A review of varicocele repair for pain
Introduction
A varicocele is an abnormal dilation of the veins within the pampiniform plexus of the spermatic cord, causing palpable or visible veins in the scrotum (1). While most varicoceles arise from abnormal retrograde flow within the internal spermatic venous system, another potential cause is venous tributaries from the external cremasteric system (2). The reported prevalence of varicoceles varies but is generally estimated to be approximately 15% (3). Indeed, a recently published, large cross-sectional study of 7,035 unselected young European men with median age of 19 reported a general population prevalence of varicocele to be 15.7% (4). The prevalence increases to up to 50% in men presenting with primary infertility (5,6), and 70% of those with secondary infertility (6).
Although most men remain asymptomatic, the most common clinical symptoms include male factor infertility and chronic scrotal pain (1). In men diagnosed with a varicocele, the incidence of pain is estimated to be up to 10% (7). While it is important to assess for other causes of chronic scrotal pain, the presence of a varicocele should be considered as a potential etiology.
The etiology of scrotal pain associated with varicoceles is not completely understood. Proposed mechanisms include compression of nearby neural fibers by the dilated venous complex, increased scrotal temperature, oxidative stress to the testicular parenchyma, and tissue ischemia secondary to venous stasis (1,8). The incidence of chronic scrotal pain secondary to varicocele is difficult to estimate, but chronic scrotal pain in general accounts for an estimated 100,000 patient visits per year in the United States (9).
Varicocele repair, also commonly known as varicocelectomy, is the accepted treatment of choice for chronic scrotal pain associated with a varicocele that has not responded to conservative measures. In this review, we discuss the evaluation and management of varicocele-associated pain, specifically focusing on the various surgical approaches and outcomes.
Presentation and evaluation
A varicocele is most commonly an incidental finding in an asymptomatic patient. Present in approximately 15% of the world’s male population (3,4), varicoceles are typically found on routine examinations, sports physicals for adolescents, or as incidental findings on imaging. For this reason, the clinician must be keenly aware of the type of pain that varicoceles may represent to avoid a misdiagnosis. Symptomatic varicoceles are typically described as a dull, aching, or throbbing pain in the testicle, scrotum, or groin; rarely, varicocele-induced pain can be acute, sharp or stabbing. A varicocele may also be described as a scrotal heaviness that worsens with exercise, activity, or after standing for prolonged periods of time. As a result, varicoceles can cause exercise intolerance that is unacceptable for many young healthy men. Hence, investigations surrounding varicocele-related scrotal pain often occur in police or military training programs (7,10,11).
During the initial evaluation, the location, severity, quality, and frequency of pain should be documented, along with exacerbating and alleviating factors. Scrotal or testicular pain from a varicocele is almost always chronic in nature, so it is important to elicit the duration of pain. Varicoceles arise during puberty, so varicocele-related scrotal or testicular pain is rarely a new diagnosis in older age men. Similarly, a varicocele is rarely the etiology for acute scrotal pain. Thus, when a patient presents to an emergency room for evaluation of acute scrotal pain, a scrotal ultrasound which is normal except for identifying a subclinical varicocele has often found nothing more than an incidental finding, and the evaluation for the true etiology of the acute pain must continue without distraction by the incidental finding.
Physical examination is the gold standard for diagnosing a varicocele (12). Inspection and palpation of the scrotum should occur with the patient in the standing and supine positions, with and without a Valsalva maneuver, in a warm room to facilitate relaxation of the cremasteric and dartos muscle fibers of the scrotum. The varicocele is graded based on the ability of the examiner to visualize and/or palpate the dilated spermatic cord veins in both the relaxed state and while inducing Valsalva. The currently accepted clinical grading scale is based on the Dubin and Amelar classification system (13,14). Grade I varicoceles are palpable only with Valsalva, grade II varicoceles are palpable without Valsalva, and grade III varicoceles are easily visible through the scrotal skin without the need for palpation or Valsalva. A subclinical varicocele is not visible or palpable, and is diagnosed incidentally with imaging. Although the grade is clinically relevant, controversy exists over the ability to predict successful outcomes after treatment for pain based on such characteristics (15-17). Additionally, a varicocele can result in testicular atrophy, which may impact testicular functions that include testosterone production and spermatogenesis; therefore, careful assessment of signs and symptoms of hypogonadism, testicular volume, and symmetry between the two testes are of particular importance during the history and physical examination.
