any redundant skin on the penile shaft to ensure that it stays outside the treated area. Despite the encouraging early results, this procedure remains investigational as no long term results have yet been reported.
Management of toxicities
As would be expected, both interstitial and contact mold techniques will cause radiodermatitis (moist desquamation) within the treated area. Side effects reach their maximum by week 3 for both techniques and require active management on the patient’s part with good local hygiene. Healing may take 4–8 weeks for plesiotherapy and 12 weeks for interstitial techniques. Frequent daily soaks in warm saline or bicarbonate solutions (made with baking soda) will help keep the area clean and aid in patient comfort. To aid with healing, topical antibiotic agents such as polysporin or flamazine may be used. Cortisone-based creams should be avoided in areas of open ulceration or moist desquamation. Regression of the tumour is very rapid, usually occurring over 1–2 weeks, and will leave behind an open area of ulceration that reflects the original depth of invasion. Use of non-adherent dressings around the penile shaft can help with patient comfort. Meatal adhesions may develop in the weeks following treatment and require separation to avoid later stenosis. Patients can be instructed to use a meatal dilator at home as required.
Meatal stenosis is the most common late effect and is reported in 4–43% of patients (25,27,30,32,36) It is more common when needles are placed in close proximity to the distal tip of the glans. Again, as for early adhesions, this can most often be managed by simple dilatation. Other late toxicities include telangiectasia in 48% of patients and atrophy in 17%. In one series, two patients (9%) required penectomy for fibrosis, but this is uncommon (36).
Soft tissue necrosis or tissue ulceration occur in 14–23% of patients, usually within the first 1–2 years (26,31). Precipitating factors include frictional trauma or chaffing and cold exposure (which compromises blood circulation). Repetitive activities, such as wood chopping or gardening, can cause frictional injuries and men should avoid wearing stiff garments to reduce chaffing. Water-based lubricants are advised for intercourse. Areas of ulceration can be treated conservatively with saline soaks (or baking soda), topical antibiotics and vitamin E ointment. Hyperbaric oxygen treatment can be very successful in refractory cases (45). Biopsy should be avoided unless there is a clinical suspicion for recurrence as this will further compromise healing.
Regional radiation of the groin and pelvis
Penile lymphatic drainage follows a stepwise progression, first involving the superficial and deep inguinal nodes and then the pelvis. Given the midline location of the penis, drainage should be assumed to be bilateral. Pathologic features such high grade, deep invasion, and lymphovascular invasion predict for lymph node involvement (46). Recommended management for the high risk patient involves surgical staging as the risk of clinically occult metastatic disease can be 20% or greater, the risk increasing as the constellation of pathologic features worsens (47). Furthermore, delayed “therapeutic” inguinal node dissection is associated with markedly inferior 10-year disease-free survival compared to immediate lymphadenectomy (30% vs. 71%, P=0.002) (48).
Guidelines for adjuvant radiotherapy in penile cancer are extrapolated from management of other genitourinary SCC sites. The encouraging results seen in trials of vulvar cancer have informed management for penile cancers: adjuvant treatment to the inguinal nodes/pelvis should be offered for multiple positive nodes or extracapsular extension. The pelvis should be included for all cases with >1 inguinal lymph node involved. If there is no evidence of gross disease, the dose can be limited to 45 Gy in 25 fractions. Higher dose to the groin or positive lymph nodes depends on pathology including margin status, presence of extracapsular disease or gross residual disease.
Definitive/neo-adjuvant chemo radiotherapy
Owing to the low incidence of penile cancer, there is an absence of direct level I evidence for definitive treatment with chemoradiotherapy for primary penile cancers. Until we have results from clinical trials such as InPACT (NCT 02305654), clinical recommendations can be extrapolated from evidence obtained from other SCC sites. We will review the information from vulvar, anal and cervical SCC and how treatment paradigms can be applied to SCC of the penis. The consistency and quality of evidence from these sites would suggest that a similar approach is reasonable for penile cancer, at least until results from InPACT are available to define the roles of adjuvant and neoadjuvant treatment
Vulvar cancer
The most frequently applied evidence applicable to penile cancer originates from clinical trials on vulvar cancer, which is a common comparator owing to several similarities, including a common HPV-mediated oncogenesis, lymph node drainage and age-demographics.
