The importance of quality control and quality assurance in SDF testing
Dr. Kadioglu and Ortac from Turkey commented about the advantages and shortcomings of SDF testing in the clinical scenarios discussed by Agarwal et al. (1), and provided overall supportive remarks for the utility of SDF testing in selected populations (2). Notwithstanding, the authors noted that there is no consensus as to whether or not measurement of SDF provides any clinical benefit in the assessment of the male patient. Moreover, they highlighted existing guidelines issued by the American Urological Association (AUA) and European Association of Urology (EAU), which indicate that varicocele repair is not recommended for infertile men with normal semen analysis (3,4). Lastly, the authors inquired about the interlaboratory variation of SDF testing and which test should be considered the gold standard. In our reply to Drs. Kadioglu and Ortac, we elaborate on these aspects to provide readers further insights into these concerns.
In our guidelines, we reviewed the existing evidence and provided practical recommendations graded according to the quality of the available evidence (1). The selected clinical scenarios are familiar to practicing clinicians, and all of them pose difficulties for management. Despite concurring with the authors that additional prospective studies are needed to further clarify the clinical role of SDF for the evaluation of the infertile male, there is a bulk of literature demonstrating an association between SDF and reproductive outcomes [reviewed by Cho et al. (5)]. Given the fact that the integrity of sperm DNA is crucial for normal fertilization, embryo development, and successful implantation, we ponder that SDF testing provides clear information that adds to conventional semen analysis results without being superfluous (6-8).
It might be argued that the prognostic clinical value of DNA integrity testing may not affect the treatment of couples, as noted by the ASRM guidelines (9). However, new evidence has emerged particularly concerning the use of testicular in preference over ejaculated sperm for ICSI among couples whose male partners have high SDF in the neat ejaculate (10,11). Along the same lines, although the AUA and EAU guidelines regarding varicocele management advocate against surgery in the face of normal semen analysis [reviewed by Shridharani et al. (12)], it is worth mentioning that these guidelines based their recommendations on the grounds of routine semen analysis. It is well known that routine semen analysis is limited as a surrogate marker for male fertility (6); despite this fact, such limitations were neglected by the AUA/ EAU guidelines. Added to this, the AUA guidelines are yet to update semen analysis reference ranges to the newest 2010 WHO manual (3,13). Noteworthy, clinical practice guidelines are evolving documents that should undergo periodic review and updates.
Lastly, it is important to recognize that efforts have been made to standardize SDF testing (14-18). As part of the standardization process, inter- and intra-laboratory precision, and coefficient inter- and intra-observer variation have been calculated for tests such as SCSA, SCD, and flow-cytometer TUNEL (15-22). In Table 1, we summarize the relevant information provided by studies as regard to standardization of the SDF testing. Overall, these tests have adequate precision and repeatability as shown by the low coefficient of variation, which validate the assays for SDF assessment.
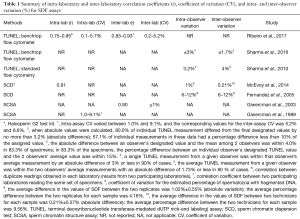
Full table
To sum up, SDF tests measure the proportion of cells with DNA damage or fragmentation. Test results reflect the quality of the whole semen specimen, and offer prognostic information as regards pregnancy, both naturally and assisted. Having emerged as a complementary tool to routine semen analysis, SDF testing may enable clinicians to better evaluate, counsel, and manage the male patient and the couple as a whole, particularly in challenging clinical scenarios like varicocele and borderline/normal semen analysis, unexplained infertility, recurrent miscarriages, and failed IUI, IVF and ICSI. We advise that SDF testing should be carried out in laboratories equipped with proper instrumentation, skilled technicians, and enrolled in internal and external quality control programs.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kadioglu A, Ortac M. The role of sperm DNA testing on male infertility. Transl Androl Urol 2017;6:S600-3.