The majority of varicoceles are left-sided due to the drainage of the left gonadal vein into the higher resistance left renal vein compared with the resistance of the right gonadal vein directly draining into the vena cava. Varicoceles found on the right side are usually identified only when bilateral varicoceles are present together. However, an isolated right varicocele, or one that is irreducible in the supine position, necessitates additional investigation into underlying retroperitoneal etiologies.
Rule out other causes of chronic scrotal pain
Essential to the diagnostic workup of any male with chronic scrotal pain, even when a clinically palpable varicocele is present on physical examination, is to evaluate for other possible causes of pain. A varicocele is a diagnosis of exclusion when considered as an etiology of chronic pain, simply owing to the frequency of the diagnosis in the general population and its often-asymptomatic presentation. Other etiologies of scrotal and testicular pain include recent testicular or groin trauma, testicular malignancy, benign intratesticular or paratesticular masses, hydroceles, spermatoceles and epididymal cysts, inguinal hernias or more elusive inguinal sports hernias, post-inguinal hernia surgery spermatic cord or nerve entrapment, post-vasectomy pain, and infection and/or inflammation including prostatitis, orchitis, and epididymitis. Each must be considered and ruled out appropriately prior to recommending intervention for a varicocele suspected to be the source of scrotal pain. For all of these reasons, very careful examination of the groin, spermatic cord, testicle, epididymis, and scrotal skin is imperative, even when a varicocele is quickly recognized.
In addition to history and physical examination, urinalysis and urine culture should be obtained if indicated. In men with a history of previous inguinal hernia or vasectomy who present with a varicocele and pain, a spermatic cord block may be beneficial (18,19). Spermatic cord block with a long acting local anesthetic may aid in diagnosing neuropathic pain in addition to providing guidance as to the type of intervention that may be warranted. For example, if a patient has a greater than 50% reduction in pain after spermatic cord block, microsurgical cord denervation may be an effective treatment option (19,20). Men who report <50% resolution of symptoms after spermatic cord block may still benefit from varicocele repair, or they may have other mechanisms for pain such as unrecognized pathology, central sensitization, a coexisting pudendal pathway, or even malingering behavior (18).
Fertility evaluation
All men of any age diagnosed with a symptomatic varicocele should undergo a complete reproductive and sexual history, even when pain is the presenting complaint. Patients considering invasive treatment for symptomatic varicoceles should be offered formal baseline assessment of their fertility regardless of age, unless future fertility is not desired. Because a varicocele can adversely affect both of the primary functions of the testicle, including spermatogenesis and testosterone production, this fertility assessment should include a semen analysis as well as a reproductive hormone profile including a morning total testosterone, luteinizing hormone, and follicle stimulating hormone (12).
Imaging
Whereas the use of scrotal ultrasonography in the workup of men with infertility and a varicocele is not recommended except in cases of an indeterminate or difficult physical examination, imaging should be considered in the evaluation of a male with chronic scrotal pain to rule out other potential intrascrotal or abdominopelvic pathology. Because varicocele as a source of chronic scrotal pain is a diagnosis of exclusion, all sources of direct and referred pain must be considered. Pelvic ultrasound is an inexpensive and non-invasive measure to consider in addition to the commonly obtained scrotal ultrasound. Additionally, computed tomography (CT) or magnetic resonance imaging (MRI) of the abdomen and pelvis could be considered to provide more detailed anatomical views of areas that can contribute to referred scrotal pain.
Initial management
The management of symptomatic varicoceles should always begin with conservative measures and a period of observation. Treatment with non-steroidal anti-inflammatory drugs, scrotal support, and activity limitations provide adequate pain relief in a sizeable patient population. Secondary treatment options for medical management of chronic orchialgia, many of which may apply to patients with varicocele-related pain, will be reviewed in another chapter within this issue. In addition to conservative measures and secondary medical management strategies, a several-month-long period of observation will allow other potential subtle sources of pain (e.g., minor trauma, strained groin muscle) to resolve. If such conservative measures fail to improve symptoms, activity limitations are not possible, and/or symptoms remain bothersome after a period of observation, procedural intervention is indicated.