In the Gynecologic Oncology Group (GOG protocol 37), 114 patients with positive inguinal nodes at groin dissection were randomly assigned to further treatment with either pelvic lymph node dissection, or adjuvant radiotherapy to the pelvis and inguinal region (49). The dose delivered was 45–50.4 Gy in 4.5–6 weeks using a simple parallel-opposed-pair of radiation beams. At 2 years there was a survival advantage for radiotherapy over the surgical arm (68% vs. 54%, P=0.02), due to a decrease in groin relapses for those who received radiotherapy (5% versus 24%). On multivariate analysis, the number and size of lymph nodes did not impact outcome. An update demonstrated a persistent survival benefit at 6 years favouring the RT arm (HR 0.61) (50). In the surgical arm (n=59), there was only one pelvic failure despite the fact that 15 patients had positive pelvic nodes, compared to 4 pelvic failures in the radiotherapy arm. Surgery appeared better at preventing pelvic relapses compared to radiation non-enhanced by concurrent chemotherapy. This study supports the routine use of adjuvant RT to the pelvis and groin for patients with a high risk groin (two or more nodes involved or extracapsular disease).
Vulvar cancer is primarily treated with surgical resection, followed by adjuvant radiotherapy when indicated. However, patients presenting with unresectable disease may be converted to surgical candidates with neoadjuvant RT or chemoradiotherapy. Furthermore, some patients may never be surgical candidates owing to their age or comorbidities. The largest trial looking at chemoradiotherapy was the GOG 101 trial. Ninety-six patients with unresectable vulvar cancer were treated with split-course radiation to 47.6 Gy (2 courses of 23.8 Gy) combined with cisplatin and 5-FU chemotherapy. This was followed by resection of the residual tumor and bilateral inguinal lymph node dissection. For patients with N2 or N3 lymphadenopathy (n=41), 95% became resectable and of these patients, 41% were found to have had a complete pathological response (51).
Since split course subsequently fell into disfavour, the subsequent GOG trial (52) used standard fractionation of 1.8 Gy daily to a total dose of 57.6 Gy combined with weekly cisplatin (40 mg/m2) and followed by surgical resection for any residual disease. A total of 58 women with T3-T4N0-3 vulvar SCC were treated, 47% of whom were over the age of 60 and 24% over the age of 70. These results were even more encouraging with complete clinical response seen in 64% (n=37).
Anal SCC
SCC of the anal canal also shares many important features with penile cancer. Since abdominoperineal resection results in significant morbidity, sphincter preserving strategies have been widely adopted with excellent success. Two major European trials have demonstrated superiority of chemoradiotherapy to radiotherapy alone. The UK Coordinating Committee on Cancer Research (UKCCCR) ACT 1 trial randomized 585 patients of any stage to RT alone (45 Gy to the pelvis with a 15–35 Gy boost to the primary) or to chemo-RT with 5-FU and mitomycin C. Both complete response (39% vs. 30%, P=0.08), and local-regional control (53.7% vs. 29.5%) were improved (HR 0.46, P<0.001) (53). Similar results were seen in the EORTC trial, where 110 patients with locally advanced anal canal SCC were randomized to chemo-radiation (dose to 45 Gy in 25 fractions and 15–20 Gy boost with infusional 5-FU and mitomycin C) or radiotherapy alone. The improvement in loco regional control was 18% at 5 years (P=0.02) and in colostomy free survival was 32% (P=0.002) (54). These two large studies have made chemoradiotherapy the standard of care in anal cancers.