- Agarwal A, Majzoub A, Esteves SC, et al. Clinical utility of sperm DNA fragmentation testing: practice recommendations based on clinical scenarios. Transl Androl Urol 2016;5:935-50. [Crossref] [PubMed]
- Jarow J, Sigman M, Kolettis PN, et al. Optimal Evaluation of the Infertile Male. Available online: [Last accessed on 2017 Mar 2].http://www.auanet.org/education/guidelines/male-infertility-d.cfm
- Jungwirth A, Diemer T, Dohle GR, et al. Guidelines on Male Infertility. Available online: [Last accessed on 2017 Feb 3].https://uroweb.org/wp-content/uploads/17-Male-Infertility_LR1.pdf
- Agarwal A, Cho CL, Esteves SC. Should we evaluate and treat sperm DNA fragmentation? Curr Opin Obstet Gynecol 2016;28:164-71. [Crossref] [PubMed]
- Esteves SC. Clinical relevance of routine semen analysis and controversies surrounding the 2010 World Health Organization criteria for semen examination. Int Braz J Urol 2014;40:443-53. [Crossref] [PubMed]
- Esteves SC, Hamada A, Kondray V, et al. What every gynecologist should know about male infertility: an update. Arch Gynecol Obstet 2012;286:217-29. [Crossref] [PubMed]
- Majzoub A, Esteves SC, Gosálvez J, et al. Specialized sperm function tests in varicocele and the future of andrology laboratory. Asian J Androl 2016;18:205-12. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Diagnostic evaluation of the infertile male: a committee opinion. opinion. Fertil Steril 2015;103:e18-25. [Crossref] [PubMed]
- Esteves SC, Sánchez-Martín F, Sánchez-Martín P, et al. Comparison of reproductive outcome in oligozoospermic men with high sperm DNA fragmentation undergoing intracytoplasmic sperm injection with ejaculated and testicular sperm. Fertil Steril 2015;104:1398-405. [Crossref] [PubMed]
- Bradley CK, McArthur SJ, Gee AJ, et al. Intervention improves assisted conception intracytoplasmic sperm injection outcomes for patients with high levels of sperm DNA fragmentation: a retrospective analysis. Andrology 2016;4:903-10. [Crossref] [PubMed]
- Shridharani A, Owen RC, Elkelany OO, et al. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl 2016;18:269-75. [Crossref] [PubMed]
- Esteves SC, Chan P. A systematic review of recent clinical practice guidelines and best practice statements for the evaluation of the infertile male. Int Urol Nephrol 2015;47:1441-56. [Crossref] [PubMed]
- Feijó CM, Esteves SC. Diagnostic accuracy of sperm chromatin dispersion test to evaluate sperm deoxyribonucleic acid damage in men with unexplained infertility. Fertil Steril 2014;101:58-63.e3. [Crossref] [PubMed]
- Evenson DP. The Sperm Chromatin Structure Assay (SCSA(®)) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim Reprod Sci 2016;169:56-75. [Crossref] [PubMed]
- Ribeiro S, Sharma R, Gupta S, et al. Inter- and intra-laboratory standardization of TUNEL assay for assessment of sperm DNA fragmentation. Andrology 2017;5:477-85. [Crossref] [PubMed]
- Sharma R, Ahmad G, Esteves SC, et al. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay using bench top flow cytometer for evaluation of sperm DNA fragmentation in fertility laboratories: protocol, reference values, and quality control. J Assist Reprod Genet 2016;33:291-300. [Crossref] [PubMed]
- Sharma RK, Sabanegh E, Mahfouz R, et al. TUNEL as a test for sperm DNA damage in the evaluation of male infertility. Urology 2010;76:1380-6. [Crossref] [PubMed]
- McEvoy A, Roberts P, Yap K, et al. Development of a simplified method of human semen storage for the testing of sperm DNA fragmentation using the Halosperm G2 test kit. Fertil Steril 2014;102:981-8. [Crossref] [PubMed]
- Fernández JL, Muriel L, Goyanes V, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84:833-42. [Crossref] [PubMed]
- Giwercman A, Richthoff J, Hjøllund H, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril 2003;80:1404-12. [Crossref] [PubMed]
- Giwercman A, Spano M, Lähdetie J, et al. Quality assurance of semen analysis in multicenter studies. Asclepios. Scand J Work Environ Health 1999;25 suppl 1:23-5. [PubMed]