Surgical management—varicocele repair
When other causes of scrotal pain have been ruled out, and conservative measures have failed, varicocele repair is often effective in the treatment of a painful varicocele. Various techniques for venous ligation have been described—including retroperitoneal, inguinal, subinguinal, and scrotal approaches. Additionally, these techniques have been augmented with the use of laparoscopy, loupe magnification, and the operating microscope for enhanced visualization. The addition of microvascular Doppler has improved identification of the testicular arteries. Each technique has its relative advantages and pitfalls; however, for reasons presented below, the open subinguinal approach utilizing the operating microscope has proven to be the gold standard treatment.
Indications for repair
Historically, guidelines have reserved operative management of varicoceles for infertile males with subnormal semen analyses (21). More recently, however, the American Society for Reproductive Medicine, together with the Society for Male Reproduction and Urology, published updated guidelines regarding the evaluation and management of varicoceles. These guidelines state that persistent scrotal pain related to a varicocele that does not improve with conservative management is an indication for repair, regardless of fertility status (12). These guidelines represent an expanding body of evidence supporting varicocele repair for unremitting scrotal pain.
Alternatives
The primary alternative to surgical varicocele repair is angiographic embolization of the refluxing internal spermatic veins. Various techniques for venous occlusion are utilized, including sclerosing solutions, balloons, and stainless steel coils. Early reports of percutaneous embolization demonstrated lower technical success rates than varicocele repair (22), but improvements in technique, equipment and thrombogenic materials have led to improved success rates. It is also important to note that while these procedures are non-operative, they can prove exceedingly difficult and may require more time and have a similar convalescence compared with surgical varicocele repair.
A recent series of 154 patients undergoing varicocele embolization for pain in 2014 reported ultrasonographic success in 69%, with rates of clinical success and relapse/persistence of 87% and 13%, respectively, at 3–6 months that persisted at a median follow-up of 39 months. Complications included technical difficulties such as “venous valves, venous rupture, coil displacement, etc.” in 4.5% and failure to complete the procedure in an additional 4.5% (for a total 9% considered to have technical difficulty or failure), and hydrocele in 5% (23). Despite these results, embolization is typically reserved for treatment of recurrent varicocele after surgical repair when the anatomy causing the varicocele needs to be radiographically defined (8,24-26).
Predictors of success
Specific characteristics of varicoceles may predict the success of pain relief after repair. One characteristic shown to have an effect is duration of pain prior to intervention (15,27). In one series of 284 patients reporting an 86% complete resolution of pain, 19 (7%) patients had persistent scrotal pain after varicocele repair. Of these 19 patients, 17 began to experience pain less than 3 months prior to presentation (28), further highlighting the need for an initial attempt at conservative therapy. Others have suggested that success in pain relief is correlated with pre-operative varicocele grade, with risk of persistent pain more likely in higher grade varicoceles (11). The character of the pain itself may also predict success. A majority of patients with pain related to varicocele described their pain as a dull ache, dragging, or heaviness isolated to the scrotum, typically after prolonged standing. Pain described as sharp or radiating to the groin or thigh may still be related to varicocele, but also may be more likely to persist after surgery (7,29). Other factors, such as pain severity and body mass index have been proposed as predictors of response to varicocele repair, but have not consistently demonstrated an association.
Surgical approaches to varicocele repair
The earliest recorded varicocele repairs were performed via a scrotal approach; however, given the intimate relationship between the pampiniform plexus and the testicular artery at the level of the scrotum, this technique was abandoned due to its high rate of testicular artery injury and subsequent testicular atrophy. Currently acceptable surgical techniques for varicocele repair include retroperitoneal (laparoscopic or open), inguinal, and subinguinal approaches.
Adjuncts to surgical repair
Within the scope of open repair, there exist several useful adjuncts to include loupe magnification, the operating microscope, and microvascular Doppler ultrasound. Each of these tools help allow for accurate identification of lymphatic, arterial, and venous vessels, dramatically reducing the incidence of the three most dreaded complications of varicocele repair: recurrent or persistent varicocele, post-operative hydrocele formation, and testicular artery injury with resulting testicular atrophy or loss.
A 2014 study compared the identification of arteries, veins, and lymphatics of the same 33 open inguinal varicocele repairs, first with dissection using 3.5× loupe magnification, followed by the use of the operating microscope on the same spermatic cord (30). The operating microscope identified significantly more internal spermatic veins (5.70 vs. 4.39), arteries (1.51 vs. 0.97), and lymphatics (3.52 vs. 1.61) compared with the loupe-assisted dissection (P<0.001, P<0.001, P<0.001, respectively), and even more concerning, 0.36±0.55 arteries were to be ligated had the microscope not have been used. This eloquent study confirms the superiority of the operating microscope for varicocele repairs (30).