Cervical cancer
SCC of the cervix is also an HPV-mediated malignancy that shares similarities with penile cancer. In the cornerstone GOG 123 trial, 374 patients with bulky cervical cancer (>4 cm), were randomized to treatment with radiotherapy (45 Gy in 1.8–2 Gy fractions followed by 30 Gy LDR brachytherapy prescribed to point A) or radiation combined with intravenous cisplatin 40 mg/m2. At 72 months, 71% of patients receiving chemo-radiation were alive and disease-free, compared to 60% for RT alone. The adjusted hazard ratio for death was 0.63 (95% CI: 0.43–0.91, P<0.015), favouring chemoradiotherapy (55).
Applications to penile cancer and indications for chemoradiotherapy
The accumulated evidence supports the notion that chemo-radiation is a very effective treatment for anogenital SCC. Due to the relative rarity of penile cancer, randomized trials have not yet been performed. Down-staging locally advanced penile cancer with neoadjuvant chemoradiotherapy has been reported for 26 patients treated with cisplatin-based chemotherapy and radiotherapy to a median dose of 49 Gy (range, 18–70 Gy). In the absence of surgical resection, progression free survival was only a median of 6 months (range, 2–7 months), but given the variable and generally low dose of radiotherapy any conclusions may be unreliable.
Currently, the International Rare Cancers Initiative, Cancer Research UK (CRUK), Institute of Cancer Research (ICR) and National Cancer Institute (NCI) are collaborating in InPACT. This international phase III trial (clinicaltrials.gov NCT02305654), randomizes node positive penile cancer patients to one of three treatment arms using a Bayesian design: (I) ILND; (II); neoadjuvant chemotherapy (TIP regimen: docetaxel, ifosfamide and platinum) (56) followed by ILND; or (III) neoadjuvant chemoradiotherapy (45 Gy + concurrent weekly cisplatinum) followed by ILND. Following ILND, patients with high risk groin pathology or radiologic evidence of pelvic adenopathy, can be randomized between pelvic lymph node dissection or pelvic chemoradiotherapy. Data on the relative effectiveness of sequencing of surgery, radiation and chemotherapy will inform future clinical decision-making and define the role of adjuvant, neoadjuvant and definitive treatments in node positive penile cancer.
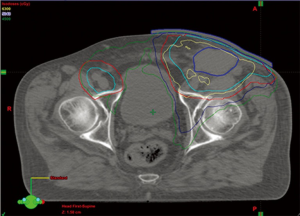
Current recommendations suggest treating locally advanced disease with radiation therapy and concurrent weekly cisplatin. Typically, the penile shaft, inguinal lymph node regions, prepubic fat and pelvic lymph node are treated to a dose of 45 Gy in 25 fractions. Intensity modulated radiation techniques are recommended to reduce toxicity to the central pelvic organs (Figure 8). This approach is recommended as neoadjuvant for inoperable loco-regionally advanced presentations who may benefit from down-staging. If patients are not-surgical candidates due to comorbidities or remain inoperable following neoadjuvant treatment, radiation would be continued to a dose appropriate for the bulk of disease, generally 54–57 Gy for nodal disease with concurrent platinum, and up to 63 Gy for the primary site. If chemotherapy is not administered, the total dose would be correspondingly increased.
Conclusions
Radiotherapy should play a key role in the management of penile SCC. Locally confined tumours may be treated with external beam radiation therapy or brachytherapy, with a good chance of penile preservation and improved quality of life. In the adjuvant setting, external beam radiation or chemo-radiation may be added to improve local-regional control after surgical nodal staging for multiple positive nodes or in the presence of extracapsular extension or incomplete resection. In non-surgical patients, a combination of radiation therapy and concurrent cisplatinum for radiation sensitization may be used for definitive treatment or for surgical down-staging, as appropriate. While direct level I evidence is evolving and we await the results of international trials such as InPACT, lessons learned from other anogenital malignancies would support the use of combination treatment.