In a separate, but equally eloquent surgical study, Guo et al. compared in a randomized trial the surgical outcomes and complications between microsurgical subinguinal varicocele repair with intraoperative vascular Doppler ultrasound-assisted microsurgical subinguinal varicocele repair for 172 infertile patients with varicoceles (31). The group undergoing Doppler ultrasound-assisted repair had an increased number of spermatic arteries identified and spared (1.9±0.8 vs. 1.3±0.7, P<0.05), and ultimately improved semen parameters after surgery (31).
Retroperitoneal approach
In the open retroperitoneal approach, an incision is made over the internal inguinal ring, the external and internal oblique musculatures are divided, transversalis fascia is entered, and the peritoneum is retracted medially. The spermatic vessels are ligated lateral to the ureter. The laparoscopic approach ligates the spermatic veins at the same level, but may provide better visualization of the testicular artery and lymphatics with less incisional pain.
The main advantage of this approach is simplicity—there are relatively few spermatic veins at this location, they are larger in size, and are easily separated from the spermatic artery. The main disadvantage of this approach is the high incidence of varicocele recurrence due to collateral venous drainage proximal to the point of ligation. The complications are discussed in more detail below.
Inguinal approach
An inguinal incision is made superior to the external inguinal ring, and the external oblique fascia is incised. The cord is isolated, and spermatic veins are ligated with or without the assistance of an operating microscope. The potential advantage of this approach over the subinguinal approach is that all of the internal spermatic veins may be safely ligated within the inguinal canal with a lower risk of arterial injury or missed veins, because fewer veins are present within the cord in the inguinal canal and the arteries are fewer, larger, and more easily identified. The primary disadvantage of this approach is the need to incise fascia and muscle, thus a longer return to convalescence. There is also a slightly higher incidence of varicocele recurrence with the inguinal approach over the subinguinal approach due to the external spermatic and cremasteric veins which are not identified with the inguinal approach.
Subinguinal approach
The microsurgical subinguinal varicocele repair is the gold standard approach due to the lack of a musculofascial incision with less post-operative pain and the lowest risk of complications (detailed below). There is a lower rate of recurrence with the subinguinal approach partially because of the ability to ligate the cremasteric veins at this level (32). The disadvantages of the subinguinal approach include a larger number of veins requiring ligation than in the more proximal approaches, and the greater potential for arterial injury, as the testicular arteries below the external inguinal ring are often densely adherent to spermatic veins at this location. This risk is minimized with the use of the operating microscope and the microvascular Doppler ultrasound (30,31).
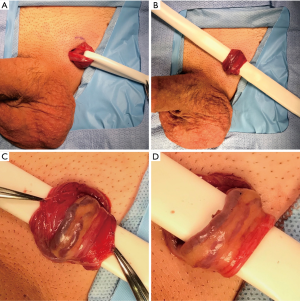
The authors of this review prefer the microsurgical subinguinal approach, and thus our preferred technique is described here. A subinguinal varicocele repair begins with a 2 cm subinguinal incision made along Langer’s lines below the external inguinal ring. Scarpa’s fascia is opened, and the spermatic cord is identified and gently grasped with a spermatic cord clamp. The cord is then delivered into the wound and secured with a 1/4” Penrose drain to allow brief exploration of the floor of the inguinal canal (Figure 1A). A 1/2” Penrose with a sterile tongue depressor within it is placed beneath the cord as a platform which is attached to the drapes (Figure 1B). The operating microscope is then docked. The external spermatic fascia is assessed for any large, dilated cremasteric veins which are ligated after carefully identifying and sparing any adjacent external spermatic arteries using the 20 MHz microvascular Doppler probe. The external spermatic fascia is opened longitudinally for 2–3 cm with electrocautery (Figure 1C). A Kittner blunt dissecting sponge is used to circumferentially free the internal cord structures from the external spermatic fascia and cremasteric fibers, after which the Penrose platform is repositioned to drop the external fascia and cremaster below (Figure 1D).