Penile cancer is uncommon in Western societies and is often managed in the community where required specific expertise may be lacking. Patients should be considered for central referral to academic centers where a team approach and organ preservation strategies are available.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Minhas S, Manseck A, Watya S, et al. Penile cancer--prevention and premalignant conditions. Urology 2010;76:S24-35. [Crossref] [PubMed]
- Centers for Disease Control and Prevention (CDC). Human papillomavirus-associated cancers - United States, 2004-2008. MMWR Morb Mortal Wkly Rep 2012;61:258-61. [PubMed]
- Djajadiningrat RS, Jordanova ES, Kroon BK, et al. Human papillomavirus prevalence in invasive penile cancer and association with clinical outcome. J Urol 2015;193:526-31. [Crossref] [PubMed]
- Antunes AA, Dall'Oglio MF, Srougi M. Organ-sparing treatment for penile cancer. Nat Clin Pract Urol 2007;4:596-604. [Crossref] [PubMed]
- Shabbir M, Muneer A, Kalsi J, et al. Glans resurfacing for the treatment of carcinoma in situ of the penis: surgical technique and outcomes. Eur Urol 2011;59:142-7. [Crossref] [PubMed]
- Crook J. Contemporary Role of Radiotherapy in the Management of Primary Penile Tumors and Metastatic Disease. Urol Clin North Am 2016;43:435-48. [Crossref] [PubMed]
- Kaye V, Zhang G, Dehner LP, et al. Carcinoma in situ of penis. Is distinction between erythroplasia of Queyrat and Bowen's disease relevant? Urology 1990;36:479-82. [Crossref] [PubMed]
- Schroeder TL, Sengelmann RD. Squamous cell carcinoma in situ of the penis successfully treated with imiquimod 5% cream. J Am Acad Dermatol 2002;46:545-8. [Crossref] [PubMed]
- Skeppner E, Windahl T, Andersson SO, et al. Treatment-seeking, aspects of sexual activity and life satisfaction in men with laser-treated penile carcinoma. Eur Urol 2008;54:631-9. [Crossref] [PubMed]
- Windahl T, Skeppner E, Andersson SO, et al. Sexual function and satisfaction in men after laser treatment for penile carcinoma. J Urol 2004;172:648-51. [Crossref] [PubMed]
- Tietjen DN, Malek RS. Laser therapy of squamous cell dysplasia and carcinoma of the penis. Urology 1998;52:559-65. [Crossref] [PubMed]
- van Bezooijen BP, Horenblas S, Meinhardt W, et al. Laser therapy for carcinoma in situ of the penis. J Urol 2001;166:1670-1. [Crossref] [PubMed]
- Feldmeyer L, Krausz-Enderlin V, Tondury B, et al. Methylaminolaevulinic acid photodynamic therapy in the treatment of erythroplasia of Queyrat. Dermatology 2011;223:52-6. [Crossref] [PubMed]
- Shindel AW, Mann MW, Lev RY, et al. Mohs micrographic surgery for penile cancer: management and long-term followup. J Urol 2007;178:1980-5. [Crossref] [PubMed]
- Hadway P, Corbishley CM, Watkin NA. Total glans resurfacing for premalignant lesions of the penis: initial outcome data. BJU Int 2006;98:532-6. [Crossref] [PubMed]
- McLean M, Akl AM, Warde P, et al. The results of primary radiation therapy in the management of squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys 1993;25:623-8. [Crossref] [PubMed]
- Maddineni SB, Lau MM, Sangar VK. Identifying the needs of penile cancer sufferers: a systematic review of the quality of life, psychosexual and psychosocial literature in penile cancer. BMC Urol 2009;9:8. [Crossref] [PubMed]
- Bullen K, Edwards S, Marke V, et al. Looking past the obvious: experiences of altered masculinity in penile cancer. Psychooncology 2010;19:933-40. [Crossref] [PubMed]