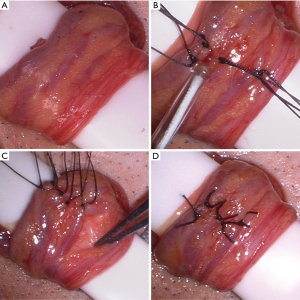
The internal cord structures, vas deferens, and vasal blood supply are carefully identified, and the vas deferens and vessels are positioned laterally and left away from the area of the dissection, as the vasal blood supply becomes the primary venous return after the repair is complete (Figure 2A). The internal spermatic fascia is opened with electrocautery and the internal arteries and veins are assessed with the microvascular Doppler ultrasound (Figure 2B). From a lateral to medial direction, maintaining the same plane of dissection, the veins are identified and each ligated with one or two 3-0 silk free ties, being sure to maintain the vas deferens and vasal blood supply lateral and protected from the area of dissection (Figure 2C). These authors prefer permanent suture ligation, although clips may be used. We also prefer to not transect the veins after ligation as to limit the risk of bleeding. Throughout the dissection, the arteries are continually assessed for patency with the Doppler ultrasound. After all of the internal veins are ligated (Figure 2D), a proximal spermatic cord and peri-incisional subcutaneous block with 0.25% bupivicaine is performed prior to closure of Scarpa’s fascia followed by a running subcuticular closure of the skin with absorbable suture (Figure 3).
Complications of varicocele repair
The three most significant complications related specifically to varicocele repair include recurrent or persistent varicocele, hydrocele formation, and testicular artery injury. The rates of these complications vary widely based on approach, though they are universally decreased when using a microsurgical inguinal or subinguinal approach.
Historically, the most common complication after repair is recurrent or persistent varicocele. However, the rates of recurrence have been reduced from upwards of 20% using retroperitoneal or unmagnified inguinal approaches to, when using microsurgical inguinal techniques, nearly 0% (22,33). An inguinal or subinguinal approach allows for identification and ligation of scrotal and inguinal venous collaterals that are left intact with a retroperitoneal approach. Similarly, unmagnified inguinal approaches fail to ligate the small veins surrounding the testicular artery, which are more easily seen with loupe magnification or especially the operating microscope (30).
Hydrocele formation after varicocele repair occurs as a result of lymphatic obstruction. Over half of hydroceles that develop as a result of varicocele repair grow to a size warranting surgical excision, either due to discomfort or size alone. The rate of hydrocele formation also varies with technique, and mirrors the trends seen with recurrent varicoceles in terms of approach. The development of hydrocele after repair occurs at a much greater frequency with retroperitoneal (7–20%) and macroscopic inguinal (7–13%) techniques, as compared to microsurgical (0–2%). The use of magnification helps to identify and preserve lymphatics, essentially eliminating the risk of hydrocele after varicocele repair (32,34).
The testicular artery supplies two thirds of the testicular blood supply, and injury can result in testicular atrophy and/or impaired spermatogenesis. Given its small size (1.0–1.5 mm in diameter) and intimate relationship to venous structures targeted for ligation, the risk of injury to the testicular artery is not trivial. Though the actual incidence of injury and subsequent testicular atrophy is unknown, the risk of injury can be made nearly negligible by using magnification (preferably an operating microscope) and Doppler ultrasound, which assist in identification and preservation of the testicular arteries (30,31).
Outcomes of varicocele repair for pain
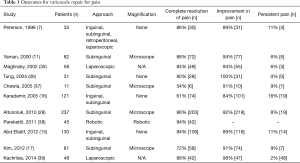
Varicocele repair performed for pain, regardless of approach, is effective in the vast majority of cases (Table 1). Contemporary series report partial or complete resolution of pain in 83–100% of patients, with the average success rate in published series approaching 92% (16,36). In the largest contemporary series, 237 patients underwent microsurgical subinguinal varicocele ligation for pain; 203 patients (86%) experienced complete resolution of pain, whereas 218 patients (92%) experienced significant improvement (28). Retroperitoneal varicocele repairs also appear to be quite effective for pain, with over 90% of patients reporting improvement in pain symptoms (36,38); however, as discussed above, the increased complication rate of this approach makes the microsurgical inguinal and subinguinal approaches far superior. Based on the reviewed literature presented in Table 1, patients undergoing a varicocele repair for pain should be quoted a risk of persistent pain after the procedure of approximately 8% on average, which was as high as 16% in one study.