- Kieffer JM, Djajadiningrat RS, van Muilekom EA, et al. Quality of life for patients treated for penile cancer. J Urol 2014;192:1105-10. [Crossref] [PubMed]
- Azrif M, Logue JP, Swindell R, et al. External-beam radiotherapy in T1-2 N0 penile carcinoma. Clin Oncol (R Coll Radiol) 2006;18:320-5. [Crossref] [PubMed]
- Zouhair A, Coucke PA, Jeanneret W, et al. Radiation therapy alone or combined surgery and radiation therapy in squamous-cell carcinoma of the penis? Eur J Cancer 2001;37:198-203. [Crossref] [PubMed]
- Sarin R, Norman AR, Steel GG, et al. Treatment results and prognostic factors in 101 men treated for squamous carcinoma of the penis. Int J Radiat Oncol Biol Phys 1997;38:713-22. [Crossref] [PubMed]
- Hasan S, Francis A, Hagenauer A, et al. The role of brachytherapy in organ preservation for penile cancer: A meta-analysis and review of the literature. Brachytherapy 2015;14:517-24. [Crossref] [PubMed]
- de Crevoisier R, Slimane K, Sanfilippo N, et al. Long-term results of brachytherapy for carcinoma of the penis confined to the glans (N- or NX). Int J Radiat Oncol Biol Phys 2009;74:1150-6. [Crossref] [PubMed]
- Crook J, Jezioranski J, Cygler JE. Penile brachytherapy: technical aspects and postimplant issues. Brachytherapy 2010;9:151-8. [Crossref] [PubMed]
- Crook J, Ma C, Grimard L. Radiation therapy in the management of the primary penile tumor: an update. World J Urol 2009;27:189-96. [Crossref] [PubMed]
- Solsona E, Iborra I, Rubio J, et al. Prospective validation of the association of local tumor stage and grade as a predictive factor for occult lymph node micrometastasis in patients with penile carcinoma and clinically negative inguinal lymph nodes. J Urol 2001;165:1506-9. [Crossref] [PubMed]
- Marinello G. Paris System for Interstitial Brachytherapy. In: Lemoigne Y, Caner A. editors. Radiotherapy and Brachytherapy. Dordrecht: Springer Netherlands, 2009:(219-25).
- Mazeron JJ, Langlois D, Lobo PA, et al. Interstitial radiation therapy for carcinoma of the penis using iridium 192 wires: the Henri Mondor experience (1970-1979). Int J Radiat Oncol Biol Phys 1984;10:1891-5. [Crossref] [PubMed]
- Delannes M, Malavaud B, Douchez J, et al. Iridium-192 interstitial therapy for squamous cell carcinoma of the penis. Int J Radiat Oncol Biol Phys 1992;24:479-83. [Crossref] [PubMed]
- Rozan R, Albuisson E, Giraud B, et al. Interstitial brachytherapy for penile carcinoma: a multicentric survey (259 patients). Radiother Oncol 1995;36:83-93. [Crossref] [PubMed]
- Soria JC, Fizazi K, Piron D, et al. Squamous cell carcinoma of the penis: multivariate analysis of prognostic factors and natural history in monocentric study with a conservative policy. Ann Oncol 1997;8:1089-98. [Crossref] [PubMed]
- Chaudhary AJ, Ghosh S, Bhalavat RL, et al. Interstitial brachytherapy in carcinoma of the penis. Strahlenther Onkol 1999;175:17-20. [Crossref] [PubMed]
- Kiltie AE, Elwell C, Close HJ, et al. Iridium-192 implantation for node-negative carcinoma of the penis: the Cookridge Hospital experience. Clin Oncol (R Coll Radiol) 2000;12:25-31. [PubMed]
- Pimenta A, Gutierrez C, Mosquera D, et al. Penile brachytherapy-Retrospective review of a single institution. Brachytherapy 2015;14:525-30. [Crossref] [PubMed]