Full table
Conclusions
Varicoceles occur in approximately 15% of the male population. Pain is a common complaint, affecting up to 10% of these patients. Many patients respond to conservative measures, but for patients with painful varicoceles who have failed non-surgical management and for whom other causes of scrotal pain have been ruled out, varicocele repair is indicated and has shown to be safe and effective. Though there exist several approaches to the procedure, an inguinal or subinguinal technique coupled with the use of the operating microscope and microvascular Doppler ultrasound has been shown to be the gold standard treatment. Using this approach, the incidence of varicocele recurrence/persistence, hydrocele formation, and testicular artery injury are negligible, and approximately 90% of patients will achieve symptomatic relief.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Lomboy JR, Coward RM. The Varicocele: Clinical Presentation, Evaluation, and Surgical Management. Semin Intervent Radiol 2016;33:163-9. [Crossref] [PubMed]
- Coolsaet BL. The varicocele syndrome: venography determining the optimal level for surgical management. J Urol 1980;124:833-9. [PubMed]
- Thomason AM, Fariss BL. The prevalence of varicoceles in a group of healthy young men. Mil Med 1979;144:181-2. [PubMed]
- Damsgaard J, Joensen UN, Carlsen E, et al. Varicocele Is Associated with Impaired Semen Quality and Reproductive Hormone Levels: A Study of 7035 Healthy Young Men from Six European Countries. Eur Urol 2016;70:1019-29. [Crossref] [PubMed]
- Gorelick JI, Goldstein M. Loss of fertility in men with varicocele. Fertil Steril 1993;59:613-6. [Crossref] [PubMed]
- Witt MA, Lipshultz LI. Varicocele: a progressive or static lesion? Urology 1993;42:541-3. [Crossref] [PubMed]
- Peterson AC, Lance RS, Ruiz HE. Outcomes of varicocele ligation done for pain. J Urol 1998;159:1565-7. [Crossref] [PubMed]
- Khera M, Lipshultz LI. Evolving approach to the varicocele. Urol Clin North Am 2008;35:183-9. viii. [Crossref] [PubMed]
- Parekattil SJ, Gudeloglu A, Brahmbhatt JV, et al. Trifecta nerve complex: potential anatomical basis for microsurgical denervation of the spermatic cord for chronic orchialgia. J Urol 2013;190:265-70. [Crossref] [PubMed]
- Biggers RD, Soderdahl DW. The painful varicocele. Mil Med 1981;146:440-1. [PubMed]
- Yaman O, Ozdiler E, Anafarta K, et al. Effect of microsurgical subinguinal varicocele ligation to treat pain. Urology 2000;55:107-8. [Crossref] [PubMed]
- Practice Committee of the American Society for Reproductive M. Society for Male R, Urology. Report on varicocele and infertility: a committee opinion. Fertil Steril 2014;102:1556-60. [Crossref] [PubMed]
- Amelar RD, Dubin L. Therapeutic implications of left, right, and bilateral varicocelectomy. Urology 1987;30:53-9. [Crossref] [PubMed]
- Dubin L, Amelar RD. Varicocele size and results of varicocelectomy in selected subfertile men with varicocele. Fertil Steril 1970;21:606-9. [Crossref] [PubMed]
- Abd Ellatif ME, Asker W, Abbas A, et al. Varicocelectomy to treat pain, and predictors of success: a prospective study. Curr Urol 2012;6:33-6. [Crossref] [PubMed]
- Karademir K, Senkul T, Baykal K, et al. Evaluation of the role of varicocelectomy including external spermatic vein ligation in patients with scrotal pain. Int J Urol 2005;12:484-8. [Crossref] [PubMed]
- Kim SO, Jung H, Park K. Outcomes of microsurgical subinguinal varicocelectomy for painful varicoceles. J Androl 2012;33:872-5. [Crossref] [PubMed]
- Benson JS, Abern MR, Larsen S, et al. Does a positive response to spermatic cord block predict response to microdenervation of the spermatic cord for chronic scrotal content pain? J Sex Med 2013;10:876-82. [Crossref] [PubMed]