- Petera J, Sirak I, Kasaova L, et al. High-dose rate brachytherapy in the treatment of penile carcinoma--first experience. Brachytherapy 2011;10:136-40. [Crossref] [PubMed]
- Sharma DN, Joshi NP, Gandhi AK, et al. High-dose-rate interstitial brachytherapy for T1-T2-stage penile carcinoma: short-term results. Brachytherapy 2014;13:481-7. [Crossref] [PubMed]
- Crook JM, Haie-Meder C, Demanes DJ, et al. American Brachytherapy Society-Groupe Europeen de Curietherapie-European Society of Therapeutic Radiation Oncology (ABS-GEC-ESTRO) consensus statement for penile brachytherapy. Brachytherapy 2013;12:191-8. [Crossref] [PubMed]
- Kellas-Sleczka S, Bialas B, Fijalkowski M, et al. Interstitial HDR Brachytherapy for Penile Cancer: A Thirteen-Year Follow Up of 55 Patients. Brachytherapy 2015;14:S33. [Crossref]
- Rouscoff Y, Falk AT, Durand M, et al. High-dose rate brachytherapy in localized penile cancer: short-term clinical outcome analysis. Radiat Oncol 2014;9:142. [Crossref] [PubMed]
- Neave F, Neal AJ, Hoskin PJ, et al. Carcinoma of the penis: a retrospective review of treatment with iridium mould and external beam irradiation. Clin Oncol (R Coll Radiol) 1993;5:207-10. [Crossref] [PubMed]
- Helou J, Morton G, Easton H, et al. Customized Penile Plesiobrachytherapy Using Latest Stereolithography Techniques. Brachytherapy 2015;14:S99-100. [Crossref]
- Matys R, Kubicka-Mendak I, Łyczek J, et al. Penile cancer brachytherapy HDR mould technique used at the Holycross Cancer Center. J Contemp Brachytherapy 2011;3:224-9. [Crossref] [PubMed]
- Gomez-Iturriaga A, Crook J, Evans W, et al. The efficacy of hyperbaric oxygen therapy in the treatment of medically refractory soft tissue necrosis after penile brachytherapy. Brachytherapy 2011;10:491-7. [Crossref] [PubMed]
- Ficarra V, Akduman B, Bouchot O, et al. Prognostic factors in penile cancer. Urology 2010;76:S66-73. [Crossref] [PubMed]
- Parra RO. Accurate staging of carcinoma of the penis in men with nonpalpable inguinal lymph nodes by modified inguinal lymphadenectomy. J Urol 1996;155:560-3. [Crossref] [PubMed]
- Ornellas AA, Kinchin EW, Nobrega BL, et al. Surgical treatment of invasive squamous cell carcinoma of the penis: Brazilian National Cancer Institute long-term experience. J Surg Oncol 2008;97:487-95. [Crossref] [PubMed]
- Homesley HD, Bundy BN, Sedlis A, et al. Prognostic factors for groin node metastasis in squamous cell carcinoma of the vulva (a Gynecologic Oncology Group study). Gynecol Oncol 1993;49:279-83. [Crossref] [PubMed]
- Kunos C, Simpkins F, Gibbons H, et al. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: a randomized controlled trial. Obstet Gynecol 2009;114:537-46. [Crossref] [PubMed]
- Montana GS, Thomas GM, Moore DH, et al. Preoperative chemo-radiation for carcinoma of the vulva with N2/N3 nodes: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys 2000;48:1007-13. [Crossref] [PubMed]
- Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol 2012;124:529-33. [Crossref] [PubMed]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Stehman FB, Ali S, Keys HM, et al. Radiation therapy with or without weekly cisplatin for bulky stage 1B cervical carcinoma: follow-up of a Gynecologic Oncology Group trial. Am J Obstet Gynecol 2007;197:503.e1-6. [Crossref] [PubMed]
- Pagliaro LC, Williams DL, Daliani D, et al. Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol 2010;28:3851-7. [Crossref] [PubMed]