- Marconi M, Palma C, Troncoso P, et al. Microsurgical Spermatic Cord Denervation as a Treatment for Chronic Scrotal Content Pain: A Multicenter Open Label Trial. J Urol 2015;194:1323-7. [Crossref] [PubMed]
- Larsen SM, Benson JS, Levine LA. Microdenervation of the spermatic cord for chronic scrotal content pain: single institution review analyzing success rate after prior attempts at surgical correction. J Urol 2013;189:554-8. [Crossref] [PubMed]
- Male Infertility Best Practice Policy Committee of the American Urological Association. Practice Committee of the American Society for Reproductive Medicine. Report on varicocele and infertility. Fertil Steril 2004;82 Suppl 1:S142-5. [Crossref] [PubMed]
- Cayan S, Shavakhabov S, Kadioğlu A. Treatment of Palpable Varicocele in Infertile Men: A Meta-analysis to Define the Best Technique. J Androl 2009;30:33-40. [Crossref] [PubMed]
- Puche-Sanz I, Flores-Martin JF, Vazquez-Alonso F, et al. Primary treatment of painful varicocoele through percutaneous retrograde embolization with fibred coils. Andrology 2014;2:716-20. [Crossref] [PubMed]
- Cassidy D, Jarvi K, Grober E, et al. Varicocele surgery or embolization: Which is better? Can Urol Assoc J 2012;6:266-8. [Crossref] [PubMed]
- Kim J, Shin JH, Yoon HK, et al. Persistent or recurrent varicocoele after failed varicocoelectomy: outcome in patients treated using percutaneous transcatheter embolization. Clin Radiol 2012;67:359-65. [Crossref] [PubMed]
- Shridharani A, Owen RC, Elkelany OO, et al. The significance of clinical practice guidelines on adult varicocele detection and management. Asian J Androl 2016;18:269-75. [Crossref] [PubMed]
- Park HJ, Lee SS, Park NC. Predictors of pain resolution after varicocelectomy for painful varicocele. Asian J Androl 2011;13:754-8. [Crossref] [PubMed]
- Altunoluk B, Soylemez H, Efe E, et al. Duration of preoperative scrotal pain may predict the success of microsurgical varicocelectomy. Int Braz J Urol 2010;36:55-9. [Crossref] [PubMed]
- Al-Buheissi SZ, Patel HR, Wazait HD, et al. Predictors of success in surgical ligation of painful varicocele. Urol Int 2007;79:33-6. [Crossref] [PubMed]
- Zhang H, Liu XP, Yang XJ, et al. Loupe-assisted versus microscopic varicocelectomy: is there an intraoperative anatomic difference? Asian J Androl 2014;16:112-4. [Crossref] [PubMed]
- Guo L, Sun W, Shao G, et al. Outcomes of Microscopic Subinguinal Varicocelectomy With and Without the Assistance of Doppler Ultrasound: A Randomized Clinical Trial. Urology 2015;86:922-8. [Crossref] [PubMed]
- Marmar JL, Kim Y. Subinguinal microsurgical varicocelectomy: a technical critique and statistical analysis of semen and pregnancy data. J Urol 1994;152:1127-32. [PubMed]
- Al-Kandari AM, Shabaan H, Ibrahim HM, et al. Comparison of outcomes of different varicocelectomy techniques: open inguinal, laparoscopic, and subinguinal microscopic varicocelectomy: a randomized clinical trial. Urology 2007;69:417-20. [Crossref] [PubMed]
- Goldstein M, Gilbert BR, Dicker AP, et al. Microsurgical inguinal varicocelectomy with delivery of the testis: an artery and lymphatic sparing technique. J Urol 1992;148:1808-11. [PubMed]
- Maghraby HA. Laparoscopic varicocelectomy for painful varicoceles: merits and outcomes. J Endourol 2002;16:107-10. [Crossref] [PubMed]
- Tung MC, Huang WJ, Chen KK. Modified subinguinal varicocelectomy for painful varicocele and varicocele-associated infertility. J Chin Med Assoc 2004;67:296-300. [PubMed]
- Chawla A, Kulkarni G, Kamal K, et al. Microsurgical varicocelectomy for recurrent or persistent varicoceles associated with orchalgia. Urology 2005;66:1072-4. [Crossref] [PubMed]
- Parekattil SJ, Brahmbhatt JV. Robotic approaches for male infertility and chronic orchialgia microsurgery. Curr Opin Urol 2011;21:493-9. [Crossref] [PubMed]
- Kachrilas S, Popov E, Bourdoumis A, et al. Laparoscopic varicocelectomy in the management of chronic scrotal pain. JSLS 2014;18.